Effect of Anti-Inflammatory Diets on Pain in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
3. Results
4. Discussion
5. Other Information
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Search Strategy: MEDLINE (OVID)
Appendix A.2. Search Strategy: Embase (Elsevier)
Appendix A.3. Search Strategy: CINAHL with Full Text (EBSCOhost)
Appendix B
| Outcome | Certainty Assessment | No. of Patients | Absolute Effect (95% CI) | Certainty | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Anti-Inflam- matory Diet | Ordinary Diet | |||
| Pain | 7 | randomized trials | very serious a | not serious | not serious | serious b | none | 172 | 154 | MD 9.22 lower (14.15 lower to 4.29 lower) | ⊕◯◯◯ very low |
| CRP | 5 | randomized trials | serious c | not serious | not serious | very serious d | none | 140 | 127 | MD 2.51 lower (6.10 lower to 1.08 higher) | ⊕◯◯◯ very low |
| ESR | 4 | randomized trials | serious e | not serious | not serious | serious f | none | 95 | 82 | MD 2.9 lower (7.67 lower to 1.87 higher) | ⊕⊕◯◯ low |
| HAQ | 4 | randomized trials | very serious g | not serious | not serious | serious f | none | 108 | 94 | MD 0.20 lower (0.36 lower to 0.05 lower) | ⊕⊕◯◯ low |
| SJC | 4 | randomized trials | very serious h | serious i | not serious | not serious | none | 112 | 102 | SMD 0.6 lower (1.08 lower to 0.11 lower) | ⊕◯◯◯ very low |
| TJC | 4 | randomized trials | very serious h | very serious j | not serious | serious f | none | 110 | 102 | SMD 0.39 lower (1.17 lower to 0.39 higher) | ⊕◯◯◯ very low |
| Weight loss | 6 | randomized trials | serious k | very serious j | not serious | not serious | none | 152 | 134 | MD 3.73 lower (5.45 lower to 2.01 lower) | ⊕◯◯◯ very low |
| BMI decrease | 4 | randomized trials | serious l | very serious j | not serious | not serious | none | 93 | 99 | MD 1.28 lower (1.89 lower to 0.67 lower) | ⊕◯◯◯ very low |
References
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Primers 2018, 4, 18001. [Google Scholar] [CrossRef]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Genel, F.; Kale, M.; Pavlovic, N.; Flood, V.M.; Naylor, J.M.; Adie, S. Health effects of a low-inflammatory diet in adults with arthritis: A systematic review and meta-analysis. J. Nutr. Sci. 2020, 9, e37. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, S.; Gautier, S.; Salem, N., Jr. Global Estimates of Dietary Intake of Docosahexaenoic Acid and Arachidonic Acid in Developing and Developed Countries. Ann. Nutr. Metab. 2016, 68, 258–267. [Google Scholar] [CrossRef] [Green Version]
- Grant, W.B. The role of meat in the expression of rheumatoid arthritis. Br. J. Nutr. 2000, 84, 589–595. [Google Scholar] [CrossRef] [Green Version]
- Pattison, D.J.; Symmons, D.P.; Lunt, M.; Welch, A.; Luben, R.; Bingham, S.A.; Khaw, K.T.; Day, N.E.; Silman, A.J. Dietary Risk Factors for the Development of Inflammatory Polyarthritis: Evidence for a Role of High Level of Red Meat Consumption. Arthritis Rheum 2004, 50, 3804–3812. [Google Scholar] [CrossRef]
- Siriwardhana, N.; Kalupahana, N.S.; Moustaid-Moussa, N. Health Benefits of n-3 Polyunsaturated Fatty Acids: Eicosapentaenoic Acid and Docosahexaenoic Acid. Adv. Food Nutr. Res. 2012, 65, 211–222. [Google Scholar] [CrossRef] [PubMed]
- James, M.; Proudman, S.; Cleland, L. Fish oil and rheumatoid arthritis: Past, present and future. Proc. Nutr. Soc. 2010, 69, 316–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.H.; Bae, S.C.; Song, G.G. Omega-3 Polyunsaturated Fatty Acids and the Treatment of Rheumatoid Arthritis: A Meta-analysis. Arch. Med. Res. 2012, 43, 356–362. [Google Scholar] [CrossRef]
- Miles, E.A.; Calder, P.C. Influence of marine n-3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br. J. Nutr. 2012, 107 (Suppl 2), S171–S184. [Google Scholar] [CrossRef] [Green Version]
- Dürholz, K.; Hofmann, J.; Iljazovic, A.; Häger, J.; Lucas, S.; Sarter, K.; Strowig, T.; Bang, H.; Rech, J.; Schett, G.; et al. Dietary Short-Term Fiber Interventions in Arthritis Patients Increase Systemic SCFA Levels and Regulate Inflammation. Nutrients 2020, 12, 3207. [Google Scholar] [CrossRef]
- Masino, S.A.; Ruskin, D.N. Ketogenic Diets and Pain. J. Child. Neurol. 2013, 28, 993–1001. [Google Scholar] [CrossRef] [Green Version]
- Korbecki, J.; Baranowska-Bosiacka, I.; Gutowska, I.; Chlubek, D. The Effect of Reactive Oxygen Species on the Synthesis of Prostanoids From Arachidonic Acid. J. Physiol. Pharmacol. 2013, 64, 409–421. [Google Scholar] [PubMed]
- Cronstein, B.N.; Sitkovsky, M. Adenosine and adenosine receptors in the pathogenesis and treatment of rheumatic diseases. Nat. Rev. Rheumatol. 2017, 13, 41–51. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Fries, J.F.; Spitz, P.; Kraines, R.G.; Holman, H.R. Measurement of Patient Outcome in Arthritis. Arthritis Rheum 1980, 23, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Prevoo, M.L.; van ’t Hof, M.A.; Kuper, H.H.; van Leeuwen, M.A.; van de Putte, L.B.; van Riel, P.L. Modified Disease Activity Scores That Include Twenty-eight-joint Counts: Development and Validation in a Prospective Longitudinal Study of Patients with Rheumatoid Arthritis. Arthritis Rheum 1995, 38, 44–48. [Google Scholar] [CrossRef] [Green Version]
- Fransen, J.; Welsing, P.; de Keijzer, R.; van Riel, P. Disease activity scores using C-reactive protein: CRP may replace ESR in the assessment of RA disease activity. Ann. Rheum. Dis. 2004, 62, 151. [Google Scholar]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.; Hernan, M.A.; Reeves, B.C.; Savovic, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [Green Version]
- Review Manager (RevMan) [Computer program]. Version 5.4, The Cochrane Collaboration, 2020. RRID:SCR_003581. Available online: www.training.cochrane.org/online-learning/core-software-cochrane-reviews/revman (accessed on 23 November 2021).
- Follmann, D.; Elliott, P.; Suh, I.; Cutler, J. Variance Imputation for Overviews of Clinical Trials with Continuous Response. J. Clin. Epidemiol. 1992, 45, 769–773. [Google Scholar] [CrossRef]
- Abrams, K.R.; Gillies, C.L.; Lambert, P.C. Meta-analysis of heterogeneously reported trials assessing change from baseline. Stat. Med. 2005, 24, 3823–3844. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Li, T.; Deeks, J.J. Choosing effect measures and computing estimates of effect. In Cochrane Handbook for Systematic Reviews of Interventions; Version 6.2 (updated February 2021); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane, 2021; Chapter 6; Available online: www.training.cochrane.org/handbook (accessed on 23 November 2021).
- Page, M.J.; Higgins, J.P.T.; Sterne, J.A.C. Assessing risk of bias due to missing results in a synthesis. In Cochrane Handbook for Systematic Reviews of Interventions; Version 6.2 (updated February 2021); Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane, 2021; Available online: www.training.cochrane.org/handbook (accessed on 23 November 2021).
- The GRADE Working Group. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations; Updated October 2013; Schünemann, H., Brożek, J., Guyatt, G., Oxman, A., Eds.; The GRADE Working Group, 2013; Available online: www.guidelinedevelopment.org/handbook (accessed on 23 November 2021).
- GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University, 2020 (developed by Evidence Prime, Inc.). RRID:SCR_021308. Available online: www.gradepro.org (accessed on 23 November 2021).
- Sköldstam, L.; Larsson, L.; Lindström, F.D. Effect of Fasting and Lactovegetarian Diet on Rheumatoid Arthritis. Scand. J. Rheumatol. 1979, 8, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen-Kragh, J.; Haugen, M.; Borchgrevink, C.F.; Laerum, E.; Eek, M.; Mowinkel, P.; Hovi, K.; Førre, O. Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. Lancet 1991, 338, 899–902. [Google Scholar] [CrossRef]
- Hafström, I.; Ringertz, B.; Spångberg, A.; von Zweigbergk, L.; Brannemark, S.; Nylander, I.; Rönnelid, J.; Laasonen, L.; Klareskog, L. A vegan diet free of gluten improves the signs and symptoms of rheumatoid arthritis: The effects on arthritis correlate with a reduction in antibodies to food antigens. Rheumatology 2001, 40, 1175–1179. [Google Scholar] [CrossRef] [Green Version]
- Sköldstam, L.; Hagfors, L.; Johansson, G. An experimental study of a Mediterranean diet intervention for patients with rheumatoid arthritis. Ann. Rheum. Dis. 2003, 62, 208–214. [Google Scholar] [CrossRef]
- Garcia-Morales, J.M.; Lozada-Mellado, M.; Hinojosa-Azaola, A.; Llorente, L.; Ogata-Medel, M.; Pineda-Juarez, J.A.; Alcocer-Varela, J.; Cervantes-Gaytan, R.; Castillo-Martinez, L. Effect of a Dynamic Exercise Program in Combination With Mediterranean Diet on Quality of Life in Women With Rheumatoid Arthritis. J. Clin. Rheumatol. 2020, 26, S116–S122. [Google Scholar] [CrossRef]
- Abendroth, A.; Michalsen, A.; Ludtke, R.; Ruffer, A.; Musial, F.; Dobos, G.J.; Langhorst, J. Changes of Intestinal Microflora in Patients with Rheumatoid Arthritis during Fasting or a Mediterranean Diet. Forsch Komplementmed 2010, 17, 307–313. [Google Scholar] [CrossRef]
- Von Koerber, K.; Männle, T.; Leitzmann, C. 4.5 Ernährung und Mikroflora des Verdauungstrakts. In Vollwert-Ernährung: Konzeption einer zeitgemäßenund nachhaltigen Ernährung, 10th ed.; Haug: Stuttgart, Germany, 2004; pp. 93–98. [Google Scholar]
- Adam, O.; Beringer, C.; Kless, T.; Lemmen, C.; Adam, A.; Wiseman, M.; Adam, P.; Klimmek, R.; Forth, W. Anti-inflammatory effects of a low arachidonic acid diet and fish oil in patients with rheumatoid arthritis. Rheumatol. Int. 2003, 23, 27–36. [Google Scholar] [CrossRef]
- Harris, J.A.; Benedict, F.G. A Biometric Study of Human Basal Metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingegnoli, F.; Schioppo, T.; Scotti, I.; Ubiali, T.; de Lucia, O.; Murgo, A.; Marano, G.; Boracchi, P.; Caporali, R. Adherence to Mediterranean diet and patient perception of rheumatoid arthritis. Complement Ther. Med. 2020, 52, 102519. [Google Scholar] [CrossRef] [PubMed]
- McDougall, J.; Bruce, B.; Spiller, G.; Westerdahl, J.; McDougall, M. Effects of a Very Low-Fat, Vegan Diet in Subjects with Rheumatoid Arthritis. J. Altern. Complement. Med. 2002, 8, 71–75. [Google Scholar] [CrossRef] [PubMed]
- McKellar, G.; Morrison, E.; McEntegart, A.; Hampson, R.; Tierney, A.; Mackle, G.; Scoular, J.; Scott, J.A.; Capell, H.A. A pilot study of a Mediterranean-type diet intervention in female patients with rheumatoid arthritis living in areas of social deprivation in Glasgow. Ann. Rheum. Dis. 2007, 66, 1239–1243. [Google Scholar] [CrossRef] [Green Version]
- Nenonen, M.T.; Helve, T.A.; Rauma, A.L.; Hänninen, O.O. Uncooked, Lactobacilli-rich, Vegan Food and Rheumatoid Arthritis. Br. J. Rheumatol. 1998, 37, 274–281. [Google Scholar] [CrossRef] [Green Version]
- Hänninen, O.; Nenonen, M.; Ling, W.H.; Li, D.S.; Sihvonen, L. Effects of Eating an Uncooked Vegetable Diet for 1 Week. Appetite 1992, 19, 243–254. [Google Scholar] [CrossRef]
- Sköldstam, L. Fasting and Vegan Diet in Rheumatoid Arthritis. Scand. J. Rheumatol. 1986, 15, 219–221. [Google Scholar] [CrossRef]
- De Lorgeril, M.; Renaud, S.; Mamelle, N.; Salen, P.; Martin, J.L.; Monjaud, I.; Guidollet, J.; Touboul, P.; Delaye, J. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet 1994, 343, 1454–1459. [Google Scholar] [CrossRef]
- Kahleova, H. (Physicians Committee for Responsible Medicine, Washington, DC, USA). Personal communication, 2021. [Google Scholar]
- Stauffer, M.E.; Taylor, S.D.; Watson, D.J.; Peloso, P.M.; Morrison, A. Definition of Nonresponse to Analgesic Treatment of Arthritic Pain: An Analytical Literature Review of the Smallest Detectable Difference, the Minimal Detectable Change, and the Minimal Clinically Important Difference on the Pain Visual Analog Scale. Int. J. Inflam. 2011, 2011, 231926. [Google Scholar] [CrossRef]
- Coenen, M.J.; Trynka, G.; Heskamp, S.; Franke, B.; van Diemen, C.C.; Smolonska, J.; van Leeuwen, M.; Brouwer, E.; Boezen, M.H.; Postma, D.S.; et al. Common and different genetic background for rheumatoid arthritis and coeliac disease. Hum. Mol. Genet. 2009, 18, 4195–4203. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.J.; Katz, J. A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain 2007, 129, 210–223. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Hazlewood, G.S.; Kaplan, G.G.; Eksteen, B.; Barnabe, C. Impact of Obesity on Remission and Disease Activity in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Arthritis Care Res. 2017, 69, 157–165. [Google Scholar] [CrossRef]
- Geneen, L.J.; Moore, R.A.; Clarke, C.; Martin, D.; Colvin, L.A.; Smith, B.H. Physical activity and exercise for chronic pain in adults: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2017, 4, CD011279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurkmans, E.; van der Giesen, F.J.; Vliet Vlieland, T.P.; Schoones, J.; van den Ende, E.C. Dynamic exercise programs (aerobic capacity and/or muscle strength training) in patients with rheumatoid arthritis. Cochrane Database Syst. Rev. 2009, CD006853. [Google Scholar] [CrossRef]
- Hagen, K.B.; Byfuglien, M.G.; Falzon, L.; Olsen, S.U.; Smedslund, G. Dietary interventions for rheumatoid arthritis. Cochrane Database Syst. Rev. 2009, CD006400. [Google Scholar] [CrossRef] [PubMed]
- Heiberg, T.; Finset, A.; Uhlig, T.; Kvien, T.K. Seven year changes in health status and priorities for improvement of health in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2005, 64, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Day, A.L.; Curtis, J.R. Opioid use in rheumatoid arthritis: Trends, efficacy, safety, and best practices. Curr. Opin. Rheumatol. 2019, 31, 264–270. [Google Scholar] [CrossRef]
- Kim, S.C.; Choudhry, N.; Franklin, J.M.; Bykov, K.; Eikermann, M.; Lii, J.; Fischer, M.A.; Bateman, B.T. Patterns and predictors of persistent opioid use following hip or knee arthroplasty. Osteoarthr. Cartil. 2017, 25, 1399–1406. [Google Scholar] [CrossRef] [Green Version]
- Kuo, Y.F.; Raji, M.A.; Chen, N.W.; Hasan, H.; Goodwin, J.S. Trends in Opioid Prescriptions Among Part D Medicare Recipients From 2007 to 2012. Am. J. Med. 2016, 129, 221.e21–221.e30. [Google Scholar] [CrossRef] [Green Version]
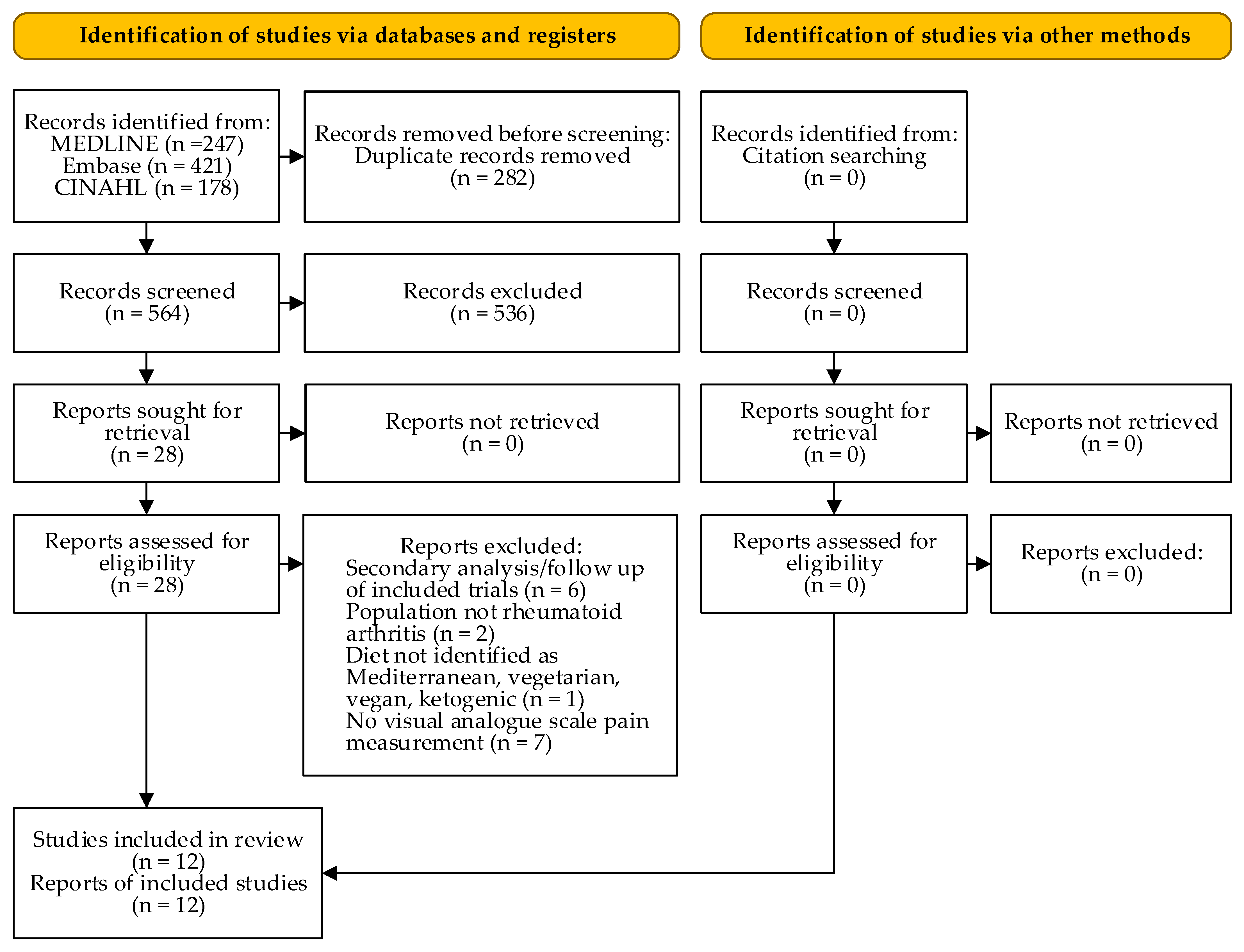
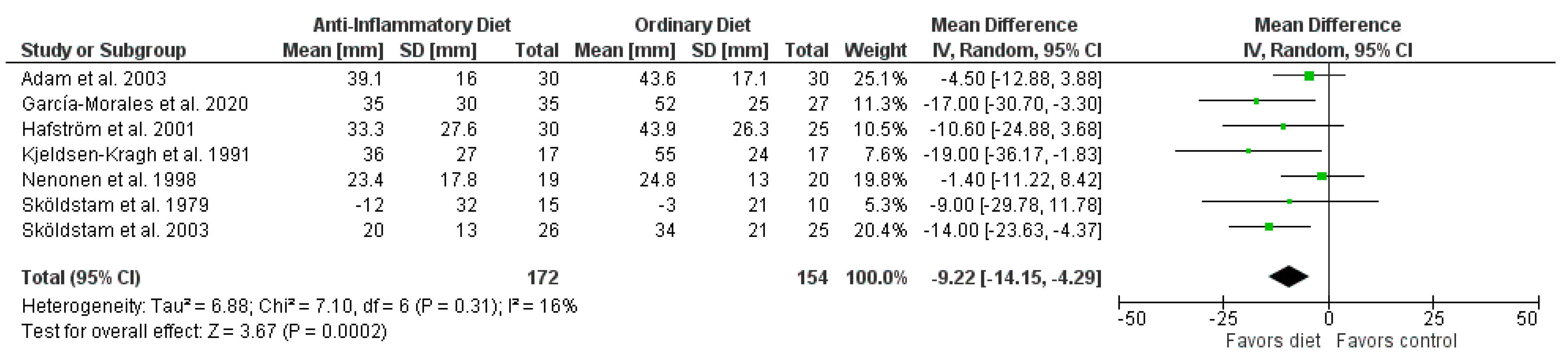
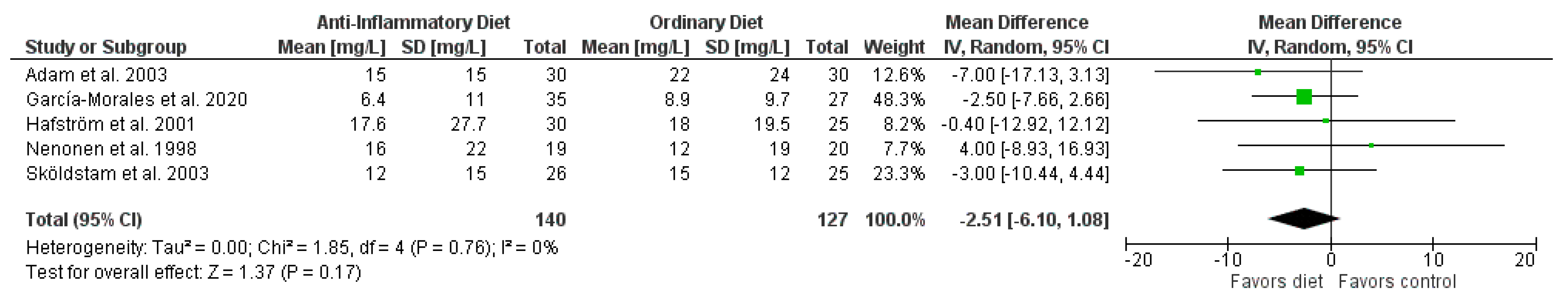
| Author Year | Population: n (% female) Age, Mean (SD/Range/IQR), y | Intervention vs. Control | Diet | Outcome, Mean (SD) Baseline; Endpoint | Study Design | ||
|---|---|---|---|---|---|---|---|
| Intervention | Control | ||||||
| Abendroth et al. 2010 [34] | MED: n = 28 (93%) Age: 60 (SD 12) | 2 weeks MED vs. 7-day fasting | MED according to Leitzmann [35]: normocaloric, mostly vegetarian, whole grain diet, fruit, and vegetables 7 p/day, abundant intake of whole grain bread, pasta and rice, fish 2 p/week, exclusive use of olive and canola oil | Pain, mm CRP, mg/L HAQ DAS28 BMI, kg/m2 | 35 (27); 33 (26) 20 (27); 16 (22) 2.4 (0.8); 2.2 (0.8) 5.4 (1.4); 4.5 (1.3) 25.5 (5.8); NA | NA | non-randomized intervention study |
| Adam et al. 2003 [36] | AID + corn oil: n = 30 (93%) Age: 58 (SD 13) WD + corn oil: n = 30 (93%) Age: 57 (SD 13) | WD vs. AID with menhaden oil vs. corn oil crossover, 3 months each | AID: modified lactovegetarian diet, only plant-derived fats and oils, no egg yolk, dairy products with reduced fat, meat maximum 2 × 120 g/week WD: usual diet, characteristic for industrialized countries, meat, and meat products >2×/week | Pain, mm CRP, mg/L SJC, n TJC, n Weight, kg BMI, kg/m2 | AID + corn oil 48 (21); 39 (16) 16 (15); 15 (15) 35 (4.9); 30 (4.5) 34 (5.1); 30 (4.7) 65 (11); 63 (9.3) 24.9 (0.7); 24.1 (0.7) | WD + corn oil 44 (18); 44(17) 22 (25); 22 (24) 34 (2.8); 36 (4.7) 36 (4.9); 36 (4.7) 62 (10); 63 (8.0) 23.2 (0.7); 23.3 (0.7) | RCT |
| García-Morales et al. 2020 [33] | MED: n = 40 (100%) Age: 46 (SD 13) Control: n = 31 (100%) Age: 49 (SD 12) | 24 weeks MED + DEP vs. DEP vs. MED vs. control | MED: individualized according to Harris-Benedict BMR [37], 50% carbohydrates, 30% fats, 20% proteins, olive or canola oil as main dietary fat, whole grains (1–2 p/meal), fruits (2–4 p/day), vegetables (2–3 p/meal), fish (>2 p/week), oilseeds (1–2 p/day), legumes (>2 p/week), red meat (<2 p/week) Control: general nutritional recommendations | Pain, mm CRP, mg/L ESR, mm/h HAQ DAS28 SJC, n TJC, n Weight, kg BMI, kg/m2 | MED: 45 (32); 35 (30) 6 (9); 6 (11) 11 (9); 11 (12) 0.5 (0.5); 0.5 (0.6) 2.2 (1.1); 2.4 (0.6) 1.0 (1.6); 0.9 (1.5) 1.4 (2.0); 2.9 (2.6) 67 (10); 64 (10) 27.2 (3.6); 26.5 (3.7) | Control: 51 (27); 52 (25) 4 (4); 9 (10) 16 (10); 18 (16) 0.9 (0.7); 0.8 (0.6) 2.6 (0.9); 2.4 (0.7) 1.4 (1.9); 2.0 (2.3) 1.5 (1.7); 0.7 (1.2) 64 (8.3); 66 (16) 27.1 (4.2); 27.6 (6.2) | RCT |
| Hafström et al. 2001 [31] | Vegan: n = 38 Age: 50 (SD 9.6) Control: n = 28 Age: 51 (SD 12) | 1 year gluten-free vegan diet vs. non-vegan diet | Gluten-free vegan: vegetables, root vegetables, nuts and fruits, buckwheat, millet, corn, rice, and sunflower seeds. Unshelled sesame seeds in the form of sesame milk were a daily source of calcium. Non-vegan diet: variety of foods from all food groups | Pain, mm CRP, mg/L HAQ Weight, kg | 46 (19); 33 (28) 23 (19); 18 (28) 1.4 (0.4); 1.1 (0.7) 66 (13); 61 (10) | 46 (21); 44 (26) 25 (22); 18 (20) 1.3 (0.5); 1.2 (0.5) 68 (20); 66 (15) | RCT |
| Ingegnoli et al. 2020 [38] | n = 205 (80%) Age: Mdn 53 (IQR 44–59) | N/A (observational study on the association between the MED score and RA disease impact, activity, and comorbidities) | Pain CRP HAQ DAS28-CRP SJC TJC BMI | Univariate analysis: association between outcomes (dependent variables) and the adherence to MED (independent variable) regression coefficient (95% CI) −0.08 (−0.15, −0.01) 0.01 (−0.03, 0.05) −0.01 (−0.02, −0.001) −0.01 (−0.04, 0.01) −0.01 (−0.03, 0.01) −0.02 (−0.06, 0.02) −0.04 (−0.15,0.07) | observational, cross-sectional study | ||
| Kjeldsen-Kragh et al. 1991 [30] | Vegetarian: n = 27 (89%) Age: 53 (range 26–63) Control: n = 26 (81%) Age: 56 (range 38–78) | 13 months vegetarian vs. usual diet | Vegetarian: initial 7–10 days fast (800–1260 kJ/day), afterwards reintroduction of a new food item every 2nd day, during the first 3–5 months no gluten, meat, fish, eggs, dairy products, refined sugar, citrus fruits, salt, strong spices, preservatives, alcoholic beverages, tea, coffee, afterwards reintroduction of milk, other dairy products Control: ordinary mixed food | Pain, mm HAQ Weight, kg | NA; 36 (27) NA; 1.0 (0.6) NA; 65 (11) | NA; 55 (24) NA; 1.1 (0.6) NA; 67 (11) | RCT |
| McDougall et al. 2002 [39] | Vegan: n = 24 (92%) Age: 54 (SD 11) | 4 weeks vegan diet | Low-fat, vegan diet: no animal products or added fats and oils of any kind, ad libitum menus based on common starches, such as beans, breads, corn, pastas, potatoes, sweet potatoes, and rice, fresh or fresh-frozen fruits and vegetables, dehydrated cereals, soups, main entrees | Pain, mm CRP, mg/L ESR, mm/h SJC, n TJC, n Weight, kg | 49 (20); 34 (20) 21 (18); 17 (17) 50 (30); 50 (28) 27 (9); 22 (8) 24 (12); 17 (16) 68 (19); 65 (18) | NA | uncontrolled, pre-post intervention study |
| McKellar et al. 2007 [40] | MED: n = 75 (100%) Age: 54 (IQR 47–64) Control: n = 55 (100%) Age: 53 (IQR 45–61) | 6 months MED vs. healthy eating | MED: 6-week cookery course on Medi-terranean-type diet, weekly 2 h cookery class, written information on a Medi-terranean-type diet, healthy eating and recipes promoting fruits, vegetables and legumes, substitution of saturated fat with olive oil or spreads containing olive oil Control: readily available written information on healthy eating | Pain, mm CRP, mg/L ESR, mm/h HAQ DAS28 SJC, n TJC, n Weight, kg BMI, kg/m2 | Median: 50; 50 10; 10 19; 16 1.8; 1.6 4.7; 4.4 6; 4 5; 4 66; 65 25.9; 25.4 | Median: 55; 63 8.5; 8.0 19; 16 1.8; 1.9 5.0; 4.8 6; 5 6; 6 70; 73 27.7; 28.2 | non-randomized intervention study |
| Nenonen et al. 1998 [41] | Vegan: n = 22 (82%) Age: 49 (SD 7) Control: n = 20 (95%) Age: 56 (SD 11) | 2–3 months vegan vs. omnivorous diet | Vegan living food diet according to Hänninen [42]: uncooked, rich in lacto-bacilli, no animal products, no refined substances, no added salt, majority of food items soaked and sprouted (seeds and grains), fermented, bread is blended and dehydrated. Control: previous omnivorous diet | Pain, mm CRP, mg/L ESR, mm/h SJC, n TJC, n Weight, kg BMI, kg/m2 | 36 (14); 23 (18) 13 (16); 16 (22) 33 (16); 41 (22) 3.4 (2.5); 3.6 (3.0) 8.6 (4.7); 6.5 (4.7) 68 (10); 62 (9) 25.5 (4.0); 23.4 (3.5) | 38 (15); 25 (13) 17 (24); 12 (19) 40 (26); 43 (26) 3.9 (3.6); 3.8 (2.8) 9.6 (4.6); 9.6 (5.2) 64 (12); 64 (11) 23.5 (3.4); 23.7 (3.5) | RCT |
| Sköldstam et al. 1979 [29] | Vegetarian: n = 16 (63%) Age: 52 (range 35–66) Control: n = 10 (90%) Age: 54 (range 43–65) | 9 weeks vegetarian vs. normal diet | Vegetarian: initial 7–10 days fast (800 kJ/day, 3 L fruit and vegetable juices), followed by plain lactovegetarian diet, no animal or fish protein (including egg), yoghurt allowed freely, fresh milk and cream discouraged, no alcohol, tobacco, coffee, tea, restriction on salt, sugar, white flour, small quantities of grain products Control: normal diet | Pain, mm ESR, mm/h Weight, kg | 35 (19), Δ-12 (32) 41 (23), Δ2.3 (11) 71 (15), Δ−2.6 (2.1) | 27 (17), Δ-3 (21) 41 (20), Δ0.7 (14) 69 (9.5), Δ0.6 (2.0) | RCT |
| Sköldstam 1986 [43] | n = 20 (90%) Age: range 35–68 | 4 months vegan diet vs. ordinary diet | Vegan: initial 7–10 days fast, followed by diet excluding meat, fish, eggs and dairy products, refined sugar, corn flour, salt, strong spices, preservatives, alcoholic beverages, tea, coffee Control: ordinary diet | Pain, mm CRP, mg/L ESR, mm/h Weight, kg | 45 (NA); 36 (NA) No change No change Δ−4.8 (0.7) | NA | uncontrolled, pre-post intervention study |
| Sköldstam et al. 2003 [32] | MED: n = 26 (81%) Age: 58 (range 33–73) Control: n = 25 (80%) Age: 59 (range 35–75) | 12 weeks MED vs. usual diet | Cretan MED according to de Lorgeril [44]: olive and canola oil for cooking, canola-based margarine, reduced consumption of dairy products or low-fat dairy products, green or black tea Control: ordinary hospital food followed by usual diet at home. | Pain, mm CRP, mg/L ESR, mm/h HAQ DAS28 SJC, n TJC, n Weight, kg BMI, kg/m2 | 32 (20); 20 (13) 17 (20); 12 (15) 24 (15); 25 (15) 0.7 (0.5); 0.6 (0.4) 4.4 (1.2); 3.9 (1.2) 7.0 (5.6); 5.2 (5.1) 6.8 (5.9); 4.5 (5.1) 79 (14); 76 (14) 28.4 (4.9); 27.3 (4.6) | 31 (20); 34 (21) 15 (14); 15 (12) 23 (15); 25 (19) 0.8 (0.6); 0.8 (0.6) 4.3 (1.4); 4.3 (1.5) 6.9 (5.0); 7.5 (5.7) 6.9 (6.3); 6.1 (6.4) 73 (13); 73 (13) 25.7 (3.6); 25.6 (3.6) | RCT |
| Author Year | Randomization Process | Deviations from Interventions | Missing Outcome Data | Outcome Measurement | Selection of the Reported Result | Overall Bias |
|---|---|---|---|---|---|---|
| Adam et al. 2003 [36] | Some concerns | Low | Low | High | Some concerns | High |
| García-Morales et al. 2020 [33] | Low | Low | Low | High | Some concerns | High |
| Hafström et al. 2001 [31] | Some concerns | Low | Low | High | Some concerns | High |
| Kjeldsen-Kragh et al. 1991 [30] | Some concerns | Low | Low | High | Some concerns | High |
| Nenonen et al. 1998 [41] | Some concerns | Some concerns | Low | High | Some concerns | High |
| Sköldstam et al. 1979 [29] | Low | Some concerns | Low | High | Some concerns | High |
| Sköldstam et al. 2003 [32] | Low | Some concerns | Low | High | Some concerns | High |
| Author Year | Confounding | Selection of Participants | Intervention Classification | Deviations from Interventions | Missing Outcome Data | Outcome Measurement | Selection of the Reported Result | Overall Bias |
|---|---|---|---|---|---|---|---|---|
| Abendroth et al. 2010 [34] | Serious | Low | Moderate | No information | No information | Serious | Moderate | Serious |
| Ingegnoli et al. 2020 [38] | Serious | Moderate | Low | No information | Low | Serious | Low | Serious |
| McDougall et al. 2002 [39] | Low | Low | Low | Low | Low | Serious | Low | Serious |
| McKellar et al. 2007 [40] | Low | Low | Low | No information | Low | Serious | Low | Serious |
| Sköldstam 1986 [43] | Low | Low | No information | Low | No information | Serious | Low | Serious |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schönenberger, K.A.; Schüpfer, A.-C.; Gloy, V.L.; Hasler, P.; Stanga, Z.; Kaegi-Braun, N.; Reber, E. Effect of Anti-Inflammatory Diets on Pain in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 4221. https://doi.org/10.3390/nu13124221
Schönenberger KA, Schüpfer A-C, Gloy VL, Hasler P, Stanga Z, Kaegi-Braun N, Reber E. Effect of Anti-Inflammatory Diets on Pain in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Nutrients. 2021; 13(12):4221. https://doi.org/10.3390/nu13124221
Chicago/Turabian StyleSchönenberger, Katja A., Anne-Catherine Schüpfer, Viktoria L. Gloy, Paul Hasler, Zeno Stanga, Nina Kaegi-Braun, and Emilie Reber. 2021. "Effect of Anti-Inflammatory Diets on Pain in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis" Nutrients 13, no. 12: 4221. https://doi.org/10.3390/nu13124221
APA StyleSchönenberger, K. A., Schüpfer, A.-C., Gloy, V. L., Hasler, P., Stanga, Z., Kaegi-Braun, N., & Reber, E. (2021). Effect of Anti-Inflammatory Diets on Pain in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Nutrients, 13(12), 4221. https://doi.org/10.3390/nu13124221






