Abstract
Cancer is one of the leading causes of death globally. Epidemiological studies have strongly linked a diet high in fruits to a lower incidence of cancer. Furthermore, extensive research shows that secondary plant metabolites known as phytochemicals, which are commonly found in fruits, have onco-preventive and chemo-protective effects. Apple is a commonly consumed fruit worldwide that is available all year round and is a rich source of phytochemicals. In this review, we summarize the association of apple consumption with cancer incidence based on findings from epidemiological and cohort studies. We further provide a comprehensive review of the main phytochemical patterns observed in apples and their bioavailability after consumption. Finally, we report on the latest findings from in vitro and in vivo studies highlighting some of the key molecular mechanisms targeted by apple phytochemicals in relation to inhibiting multiple ‘hallmarks of cancer’ that are important in the progression of cancer.
Keywords:
fruit; apple; phytochemicals; cancer; chemoprevention; antioxidants; phenolics; triterpenoids 1. Introduction
Chronic diseases including cancer continue to remain a public health burden globally [1,2,3,4,5]. In 2020, cancer was the second leading chronic illness following cardiovascular disease, with an estimate of 19 million new cases and accounting for 10 million deaths per year, globally [6,7]. Data from GLOBOCAN [7] show that cancers of the breast are the most commonly diagnosed followed by cancers of the lung, colorectal, and prostate.
To reduce cancer’s global health burden, it is necessary to promote both cancer treatment and cancer prevention. There is growing evidence that phytochemicals found in vegetables and fruits play a major role in cancer aetiology. Furthermore, diet and simple dietary changes incorporating fruits and vegetables can influence the risk of cancer [8]. Apples are an example of commonly available fruits worldwide that are a rich source of phytochemicals. There is a milieu of studies around the health benefits of apples including onco-preventive effects. However, translating this information into an appropriate intervention requires understanding of how the different components of apples contribute to their health benefit. These components include differences in the phytochemical concentration within the skin vs. flesh of the apple, the impact of the apple food matrix, and absorption and bioavailability of apple phytochemicals. In this review, we provide detailed insight into selected dietary phytochemicals found in apples, their onco-protective role in cancer, and their effect on different pathways implicated in cancer development and progression. This review collates details from different studies related to apples and apple phytochemicals in order to provide a more holistic understanding around the effects of apple consumption on cancer. We also highlight the gaps in the literature to promote more relevant studies in this field including clinical trials.
2. Cancer
Cancer is a group of heterogeneous diseases that can occur in multiple parts of the body [9]. Triggers for cancer are complex and include genetic predispositions, environment, and lifestyle [10,11]. In this section, we briefly describe the different steps involved in cancer development (carcinogenesis) to better understand the pathways targeted by different phytochemicals described in subsequent sections of this review.
Carcinogenesis is a multi-step process involving initiation, promotion, and progression of cancer [12,13,14]. Initiation is the first irreversible step of carcinogenesis and refers to the genetic and epigenetic alterations in somatic cells mainly involving proto-oncogenes such as RAS, c-Myc, and tumor suppressor genes such as Rb and p53 [15,16,17,18,19]. Promotion is the second phase of carcinogenesis where non-mutagenic promoting agents result in reversible changes in the genome, giving cells the ability to proliferate uncontrollably and expand [14,20]. Growth factors such as epidermal growth factors (EGF), hormones including estrogen, and external factors such as chemical compounds from diet are examples of agents promoting these non-mutagenic events within the cell [21,22]. Progression represents a later stage of tumor development and is characterized by accumulation of multiple genetic changes, including increased mutational load, number, and arrangement of chromosomes and epigenetic changes [23]. Continuous accumulation of genomic changes within the cells allows them to acquire multiple ‘hallmarks of cancer’ including uncontrolled growth, ability to resist cell death, alter metabolism, evade the immune system, and invade and spread to other tissues and organs (reviewed in detail [24]).
3. Importance of Phytochemicals from Diet in Management of Cancer
Diet can influence cancer development in both positive and negative ways. It is estimated that a healthy lifestyle and healthy dietary practices could help lower incidence of all cancers by 30-40% [25]. Furthermore, a diet rich in vegetables, fruits, whole grains, dietary fiber, omega-3 fatty acids, and certain micronutrients (e.g., vitamins and calcium) protects against some cancers [26,27,28]. In contrast, diets rich in meat, processed foods, fried foods, and smoked foods can increase the risk of developing some cancers [29,30,31].
For a healthy diet, the World Health Organization (WHO) recommends the consumption of a minimum of five portions or 400 g of fruit and vegetables per day, of which two portions should constitute fruit [32,33]. Studies have shown differences in fruit consumption across the world based on the socioeconomic status, sex, and geographic location. From these studies, cancer incidence was estimated to decrease by 14% with consumption of 550–600 g of fruit and vegetable per day, which is greater than the current recommendations made by WHO [34,35]. These results suggest that a healthy diet such as one high in fruits and vegetables has protective effects against different types of cancer.
Protective effects of fruit against cancer are related to their high content of bioactive compounds (phytochemicals). Phytochemicals are secondary metabolites from plants responsible for the taste, color, and aroma of fruit. Research suggests that phytochemicals are beneficial in preventing and treating oxidative damage and inflammation, which are important risk factors in cancer development [36,37,38,39]. Moreover, phytochemicals have significant onco-preventive and chemo-protective effects, and this has been reviewed extensively elsewhere [38,40,41,42,43,44,45].
One fruit that is a rich and important source of bioactive phytochemicals in Western diets is the apple [46,47,48,49]. Apples are globally consumed due to their year-round availability, their cultivar diversity, low price, and easy storage [46,50]. The subsequent sections of this review will focus specifically on the onco-preventive and chemo-protective properties of phytochemicals found in apples.
4. Apple Phytochemical Profile and Bioavailability
To better understand the health benefits of apples, in this section we provide a comprehensive review of the main phytochemical patterns observed in apples and their potential health benefits depending on variety and the consumed part of the fruit (skin/peel versus the flesh of the apple).
Apples contain a wide variety of phytochemicals, including triterpenoids, organic acids, fatty acids, and apple phenolic compounds (Figure 1) [51]. Triterpenoids are components mainly of apple waxes [52]. The main triterpenoids found in apples are oleanolic, betulinic, and ursolic acid and their derivatives such as maslinic, corosolic, euscaphic, pomaceic, and pomolic acids [53].
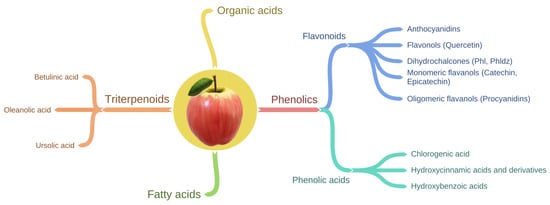
Figure 1.
Schematic showing the classification of phytochemicals present in apple.
The most well-studied group of apple phytochemicals for their health benefits are phenolic compounds [54]. Studies show that apples are an important source of phenolic compounds in our diet contributing to 22% of phenolic intake [55,56]. Most of the phenolic compounds in the fruit are usually present in the conjugated form such as glycosides or esterified carboxylic acids. However, compared to other fruit, apples contain more of the readily bioavailable free forms of phenolic compounds [57,58,59]. For instance, the ‘Red Delicious’ apple had the highest level of free forms of phenolic compounds compared to pear, plum, kiwifruit, and peach [60].
Phenolic compounds in apple can be sub-divided into two main groups (Table 1, Figure 1) known as flavonoids and phenolic acids. Flavonoids can be further divided into four structural subclasses including anthocyanidins, flavonols, dihydrochalcones, and flavan-3-ols (flavanols) which can exist in the monomeric and oligomeric form [49,61] (Table 1). Phenolic acids include chlorogenic acid, hydroxycinnamic acid, and hydroxybenzoic acid [49,61]. In general, chlorogenic acid and monomeric and polymeric flavanols are the major phenolic compounds, whereas anthocyanins and dihydrochalcones are minor phenolic compounds of apples [62]. Moreover, anthocyanidins are responsible for the apple redness [63,64]. Therefore, anthocyanidins are abundant in the apple cultivars with red skin (e.g., ‘Red Delicious’) and are either present in low concentrations or absent in green skinned apple cultivars (e.g., ‘Granny Smith’) [64,65].

Table 1.
Classification of apple phenolics.
There are differences in the distribution and type of phenolic compounds within various parts of an apple such as peel, flesh, core, and seeds. The phenolic compounds distribution and concentration in the peel and flesh of apples vary greatly due to genetic diversity, maturity stage, growing conditions and geographical location, harvest, and storage conditions [59,66,67,68,69,70]. However, studies have highlighted that there is a similar phenolics distribution pattern for most apple cultivars (Figure 2).
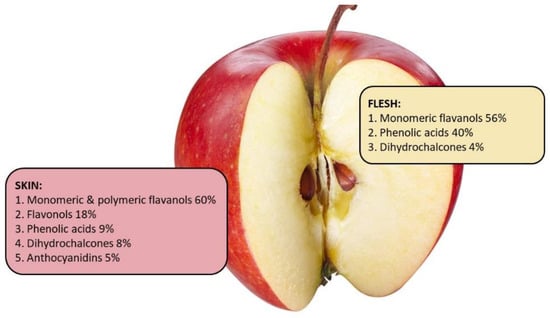
Figure 2.
Phenolics distribution pattern in the peel and flesh of an average apple based on the data from Lata, Trampczynska, and Paczesna [71], Tsao et al. [78], Lata [79], and McGhie et al. [62].
In general, the apple peel contains about 2–4 times higher concentration of phenolic compounds, and higher concentration of total procyanidins and total flavonoids, compared to flesh [71]. Kalinowska et al. [72] demonstrated that apple peel from the ‘Gold Millennium’ apple contains five times the concentration of phenolic compounds compared to the flesh [68]. Similarly, the peel of 15 different apple cultivars had greater concentration of all phenolic compounds compared to the apple’s flesh [73]. In general, evidence from multiple studies comparing various cultivars of apples has shown that apple peel contains all groups of phenolic compounds and has greater concentration of procyanidins and total flavonoids compared to the flesh [59,74,75,76]. However, there are some exceptions. Procyanidin B1 concentration in the flesh of ‘Gloster’, ‘Elstar’, and ‘Gala’ is higher compared to their peel [59] and apple flesh of ‘Lodel’ is higher in phloridzin than its peel [77]. Quercetin glycosides are usually found only in the peel [73]. On the other hand, chlorogenic acid can be found in both the flesh and the peel of ‘Golden Delicious’, ‘Granny Smith’, and ‘Idared’ apples, but tends to be higher in the flesh [59,69]. These findings are in accordance with the study from Kschnosek et al. [73], where the predominant group found in the flesh was the phenolic acids (43%), while the flavonols, namely quercetin and its glycosides, were enriched in the peel (72%) and not detected in the flesh. Taken together, these data suggest that apple peel of most apple varieties contain more phenolic compounds than the flesh.
It is important to consider that apple peel contributes only up to 10% of the weight of the whole fruit; therefore, the intake of some phenolic compounds from the peel after consumption of a whole apple might not be as significant as the intake from the flesh. Only a few studies have reported on the phenolic compounds content relative to the weight of the peel compared to the whole apple [62,71]. McGhie et al. [80] demonstrated that peel of ‘Braeburn’, ‘Royal Gala’, and ‘Red Delicious’ contributed 55%, 50%, and 52%, respectively, of the apple’s total phenolic compounds. Similarly, apple peel of ‘Granny Smith’, ‘Idared’, ‘Red Rome’, ‘Jonmac’, ‘Gloster’, and ‘Starking Delicious’ contributed 50% or more to the apple’s total phenolic compounds content. In contrast, the peel of ‘Pilot’, ‘McIntosh’, and ‘Prima’ contributed less to the total phenolic compounds of the whole apple [71]. Data from New Zealand heritage apple cultivar ‘Monty’s Surprise’ and the commercial varieties ‘Braeburn’ and ‘Red Delicious’ (Table 2) showed that the contribution of total phenolics was lower from the peel compared to the flesh. However, anthocyanidins were only present in the peel and flavonols were only found in small quantities in the flesh. Taken together, a combination of unpublished data from New Zealand (Table 2) and other published studies suggests that for most apple varieties the peel is a significant source of phenolic compounds. Therefore, discarding the peel during production of some traditional apple products, such as apple sauce [81], may decrease the health potential of the apples.

Table 2.
Estimated apple peel contribution to the total phenolics content in whole apple. Phenolic compounds were measured using liquid chromatography-mass spectrometry (LC-MS, Dionex Ultimate RS3000 UHPL and a Bruker micrOTOF-QII) in 2019, plant and food research, for 3 apple varieties—Monty’s surprise, Braeburn, and Red Delicious. Each compound concentration was quantified by comparison with an authentic standard where possible or as equivalents to standard compounds. Each phenolic compound in the table is presented as a percentage of total concentration measured using LC-MS. Percentage total phenolics (percentage values presented in bold) was calculated based on the average weight of whole apple (180 g) where apple skin contributed 18 g.
The health benefits of an apple’s bioactive compounds depend on their absorption, metabolism, and distribution within the human body [82]. The bioavailability of phenolic compounds (the fraction of the bioactive that has been absorbed and is available for biological activity) is affected by pH, enzymatic activity, their chemical structure, solubility, free and bound form, and synergistic effects with the food matrix (see Section 5.2) [83,84,85].
Despite absorption of phenolic compounds beginning in the small intestine [85], most of the compounds are released in and absorbed from the large intestine with aid from the gut microbiota [84,86,87]. The gut microbiota is capable of transforming complex phenolic compounds into metabolites that are more easily absorbed [88]. It was demonstrated that once absorbed, phenolic compounds can be detected in human plasma and urine after consumption of apple [89], apple juice [90], and apple cider [91]. Bioavailability of the main apple phytochemicals is described in Section 5.2.
While apple is a rich source of nutrients and phytochemicals, there is evidence to suggest that the apple food matrix (non-nutrient component) plays an important role in the absorption and bioavailability of apple phytochemicals. Aprikain et al. [87] demonstrated that the ingestion of phenolics-rich apple extract and apple fiber (pectin) together had greater effect on gut microbiota metabolism in the large intestine and lipid metabolism than ingestion of the phenolics-rich apple extract alone, suggesting a beneficial interaction between fiber and phenolic compounds [87]. Recent studies have shown that a whole apple has strong prebiotic effects [92,93] and that its fiber content promotes the bioaccessibility of other beneficial phytochemicals [94,95]. Additionally, dietary fiber fermentation by the gut microbiota releases short chain fatty acids that have been shown to modulate expression of cell cycle-regulating proteins and induce apoptosis in colon cancer cells [96]. The majority of dietary fiber originates in the plant cell wall and many phytochemicals are known to bind plant cell wall components [94]. As such, processing of apples (including juicing and cooking) will influence the plant cell wall integrity and fiber content of the fruit, which will consequently change the phytochemical’s bioaccessibility, bioavailabilty, and interactions with the gut microbiota. However, processing and ultra-processing techniques are diverse and complex and their impact on health is outside the scope of this review. The influence of the food matrix (mainly fiber and carbohydrates) on the main apple phytochemicals is described in Section 5.2.
The studies reviewed in this section highlight that there are large variabilities in the composition of phytochemicals in apples and that the phytochemical patterns and profiles can vary in relation to cultivar and apple part. Therefore, the type and level of health benefit will vary in relation to the phytochemical profile of the apple consumed. Ultimately, the phytochemical compounds from apple will only achieve benefit once they become bioavailable and reach the cells and tissue of interest.
5. Health Benefits of Apple Phytochemicals: Cancer
Current research attributes the health benefits of apples mainly to the phenolic compounds which exhibit several biological functions beneficial for human health [54]. Apple phenolic compounds are believed to lower incidence of chronic conditions such as cardiovascular disease, cancer, asthma and pulmonary disease, diabetes, and obesity [49,97,98,99,100,101,102,103].
Apple phytochemicals are suggested to have many chemo-preventive and chemo-protective effects (Figure 3) against various types of cancer. These effects include regulation of proliferation, cell cycle, apoptosis, reactive oxygen species (ROS), and anti-inflammatory activities [36,47,86,104,105]. In this section, we discuss the health benefits of apple phytochemicals in relation to cancer from epidemiological studies, their ability to alter ROS in cancer cells, and impact on cancer biology from in vitro and in vivo studies.
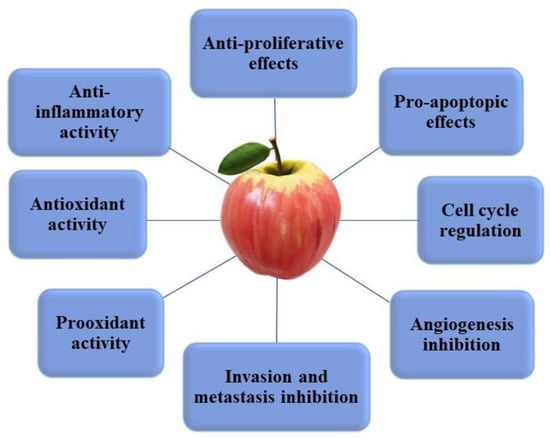
Figure 3.
Main mechanisms of action of apple phytochemicals on cancer cells.
5.1. Epidemiological Evidence of Apple Consumption and Cancer Incidence
Epidemiological studies have associated apple and pear consumption with lower incidence of different cancers. Reports from the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study demonstrated that consumption of apples and pears is associated with lower lung [106] and bladder [107] cancer incidence. Consumption of apples and pears was also associated with lower lung cancer incidence in the American Nurses’ Health Study [108]. Apple consumption in particular was associated with lower lung cancer incidence in epidemiological studies from [109], Hawaii [110], and the Zutphen elderly study (Netherlands) [111]. Furthermore, consumption of apples and pears has been associated with lower breast cancer risk from a pooled analysis of two large prospective studies (Nurses’ Health Study—NHS and NHSII) [112]. In one case-control trial from Italy, it was reported that consumption of more than three apples or pears a day was inversely related to pancreatic cancer [113], which is an extra fruit portion above the dietary recommendation by WHO. Both apples and pears are rich in polyphenols, are popular fruit, and are widely available all year round in many countries. Therefore, it is not surprising that apples and pears are identified together in many observational studies that assess dietary habits.
Apple consumption specifically was associated with lower cancer incidence in several observational studies. Apple consumption was associated with decreased breast cancer incidence in a fruit and vegetable study conducted on pooled cohorts [114] and a case-control study from Mexico in pre-menopausal women [115]. Similarly, consumption of apples was associated with reduced incidence of colorectal [116], oral cavity and pharynx [47,117], esophagus [47], larynx [47], ovary [47], renal [118,119], and prostate [47,120] cancers. One case-control study looking at fruit and vegetable consumption in pre-menopausal women in Shanghai showed an inverse association with fruit intake and breast cancer [121]. While the study found the strongest association was with citrus fruit, consumption of 57 g/day of apple or more was also reported to reduce incidence of breast cancer in the study [121]. In a meta-analysis of 20 case-control studies and 21 cohort studies, it was shown that apple consumption was associated with a reduced risk of lung, colorectal, oral cavity, and breast cancers [122].
Findings from the epidemiological studies reported in this review suggest that the consumption of apples reduce cancer risk. However, the cohort size and composition as well as the intervention vary between the different studies. Additionally, other dietary and lifestyle factors could influence cancer outcome in these cohort and observational studies. Further studies are needed to specifically clarify the effect of apple consumption on the incidence of cancer. In addition, most of these studies are observational and to date, there are no clinical intervention trials reported demonstrating the link between apple consumption and cancer incidence. Further research and clinical studies would help to better understand and confirm the effect of apple phytochemicals on cancer in humans.
5.2. In Vitro and In Vivo Evidence of the Anticancer Properties of Apple Phytochemicals
Apple phytochemicals were reported to have significant effects on inhibiting multiple ‘hallmarks of cancer’ (detailed below) which are important in the progression of cancer [123].
Phenolic compounds from different apple cultivars were positively associated with the higher degree of inhibition of breast cancer cell proliferation [124,125,126] and induction of cell cycle arrest [125,127]. Additionally, apple extracts inhibited growth of prostate [127] and lung [128] cancer cells. Extracts of phenolics from apple pomace of different apple cultivars were reported to inhibit proliferation of oral [129] and colon cancer cells [58]. In addition to in vitro studies, apple polyphenol extracts also inhibited ex vivo proliferation of a hepatoma cell line [130].
Multiple studies have demonstrated that apple phytochemicals can inhibit the activity of p21, growth factors, pyruvate dehydrogenase kinases (PDKs), cyclin-dependent kinases (CDKs), and extracellular protein kinases (ERKs) essential for cell cycle progression [37,126,131,132]. Furthermore, apple phytochemicals can also prevent cell cycle progression by activation of maspin, a tumor suppressor gene [127]. Reduced expression of the key molecules essential for regulating cell cycle such as phosphorylated Rb, Cyclin D1, and CDK4 by apple phytochemicals lead to cancer cells’ arrest [125,127]. Apple extracts were reported to inhibit apoptosis in breast cancer cells [124,128]. Apple phenolic compounds were shown to elevate the expression of pro-apoptotic genes such as p53 and Bax and reduction in the expression of anti-apoptotic genes such as p21 and Bcl-2 [133]. In addition to inhibiting cell proliferation and promoting apoptosis, apple phytochemicals have also been implicated in inhibiting angiogenesis by regulating VEGF [123] and inhibiting invasion and metastasis [58,130] by regulating matrix metalloproteinases-2,-9 (MMP-2,-9), cadherins and integrins [123], and regulating COX-2 a marker of inflammation [123]. Additionally, the ability of apple phytochemicals to inhibit cell proliferation and in turn reduce incidence of cancer was also observed in rats fed with one human apple equivalent. These rats had reduced appearance of different precancerous markers (ACF, MDF, genes, and proteins related to colorectal cancer progression) [134]. Similarly, the incidence of mammary tumors in rats was reduced after two weeks of oral administration of 3.3, 10, and 20 g of apple extract/kg of body weight, which correspond to the human consumption of one (200 g), three, and six apples per day, respectively [135].
There is evidence that anticancer properties of apples are due to the synergistic effects between apple phytochemicals and the food matrix [136,137,138,139]. Veeriah et al. [136] demonstrated that colon cancer cells treated with an apple extract (extract from a mixture of different apples) reduced cell proliferation to a greater extent compared to a synthetic apple extract composed of eight apple phenolic compounds or individual apple phenolic compounds. They further demonstrated that colon cancer cells treated with a synthetic apple extract composed of eight apple phenolic compounds also reduced cell proliferation to a greater extent compared to individual apple phenolic compounds. [136]. Results from this study indicate the importance of the apple food matrix, which may contain other bioactive compounds present in the apple extract but not in the synthetic mixture.
Taken together, evidence from in vitro, ex vivo, and in vivo studies suggest that apple phytochemicals work synergistically to inhibit multiple ‘hallmarks of cancer’, which in turn can influence cancer incidence and improve outcomes to chemotherapeutic treatments.
Oxidative stress can result in direct or indirect ROS-mediated damage of macromolecules such as DNA, proteins, and lipids, allowing cells to acquire multiple ‘hallmarks of cancer’ and facilitating carcinogenesis [55,140,141]. Phenolic compounds such as quercetin, epicatechin, procyanidin B2, phloretin, and chlorogenic acid were identified as the biggest contributors to the apple’s antioxidant activity [142]. In one study, apples showed the second highest antioxidant activity in vitro after cranberries among 11 common fruits tested [57]. Both apple peel and flesh extracts from dried and lyophilized apples of four different cultivars reduced ROS in the lipopolysaccharide (LPS)-induced mouse brain microglia cells (BV-2), with apple peel having a greater antioxidant effect [143]. While these studies have indicated the antioxidant capacity of apples, it is important to note that the methods used to measure antioxidant activity (such as total antioxidant capacity) are synthetic assays and do not necessarily capture the complexity of a physiological system [144]. Nevertheless, it has been proposed that antioxidant activity of apple phytochemicals can inhibit or reduce cancer cell proliferation [124,128,129,145,146,147] and promote apoptosis [148] of cancer cells based on in vitro studies (Table 3).
On the other hand, many bioactive compounds can work as prooxidants and under certain conditions (high concentration, presence of metal ions, and low pH) can induce ROS production and promote cell death [149,150,151]. In cancer therapy, prooxidants may have a beneficial effect by working as cytotoxic agents for fast growing cells and inducing cancer cell death [152,153,154]. Prooxidant activity of many phenolic compounds has been associated with their ability to induce apoptosis and cell cycle arrest in different cancer cells [155,156,157]. For example, Mendoza-Wilson et al. [158] demonstrated that phloridzin exhibited prooxidant activity [158]. Catechin and epicatechin are well known antioxidants, however, they can act also as prooxidants [159,160]. Epicatechin induced ROS production in colon cancer cells led to activation of pro-apoptotic enzymes and therefore induced apoptosis of these cells [159].
These examples suggest that some phytochemicals have a biphasic or hormetic response depending on the dose administered [161]. Therefore, phytochemicals such as the ones present in apple can be used to produce an antioxidant effect for cancer prevention, but also induce prooxidant effects with benefits in cancer prevention.
In the subsequent sections. the review will focus on the anticancer mechanisms of the main apple phenolic compounds (quercetin, phloretin, chlorogenic acid, catechins, epicatechins, and procyanidins) and triterpenoids. The data on in vitro activity of apple phenolics with effective concentrations are summarized in Table 3.
5.1.1. Quercetin Anticancer Properties
Of all the apple phenolic compounds, quercetin glycosides are the most efficiently absorbed compounds from apples [162,163,164], mainly absorbed in the large intestine. Quercetin in apples is present in glycoside forms, and interestingly, these are more readily absorbed compared to quercetins from tea [165] but less readily absorbed compared to the quercetins from onion [166]. Quercetin glycosides can be absorbed as intact molecules where the sugar moiety helps to improve absorption through the gut lumen [167]. Furthermore, the presence of carbohydrates and pectin in the apple food matrix significantly increases quercetin absorption [168,169,170,171]. Quercetin has been detected in the plasma of humans (Cmax 0.30 μM/92 ng/mL, Tmax 2.5 h) [166] and rats (Cmax 118 μM ± 08) [172] after apple consumption. These studies suggest that quercetin is absorbed and present in the plasma at a sufficient concentration to elicit the anticancer effects.
Recent in vitro and in vivo studies have reported that quercetin is one of the flavonoids responsible for the apple’s anticancer properties [173,174]. Quercetin has been shown to inhibit cell proliferation of breast [175,176,177,178], ovarian [179], lung [180,181], and liver [180] cancer cells in vitro (Table 3). Additionally, studies have demonstrated quercetin’s ability to induce apoptosis in multiple cancer cell lines ([177,178,182,183], Table 3) and in vivo using colon cancer cell line xenografts in mice [182]. The ability of quercetin to promote apoptosis and cell cycle arrest is believed to be due to its regulation of p53, GADD45, and AMPK [182,184]. Autophagy is another form of cell death in which damaged organelles are degraded [185]. Interestingly, quercetin is also shown to induce autophagy in lung cancer cells [181] and in breast cancer bearing mice by reducing the activity of AKT-mTOR pathway [186]. Additionally, quercetin inhibited invasion and migration of breast [186] and colorectal [187] cancer cells (Table 3). Quercetin was also shown to inhibit the activity of VEGFR2 and therefore inhibit angiogenesis in breast [188], prostate [189], and retinoblastoma [190] cancer cells.
Based on the results from in vitro studies, quercetin has strong anticancer properties in different cancer cells. However, the absorption and metabolism of quercetin glycosides from whole foods such as apples are not well known. Therefore, to better understand the anticancer effects of quercetin in humans, further studies are needed to identify factors that influence quercetin mechanisms of action and bioavailability in vivo.
5.1.2. Phloretin and Phloridzin Anticancer Properties
Phloretin and its glucoside form (phloridzin) are other flavonoids found in apple. Phloretin and phloridzin are metabolized into phloretin glucuronides and phloretin sulfate glucuronides in the human intestine [169]. Phloridzin has been detected in the plasma (Cmax 66.9.0 μM ± 19.4, Tmax 10 h) and urine of rats [191,192]. It has also been detected in the plasma of humans (Cmax 73 nM ± 11, Tmax 0.6 h) [193]. Phloretin and its glycosides has been detected in ileal fluid in humans after consumption of apple juice [194] and cider [193], whereas phloridzin was not detected, suggesting that phloridzin is more readily absorbed.
Based on the available evidence, it is unclear how the apple food matrix and composition of the gut microbiota would affect uptake and metabolism of phloretin, which in turn may impact its anticancer properties against cancer cells. Despite limited information on the bioavailability of phloretin, several lines of evidence support its anticancer properties from in vitro and in vivo studies. Phloretin was shown to inhibit proliferation of breast cancer [195] and colorectal [196] cancer cell lines via inhibition of glucose transporter 2 (Glut-2) in vitro and in vivo [197]. Additionally, phloretin inhibited proliferation by promoting cell cycle arrest [198,199], ROS production [200], apoptosis [198,201,202,203], and by inhibition of autophagy via mTOR/ULK1 [204]. Phloretin also inhibited invasion and migration [197,200,205,206]. Moreover, phloretin promoted an anti-inflammatory environment by inhibiting the expression of pro-inflammatory molecules PGE2, IL-8 and advanced glycation end products (AGEs) receptor [207]. Finally, evidence from in vivo studies suggest that phloretin may enhance the effect of commercial chemotherapeutic drugs such as Paclitaxel [208].
Details of these in vitro and in vivo studies are provided in Table 3. It is important to note that most of the in vitro cancer studies use synthetic phloretin, thus, to maximize the anticancer benefits of phloretin, there is a need to further our understanding of phloretin bioavailability.
5.1.3. Chlorogenic Acid Anticancer Properties
Chlorogenic acid is one of the phenolic acids abundant in apples and is reported to have anticancer potential, details of which have been summarized in Table 3. Chlorogenic acid is metabolized mainly in the large intestine by the gut microbiota from its aglycone form to its microbial metabolites, some of which include caffeic acid, 3-phenylpropionic acid, 3-phenylpropionic acid, hippuric acid, and quinic acid [209,210,211,212]. Interestingly, after consumption of foods rich in chlorogenic acid, its microbial products have been detected in the plasma of rats (Cmax 0.34 μM, Tmax 1 h) and human urine [209,210,213] but not its aglycone forms [209,210]. Studies in vitro and in vivo have demonstrated that chlorogenic can inhibit cell proliferation [214,215,216,217,218], promote cell cycle arrest [214,218,219] via affecting miR-17 family [214], induce apoptosis [26,218] by binding to annexin and suppressing the NF-κB pathway [220,221], and inhibit invasion and metastasis via downregulation of MMP-2 and MMP-9 [216]. Chlorogenic acid also inhibited growth of the liver [216] and breast [220,221] cancer tumor in xenograft mice in vivo.
Chlorogenic acid possesses different anticancer properties in vitro. However, the limiting factor for its anticancer properties in humans is its low bioavailability. The microbial produced metabolites of the gut might be contributors to the chemo-preventive effects of chlorogenic acid, however, further research in this area is needed.
5.1.4. Catechins and Epicatechins Anticancer Properties
Catechins and epicatechins are monomeric flavanols that are unstable in the gastrointestinal tract and poorly absorbed [222], with only 1.6% of the ingested catechins/epicatechins from tea, detected in human plasma, feces, and urine (plasma epicatechin Cmax 174 nM, Tmax 7 h) [223]. Similar results were obtained in the plasma of rats where the oral bioavailability of radioactively labeled catechin was about 3% and for radioactively labeled epicatechins was 4% of the orally administrated dose (catechins: Cmax 30.40 ± 1.80 ng/mL, Tmax 1.25 h; epicatechins: Cmax 196.5 ± 18.1 ng/mL, Tmax 1.1 h) [224,225]. Notwithstanding the limitation with their bioavailability, catechins and epicatechins have been reported to have a multitude of anticancer properties summarized in Table 3. Both catechins and epicatechins in vitro and in vivo have inhibited cancer cell proliferation [226,227,228], induced cell cycle arrest by upregulating the expression of p21 [229] and inhibition of CDC25A [226], induced apoptosis [227,229,230,231,232,233], and reduced invasion and migration [233].
In several of these studies, the catechins and epicatechins used were derived from sources other than apple. However, the impact of the apple matrix and the gut microbiome on the bioavailability of catechins and epicatechins remains unknown. Thus, further studies are required to investigate the bioavailability and anticancer properties of epicatechins and catechin from apples.
5.1.5. Procyanidins Anticancer Properties
Procyanidins are another group of oligomeric flavanols, which are abundant in apples and have many anticancer properties. Procyanidin dimers from grape seed extract and cocoa were detected in human plasma (Cmax 16 ± 5 nM, Tmax 0.5 h; Cmax 10.6 ± 2.5 nM, Tmax 2 h) [234,235] but not rat plasma [236]. Moreover, larger procyanidins were not absorbed efficiently by intestinal epithelial cells and are metabolized to low weight phenolic acids by the gut microbiota [237]. It was reported that compared to other apple phenolics, procyanidins have a greater effect on cancer cell proliferation and apoptosis in vitro [238,239]. Like their monomeric counterparts (catechins and epicatechins), procyanidins have been shown in vitro and in vivo to inhibit proliferation [238,240,241,242], induce cell cycle arrest [238,240,241], promote apoptosis [238,239,243,244], induce ROS [245,246], inhibit migration [242], and angiogenesis [247]. In addition, procyanidins isolated from apple also inhibited breast [239] and liver [242] cancer tumor growth in xenograft mouse. Table 3 provides details of the in vitro studies.
The bioavailability of procyanidins varies and is dependent on their structure and the composition of the gut microbiome, where the metabolites may elicit the anticancer effects. Therefore, to further understand the anticancer properties of procyanidins, studies are needed to investigate the anticancer properties of their metabolites and their bioavailability in humans.
5.1.6. Triterpenoids Anticancer Properties
Triterpenoids are another group of bioactive compounds found predominantly in apple peel. Triterpenoids are suggested to contribute to anticancer activity, details of which are summarized in Table 3. Triterpenoids consist of oleanolic acid and its isomer ursolic acid and betulinic acid. Triterpenoids are known to have low bioavailability and are poorly absorbed in the intestine [248,249]. It has been shown that only 2.3% of orally administered betulinic acid was detected in mouse plasma (Cmax 3.1 μg/mL, Tmax 2 h) [250], while only 0.7% of orally administered oleanolic acid was detected in rat plasma samples (Cmax 74.0 ± 57.2 ng/mL, Tmax 25 mins) [251]. In contrast, oleanolic acid has been detected in human plasma four hours after raisin consumption (Cmax 24.4 ± 14.4 ng/mL, Tmax 4 h) [252]. Like other apple phytochemicals, triterpenoids were shown to inhibit proliferation [253,254,255,256,257,258,259,260], induce apoptosis [80,253,254,255,257,258,259,261,262,263,264,265,266,267], alter ROS production [262,268,269], inhibit invasion and metastasis [255,256,257,270,271,272,273], and angiogenesis [270].
Current research on apple phytochemicals is focused more on the anticancer properties of apple phenolic compounds with relatively few studies looking at apple triterpenoids. Future research on other triterpenoids and their derivatives present in apples such as pomaceic acid (which is unique to the apples) or pomolic, euscaphic, and maslinic acid is necessary to better understand the anticancer properties specific to apples.

Table 3.
Summary of mentioned in vitro studies with the effective concentration.
Table 3.
Summary of mentioned in vitro studies with the effective concentration.
| In Vitro | Effect | Expression Markers Affected | Effective Concentration | Cell Line | Ref. |
|---|---|---|---|---|---|
| QUERCETIN | Lung cancer | ||||
| Anti-proliferative | ↓ PDK3 | 55.90 ± 2.25 µM | A549 | [180] | |
| Anti-proliferative, pro-apoptotic, autophagy inhibition | ↑LC3-II, SIRT 1, AMPK, beclin 1 ↓ p62 | 100 µM | A549, H1299 | [181] | |
| Breast cancer | |||||
| Anti-proliferative, cell cycle arrest | ↑cyclin B1 and CDK-1 ↓p21 | 10 µM | SK-BR3, MDA-MB-453 | [175] | |
| Anti-proliferative, pro-apoptotic | ↑ miR-146a, bax, caspase-3 ↓ EGFR | 80 µM/mL, 50 µM/mL (respectively) | MCF-7, MDA-MB-231 | [177] | |
| Anti-proliferative, pro-apoptotic | ↓ survivin | 40 mg/mL | MCF-7 | [178] | |
| Pro-apoptotic, cell cycle arrest | ↓ Foxo3a, p53, GADD45 | 20 µM | MDA-MB-231 | [182] | |
| Metastasis and invasion inhibition | ↓ MMP-2,9, VEGF, PKM2, GLUT1, LDHA, Akt, mTOR | 30 µM | MCF-7, MDA-MB-231 | [186] | |
| Angiogenesis inhibition | ↓ VEGF, Pin1 | 30 µM | MCF-7 | [188] | |
| Colorectal cancer | |||||
| Metastasis and invasion inhibition, anti-inflammatory | ↑ E-cadherin ↓ MMP-2,9, p65, TLR4, TNF-α, COX-2, IL-6 | 5,10, 20 μM | Caco-2 | [187] | |
| Liver cancer | |||||
| Anti-proliferative | ↓ PDK3 | 49.10 ± 1.45 µM | HepG2 | [180] | |
| Ovarian cancer | |||||
| Pro-apoptotic | ↑ phospho-eIF2α, p53 ↓ Rad51 | 25, 50, 75, 100 µM | OV2008, A2780, GM9607 | [183] | |
| Anti-proliferative, pro-apoptotic, cell cycle arrest | ↓ survivin | 30 mg/ml | SKOV-3 | [179] | |
| Prostate cancer | |||||
| Anti-proliferative, angiogenesis inhibition | ↓ Akt, mTOR, VEGFR2, S6 kinase | 10-40 mmol/L | HUVECs | [189] | |
| Retinoblastoma | |||||
| Angiogenesis inhibition | ↓ VEGFR | 25, 50, 200 µM | Y79 | [190] | |
| PHLORETIN AND PHLORIDZIN | Lung cancer | ||||
| Anti-proliferative, pro-apoptotic, invasion and migration inhibition | ↑ caspase-3,-9 ↓ Bcl-2, MMP-2,-9 | 25, 50, 75 µg/mL | A549, H838, H520, Calu-1 | [205] | |
| Pro-apoptotic, cell cycle arrest | ↑ Bax, caspase-3, -9 ↓ Bcl-2 | 50, 100, 200 µM | A549 | [199] | |
| Breast cancer | |||||
| Anti-proliferative, cell cycle arrest | ↓ GLUT-2 | 25, 50, 100, 150 µM | MDA-MB-231 | [195] | |
| Anti-proliferative, autophagy inhibition | ↓ mTOR, ULK1, LC3B-II | 100, 200 µM | MDA-MB-231, MCF7, ERα+ | [204] | |
| Colorectal cancer | |||||
| Anti-proliferative, cell cycle arrest | ↑ E-cadherin, p53 ↓ GLUT-2 | 100, 200 µM | Colo 205, HT-29 | [196] | |
| Pro-apoptotic | ↑ caspase-3,-7, -9, Bax, cytochrome C ↓ Bcl-2 | 100 μmol/L | HT-29 | [206] | |
| Anti-inflammatory | ↓ PGE2, IL-8, AGEs | 50 μM | CCD-18Co | [207] | |
| Liver cancer | |||||
| Pro-apoptotic, invasion and migration inhibition | ↓ GLUT-2, Bcl-2, Akt | 200 μM | HepG2 | [197] | |
| Pro-apoptotic | ↑ SHP-1 ↓ p-Akt, pERK, mTOR, VEGFR2, p-JNK | 25, 50, 100 μM | SK-Hep1, Hep3B2.1-7, Huh7, PLC5, HepG2 | [201] | |
| Prostate cancer | |||||
| Prooxidant, anti-proliferative, migration inhibition | ↑ ROS ↓ β-catenin, TCF4, FoxA2, c-Myc, CISD2 | 20, 50, 100 μM | PC3, DU145 | [200] | |
| Gastric cancer | |||||
| Anti-proliferative, pro-apoptotic, cell cycle arrest | ↓ p-JNK, p-38 | 4, 8, 16 μM | AGS | [198] | |
| Esophageal cancer | |||||
| Anti-proliferative, pro-apoptotic | ↑ BAX, p53 ↓ Bcl-2 | 60, 70, 80, 90, 100 μg/mL | EC-109 | [203] | |
| Brain cancer | |||||
| Anti-proliferative, pro-apoptotic, cell cycle arrest | ↑ p27 ↓ CDK-2,-4,-6, cyclin-D,-E | 100, 200, 300 μM | U87, U251 | [202] | |
| CHLOROGENIC ACID | Lung cancer | ||||
| Anti-proliferative, cell cycle arrest | ↑ p21, p53, and KHSRP ↓ c-Myc, miR-17 family | 25, 50 μM | H446 | [214] | |
| Anti-proliferative, pro-apoptotic | ↓ cIAP1, cIAP2, binding of annexin A2 to p50 and actin => ↓ NF-κB | 25, 50, 100, 200, 400, 800 μM | A549 | [220] | |
| Anti-proliferative, pro-apoptotic | ↑ Bax, caspase-3, p38, JNK, annexin V ↓ Bcl-2, SOX2 | 30, 50 μM | A549 | [215] | |
| Breast cancer | |||||
| Anti-proliferative, pro-apoptotic, migration and invasion inhibition | ↓ annexin, NF-κB, p65 | 10,20 μM | MDA-MB-231, MDA-MB-453 | [221] | |
| Colorectal cancer | |||||
| Anti-proliferative, cell cycle arrest, prooxidant | ↑ ROS, p53 ↓ ERK | 125, 250, 500, 1000 μmol/L | HCT116, HT29 | [217] | |
| Cell cycle arrest, pro-apoptotic | ↑ caspase-3 | 250, 500, 1000 μM | Caco-2 | [219] | |
| Liver cancer | |||||
| Anti-proliferative, cell cycle arrest, invasion, and metastasis inhibition | ↓ MMP-2,-9, ERK1/2 | 250, 500, 1000 μM | HepG2 | [216] | |
| Anti-proliferative, cell cycle arrest | ↑ p21, p53, and KHSRP ↓ c-Myc, miR-17 family | 25, 50 μM | Huh7 | [214] | |
| Kidney cancer | |||||
| Anti-proliferative, pro-apoptotic | ↑ caspase, Bax ↓ Bcl-2, PI3K, Akt, mTOR | 40 μM | A498 | [26] | |
| Osteosarcoma | |||||
| Anti-proliferative, pro-apoptotic, cell cycle arrest | ↑ ERK1/2 | 200, 400 μM | U2OS, Saos-2 | [218] | |
| CATECHIN AND EPICATECHIN | Breast cancer | ||||
| Pro-apoptotic | ↑ ZIP9 ↓ cAMP agonists to membrane androgen receptors | 200 nM | MDA-MB-468 | [230] | |
| Pro-apoptotic | agonists to membrane androgen receptors | 21.4 nM | T47D | [231] | |
| Anti-proliferative, pro-apoptotic, antioxidant | ↑ IRK ↓ ROS | 40, 100 μg/mL | MCF-10A | [227] | |
| Pro-apoptotic, prooxidant | ↑ ROS, Bad, Bax | 150, 200, 250, 300, 350, 400, 450, 500 μM | MDA-MB-231 | [232] | |
| Colorectal cancer | |||||
| Pro-apoptotic, migration and invasion reduction | ↑ E-cadherin ↓ ERK1/2, c-Myc, β-catenin | 12.5, 20 μM | HT-29 | [233] | |
| Liver cancer | |||||
| Anti-proliferative, cell cycle arrest | ↑ p21, waf1/cip1 ↓ CDC25A | 50, 75, 100, 125, 150 μM | HepG2, Huh7 | [226] | |
| Biliary tract cancer | |||||
| Pro-apoptotic, cell cycle arrest | ↑ caspase, p21, gene dr5 | 20, 50 μM | CCSW-1, BDC, EGI-1, SkChA-1, TFK-1, MzChA-1, MzChA-2, GBC | [229] | |
| Prostate cancer | |||||
| Pro-apoptotic | ↑ ZIP9 ↓ cAMP agonists to membrane androgen receptors | 200 nM | PC-3 | [230] | |
| Pancreatic cancer | |||||
| Anti-proliferative, pro-apoptotic, cell cycle arrest | ↑ Bax ↓ Ras, NF-κB, p65, Bcl-2, Pi3K, Akt | 25, 50 μM | E6E7-Kras-st | [228] | |
| PROCYANIDINS | Breast cancer | ||||
| Pro-apoptotic | ↑ cytochrome-c, caspase-3,-9 | 25 μg/m | B16, BALB-MC.E12 | [239] | |
| Pro-apoptotic, migration and invasion reduction | ↑ maspin, E-cadherin, BRCA1 ↓ DNA methyltransferases | 50, 100, 150, 200, 250 μM | MDA-MB-231 | [243] | |
| Pro-apoptotic | n/a | 50 μM | MCF-7 | [244] | |
| Pro-apoptotic, cell cycle arrest | ↑ Bax, caspase-3,-9 ↓ Bcl-2 | 31.5, 36.6 mg/mL | MDA-MB-231, MCF-7 | [274] | |
| Colorectal cancer | |||||
| Anti-proliferative, pro-apoptotic, cell cycle arrest | ↑ caspase-3, ERK1/2, JNK ↓ PKC | 45 μg/mL | SW620 | [238] | |
| Anti-proliferative, pro-apoptotic, cell cycle arrest | ↑ MMP-2,-9, caspase-3,-9, ERK 1/2, MEK, Akt, PI3K ↓ EGFR | 10–60 μM | Caco-2, HT-29, HCT-15, HCT-116 | [240] | |
| Pro-apoptotic, prooxidant | ↑ caspase-3,-8,-9, Bax, ROS, cytochrome-c ↓Bcl-2 | 80 µg/mL | SW480 and SW620 | [246] | |
| Anti-proliferative, cell cycle arrest, pro-apoptotic | ↑ ERK1/2, MEK, PI3K, Akt ↓ EGFR | 10, 20, 30 μM | Caco-2 | [241] | |
| Pro-apoptotic | ↑ PKB, Akt, ERK1/2, p38 | 2.5–20 μM | Caco-2 | [275] | |
| Pro-apoptotic | ↑ caspase-3,-9, cytochrome-c ↓ PI3K, Akt, bad | 2.5–50 μM | Caco-2 | [276] | |
| Liver cancer | |||||
| Anti-proliferative, migration inhibition | ↓ Kv10.1 | 10, 100, 1000 μM | HepG2 | [242] | |
| Prostate cancer | |||||
| Pro-apoptotic, prooxidant | ↑ ROS, ERK1/2, AMPKα | 25, 50 μM (PCa LNCaP); 50, 100, 200 μM (22Rv1) | PCa LNCaP, 22Rv1 | [245] | |
| TRITERPENOIDS | Breast cancer | ||||
| Anti-proliferative | n/a | n/a | MCF-7 | [260] | |
| Anti-proliferative, pro-apoptotic | ↑ Bax, cytochrome-c, p53 ↓ Bcl-2 | 2.57, 5.45 μM (respectively) | MDA-MB-231, MCF-7 | [254] | |
| Anti-proliferative, migration and invasion inhibition | ↑ caspase-3 ↓ MMP-2,-9, TIMP-2 | 5, 10, 20 μM | MCF-7, 4T1, MDA-MB-231 | [256] | |
| Anti-proliferative, pro-apoptotic, migration and invasion inhibition | ↓ aerobic glycolysis, c-Myc, lactate dehydrogenase A (LDH-A), p-PDK1, Caveloin-1 | 48.55, 19.06 μM (respectively) | MDA-MB-231, MCF-7 | [255] | |
| Anti-proliferative, pro-apoptotic, migration and invasion inhibition | ↑ GRP78, PERK ↓ aerobic glycolysis, c-Myc, β-catenin | 5-50 μM | MDA-MB-231, BT-549, HBL-100 | [257] | |
| Anti-proliferative, pro-apoptotic, autophagy inhibition, anti-inflammatory | ↓ PI3K, Akt, NF-κB | 232, 221, 240 μg/mL (respectively) | T47D, MCF-7, MDA-MB-231 | [258] | |
| Cell cycle arrest, pro-apoptotic, autophagy | ↑ p53, p21, AMPK ↓ ERK1/2, glycolysis, PKM2, HK2 | 20 μM | MCF-7, MDA-MB-231, SK-BR-3 | [80] | |
| Lung cancer | |||||
| Pro-apoptotic, angiogenesis inhibition | ↑ Bax ↓ VEGF | 25, 50 μg/ml | A549, H460 | [270] | |
| Colorectal cancer | |||||
| Anti-proliferative | n/a | n/a | Caco-2 | [260] | |
| Invasion and metastasis inhibition | ↓ cadherins, integrins | 10, 20, 40, 80 μM | SW620 | [272] | |
| Liver cancer | |||||
| Anti-proliferative, prooxidant | ↑ ROS ↓ PI3K, Akt1, mTOR | 10, 30, 100 μM | [268] | ||
| Anti-proliferative | n/a | n/a | HepG2 | [260] | |
| Invasion and metastasis inhibition | ↓ cadherins, integrins | 10, 20, 40, 80 μM | HepG2 | [272] | |
| Anti-proliferative, pro-apoptotic | ↑ p53, caspase-3 ↓ Bcl-2, Mcl-a mRNA | 10, 20, 30 μM | HUH7, PLC/PRF/5, L02 | [265] | |
| Pancreatic cancer | |||||
| Cell cycle arrest, pro-apoptotic, autophagy induction | ↑ Bax, ATG5, LC3-II ↓ Bcl-2, RAGE | 25, 50, 75, 100 μM | MIA Paca-2 | [266] | |
| Prostate cancer | |||||
| Pro-apoptotic | ↑ cytochrome-c, PARP, p21, p53 ↓ NF-κB, Bcl-2, p65 | 10, 25 μM | LNCaP, DU145 | [263] | |
| Anti-proliferative, pro-apoptotic |
↑ survivin ↓ Bcl-2, Bcl-xl, survivin, PI3K, Akt, mTOR | LNCaP, PC-3 | [259] | ||
| Cervical cancer | |||||
| Pro-apoptotic, cell cycle arrest, prooxidant | ↑ ROS, p21, Bad, caspase-9 ↓ PI3K, Akt | 30 μmol/: | HeLa | [262] | |
| Ovarian cancer | |||||
| Anti-proliferative, pro-apoptotic | ↑ Bax, caspase-3,-8,-9 ↓ Bcl-2 | 44.47 μM | A2780 | [264] | |
| Gallbladder cancer | |||||
| Anti-proliferative, cell cycle arrest, pro-apoptotic | ↑ Bax, cytochrome-c, caspase-3,-9 ↓ Bcl-2 | 50 μmol/L | GBC-SD, NOZ | [267] | |
| Brain cancer | |||||
| Pro-apoptotic, migration and invasion reduction | ↑ JNK signaling pathway, caspases ↓ enzyme MGMT | 20 μM | U373MG | [273] | |
| Anti-proliferative, pro-apoptotic | ↓ enzyme MGMT, STAT3 | 20, 30, 40, 50 μM | LN229, LN18, T98G | [253] | |
| Osteosarcoma | |||||
| Anti-proliferative, pro-apoptotic, antioxidant | ↑ caspase-3 ↓ Notch signaling pathway, Bcl-2, ROS | 50, 80 μM (respectively) | Saos-2, MG63 | [269] | |
| Melanoma | |||||
| Invasion and metastasis inhibition | ↓ cadherins, integrins | 10, 20, 40, 80 μM | B16-F10 | [272] | |
↑: increased expression; ↓: decreased expression.
6. Conclusions
Cancer is a leading cause of death globally and represents one of the greatest health challenges. Therefore, it is necessary to find effective prevention tools to lower incidence of this chronic disease. Phytochemicals are secondary metabolites present in vegetables and fruit that provide many health benefits such as chemo-preventive and chemo-protective effects in different cancers. As highlighted in this review, apples are a promising fruit to consider in dietary plans for cancer prevention as they are widely available and contains various beneficial phytochemicals. There is evidence from epidemiological studies that regular apple consumption decreases the incidence of different cancers. However, from these observational studies, it is difficult to distinguish the effects of apple specifically from other lifestyle factors that influence cancer risk. The anticancer effects of apples are believed to be mainly due to their phenolic compounds such as phloretin, quercetin and its glycosides, chlorogenic acid, catechin, and epicatechin. However, while most of the research is focused on phenolics, there is evidence that triterpenoids, which are present mainly in apple skin, have significant chemo-preventive and chemo-protective effects. A limiting factor of the apple’s anticancer benefits is their low bioavailability in vivo and in humans.
Apart from the in vivo and in vitro studies that overwhelmingly support the impact of apples in cancer prevention and inhibition, clinical interventions are lacking. Therefore, in this review article, we describe some of the considerations that need to be accounted for when using apples in an interventional setting or dietary plans. The levels and profiles of phytochemicals in apple depends on many factors such as cultivar and maturity stage, but also vary greatly within the apple parts (peel, flesh). Apple’s skin is known to be a rich source of phenolics and significantly contributes to the health benefits of apples, therefore, consumption of whole apple with skin and consumption of different apple cultivars might help to obtain greater anticancer effects. Furthermore, the food matrix components such as fiber have an important role to play in the health benefits of apples and influence the bioavailability of the apple phytochemicals.
Apple phytochemicals provide many beneficial health effects and could work as a preventive tool in cancer. However, more research (especially in vivo and clinical studies) is needed to confirm apple’s anticancer effects and bioavailability in humans.
Author Contributions
Conceptualization, L.N., N.A.N., S.M. and J.H.; writing—original draft preparation, L.N.; writing—review and editing, T.M., M.C., L.N., N.A.N., S.M. and J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Heritage Food Crops Research Trust. S.M. was supported by Maurice Wilkins Centre for Biodiscovery and HRC: Sir Charles Hercus Fellowship (21-030). L.N. is supported by a Massey University Doctoral Scholarship.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
Massey University Doctoral Scholarship support for L.N., Heritage Food Crops Research Trust.
Conflicts of Interest
The authors declare no conflict of interest. Funding by Heritage Food Crops Research Trust.
Abbreviations
| AGEs | Advanced glycation end products |
| Akt | Protein kinase B |
| AMPK | Activated protein kinase |
| ATG5 | Autophagy protein 5 |
| Bad | Bcl2 Associated Agonist Of Cell Death protein |
| Bax | Bcl2 Associated X protein |
| Bcl-2 | B-cell lymphoma 2 |
| BRCA1 | Breast cancer 1 gene |
| cAMP | Cyclic adenosine monophosphate |
| CDC25A | Cell division cycle 25 A gene |
| CDK-1,-2,-4,-6 | Cyclin dependent kinase |
| cIAP1,2 | Cellular Inhibitor of Apoptosis Protein 1 |
| cip1 | CDK- interacting protein |
| CISD2 | CISD2 gene |
| c-Myc | Cellular myelocytomatosis oncogene |
| COX-2 | Cyclooxygenase-2 |
| EGFR | Epidermal growth factor receptor |
| ERK, ERK1/2 | Extracellular regulated kinase |
| FoxA2 | Forkhead box protein A2 |
| FoxO3a | Forkhead box protein o3a |
| GADD45 | Growth arrest and DNA damage-inducible protein |
| Glut1 | Glucose transporter 1 |
| GRP78 (BiP) | Binding immunoglobulin protein |
| HK2 | Hexokinase-2 |
| IL-6,-8 | Interleukin-6,-8 |
| IRK | The arabidopsis receptor kinase |
| JNK | C-Jun N-terminal Kinase |
| KHSRP | Kh-type splicing regulatory protein |
| Kv10.1 | Kv10.1 potassium channel |
| LC3-II | Light chain membrane protein |
| LDHA | Lactate dehydrogenase A |
| LDH-A | Lactate dehydrogenase A |
| Mcl-1 | Induced myeloid leukemia cell differentiation protein |
| MEK | Mitogen-activated protein kinase kinase |
| MGMT | Methylated-DNA—protein-cysteine methyltransferase |
| miR-146a | Micro Ribonucleic Acid 146a |
| MMP-2,-9 | Matrix metalloproteinase-2,-9 |
| mTOR | Mammalian target of rapamycin protein |
| NF-κB | Nuclear Factor kappa B |
| p21, p65, p62, p53, p38, p27 | Protein 21,65,62,53,38,27 |
| PARP | Poly [ADP-ribose] polymerase 1 |
| PDK3,1 | Pyruvate dehydrogenase |
| pERK | R-like endoplasmic reticulum kinase |
| PGE2 | Prostaglandin E2 |
| Phosphor-eIF2α | Phosphorylation of eukaryotic initiation factor-2 alpha |
| PI3K | Phosphoinositide 3-kinase |
| Pin1 | Prolyl isomerase |
| p-JNK | Phosphorylated c-Jun N-terminal Kinase (JNK) |
| PKB | Protein kinase B |
| PKC | Protein kinase C |
| PKM2 | Pyruvate kinase m2 |
| rad51 | RAD51 Recombinase (gene) |
| RAGE | Advanced glycosylation end product-specific receptor |
| Ras | Ras protein |
| ROS | Reactive oxygen species |
| SHP-1 | Protein tyrosine phosphatase shp 1 |
| SIRT-1 | Sirtiuin 1 |
| SOX2 | Sry-box transcription factor 2 |
| STAT3 | Signal transducer and activator of transcription 3 |
| TCF4 | Transcription factor 4 (gene) |
| TIMP-2 | Tissue Inhibitor of Metalloproteinase 2 |
| TLR4 | Toll-like receptor 4 |
| TNF- α | Tumor necrosis factor alpha |
| ULK-1 | Unc-51 like autophagy activating kinase 1 |
| VEGF | Vascular endothelial growth factor |
| VEGFR | Vascular endothelial growth factor receptor |
| Waf1 | Wild type p53 activated protein-1 |
| ZIP9 | Zinc transporter 9 |
References
- Harris, R.E. Epidemiology of Chronic Disease: Global Perspectives; Jones & Bartlett Learning: Burlington, MA, USA, 2019. [Google Scholar]
- Wilkins, E.; Wilson, L.; Wickramasinghe, K.; Bhatnagar, P.; Leal, J.; Luengo-Fernandez, R.; Burns, R.; Rayner, M.; Townsend, N. European Cardiovascular Disease Statistics 2017; European Heart Network: Brussels, Belgium, 2017. [Google Scholar]
- Bowry, A.D.; Lewey, J.; Dugani, S.B.; Choudhry, N.K. The Burden of Cardiovascular Disease in Low- and Middle-Income Countries: Epidemiology and Management. Can. J. Cardiol. 2015, 31, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Magliano, D.J.; Islam, R.M.; Barr, E.L.M.; Gregg, E.; Pavkov, M.E.; Harding, J.L.; Tabesh, M.; Koye, D.N.; Shaw, J.E. Trends in incidence of total or type 2 diabetes: Systematic review. BMJ 2019, 366, l5003. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, O.; Kayode, A.A.; Olusola, Y.O.; Joshua, A.I.; Chinedu, O.V. Lung Cancer: A Chronic Disease Epidemiology; Prevalence Study. Asian J. Adv. Res. Rep. 2019, 3, 1–7. [Google Scholar] [CrossRef]
- World Health Organization. Noncommunicable Diseases: Progress Monitor 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2018; Available online: https://gco.iarc.fr/today (accessed on 23 July 2021).
- Reiss, R.; Johnston, J.; Tucker, K.; DeSesso, J.M.; Keen, C.L. Estimation of cancer risks and benefits associated with a potential increased consumption of fruits and vegetables. Food Chem. Toxicol. 2012, 50, 4421–4427. [Google Scholar] [CrossRef] [PubMed]
- Mathur, G.; Nain, S.; Sharma, P.K. Cancer: An Overview. Acad. J. Cancer Res. 2015, 8, 1. [Google Scholar]
- Sun, Y.S.; Zhao, Z.; Yang, Z.N.; Xu, F.; Lu, H.J.; Zhu, Z.Y.; Shi, W.; Jiang, J.; Yao, P.P.; Zhu, H.P. Risk factors and preventions of breast cancer. Int. J. Biol. Sci. 2017, 13, 1387. [Google Scholar] [CrossRef]
- Rawla, P.; Barsouk, A. Epidemiology of gastric cancer: Global trends, risk factors and prevention. Prz. Gastroenterol. 2019, 14, 26. [Google Scholar] [CrossRef]
- Botelho, M.C.; Teixeira, J.P.; Oliveira, P.A. Carcinogenesis. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; pp. 713–729. [Google Scholar]
- Fan, A.M. Chapter 11—Cancer. In Information Resources in Toxicology, 4th ed.; Wexler, P., Gilbert, S.G., Hakkinen, P.J., Mohapatra, A., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 103–121. [Google Scholar]
- Basu, A.K. DNA damage, mutagenesis and cancer. Int. J. Mol. Sci. 2018, 19, 970. [Google Scholar] [CrossRef]
- Bajaj, J.; Diaz, E.; Reya, T. Stem cells in cancer initiation and progression. J. Cell Biol. 2019, 219, e201911053. [Google Scholar] [CrossRef]
- Mäki-Nevala, S.; Valo, S.; Ristimäki, A.; Sarhadi, V.; Knuutila, S.; Nyström, M.; Renkonen-Sinisalo, L.; Lepistö, A.; Mecklin, J.P.; Peltomäki, P. DNA methylation changes and somatic mutations as tumorigenic events in Lynch syndrome-associated adenomas retaining mismatch repair protein expression. EBioMedicine 2019, 39, 280–291. [Google Scholar] [CrossRef]
- Takeshima, H.; Ushijima, T. Accumulation of genetic and epigenetic alterations in normal cells and cancer risk. Npj Precis. Oncol. 2019, 3, 1–8. [Google Scholar] [CrossRef]
- Pogribny, I.P.; Rusyn, I. Environmental Toxicants, Epigenetics, and Cancer. In Epigenetic Alterations in Oncogenesis; Springer: New York, NY, USA, 2013; pp. 215–232. [Google Scholar]
- Botezatu, A.; Iancu, I.V.; Popa, O.; Plesa, A.; Manda, D.; Huica, I.; Vladoiu, S.; Anton, G.; Badiu, C. Mechanisms of Oncogene Activation. New Aspects in Molecular and Cellular Mechanisms of Human Carcinogenesis; Bulgin, D., Ed.; BoD—Books on Demand: Norderstedt, Germany, 2016; pp. 1–52. [Google Scholar]
- Kinsella, A.R. Multistage Carcinogenesis and the Biological Effects of Tumor Promoters. In Naturally Occurring Phorbol Esters; CRC Press: Boca Raton, FL, USA, 2018; pp. 33–61. [Google Scholar]
- Mulshine, J.L.; Treston, A.M.; Brown, P.H.; Birrer, M.J.; Shaw, G.L. Initiators and promoters of lung cancer. Chest 1993, 103, 4S–11S. [Google Scholar] [CrossRef] [PubMed]
- Weston, A.; Harris, C.C. Multistage Carcinogenesis. In Cancer Medicine; Kufe, D.W., Pollock, R.E., Weichselbaum, R.R., Bast, R.C., Jr., Gansler, T.S., Holland, J.F., Frei, E., III, Eds.; BC Decker: Hamilton, ON, Canada, 2003. [Google Scholar]
- Klein, C.A. Cancer progression and the invisible phase of metastatic colonization. Nat. Rev. Cancer 2020, 20, 681–694. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Divisi, D.; Di Tommaso, S.; Salvemini, S.; Garramone, M.; Crisci, R. Diet and cancer. Acta Bio-Med. Atenei Parm. 2006, 77, 118–123. [Google Scholar]
- Wallace, T.C.; Bailey, R.L.; Blumberg, J.B.; Burton-Freeman, B.; Chen, C.-Y.O.; Crowe-White, K.M.; Drewnowski, A.; Hooshmand, S.; Johnson, E.; Lewis, R.; et al. Fruits, vegetables, and health: A comprehensive narrative, umbrella review of the science and recommendations for enhanced public policy to improve intake. Crit. Rev. Food Sci. Nutr. 2019, 60, 2174–2211. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; A Martínez-González, M.; Fung, T.T.; Lichtenstein, A.H.; Forouhi, N. Food based dietary patterns and chronic disease prevention. BMJ 2018, 361, k2396. [Google Scholar] [CrossRef] [PubMed]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Lippi, G.; Mattiuzzi, C.; Cervellin, G. Meat consumption and cancer risk: A critical review of published meta-analyses. Crit. Rev. Oncol. 2015, 97, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Johnson, I. The cancer risk related to meat and meat products. Br. Med Bull. 2016, 121, 73–81. [Google Scholar] [CrossRef]
- Yu, H.; Xu, Q.; Xiong, W.; Liu, Z.; Cai, L.; He, F. Association of pickled food, fired food and smoked food combined with smoking and alcohol drinking with lung cancer: A case-control study. Wei Sheng Yan Jiu J. Hyg. Res. 2019, 48, 925–931. [Google Scholar]
- WHO; FAO. Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a Joint Who/Fao Expert Consultation; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- WHO. Healthy Diet—Fact Sheet No. 394 2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Hurtado-Barroso, S.; Trius-Soler, M.; Lamuela-Raventós, R.M.; Zamora-Ros, R. Vegetable and Fruit Consumption and Prognosis among Cancer Survivors: A Systematic Review and Meta-Analysis of Cohort Studies. Adv. Nutr. 2020, 11, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—A systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [PubMed]
- Basli, A.; Belkacem, N.; Amrani, I. Health Benefits of Phenolic Compounds against Cancers. In Phenolic Compounds–Biological Activity; IntechOpen: London, UK, 2017; pp. 193–210. [Google Scholar]
- Davidson, K.T.; Zhu, Z.; Fang, Y. Phytochemicals in the Fight against Cancer. Pathol. Oncol. Res. 2016, 22, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Johnson, I.T. Phytochemicals and cancer. Proc. Nutr. Soc. 2007, 66, 207–215. [Google Scholar] [CrossRef]
- Shree, T.J.; Poompavai, S.; Begum SM, F.M.; Gowrisree, V.; Hemalatha, S. Cancer-fighting phytochemicals: Another look. J. Nanomedine Biother. Discov. 2019, 8, 162. [Google Scholar]
- Scarpa, E.-S.; Ninfali, P. Phytochemicals as Innovative Therapeutic Tools against Cancer Stem Cells. Int. J. Mol. Sci. 2015, 16, 15727–15742. [Google Scholar] [CrossRef] [PubMed]
- Talalay, P.; Fahey, J.W. Phytochemicals from Cruciferous Plants Protect against Cancer by Modulating Carcinogen Metabolism. J. Nutr. 2001, 131, 3027S–3033S. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-F.; Weng, C.-J.; Sethi, G.; Hu, D.-N. Natural Bioactives and Phytochemicals Serve in Cancer Treatment and Prevention. Evid.-Based Complement. Altern. Med. 2013, 2013, 698190. [Google Scholar] [CrossRef]
- Meybodi, N.M.; Mortazavian, A.M.; Monfared, A.B.; Sohrabvandi, S.; Meybodi, F.A. Phytochemicals in Cancer Prevention: A Review of the Evidence. Iran. J. Cancer Prev. 2017, 10, e7219. [Google Scholar] [CrossRef]
- Ranjan, A.; Ramachandran, S.; Gupta, N.; Kaushik, I.; Wright, S.; Srivastava, S.; Das, H.; Srivastava, S.; Prasad, S.; Srivastava, S.K. Role of phytochemicals in cancer prevention. Int. J. Mol. Sci. 2019, 20, 4981. [Google Scholar] [CrossRef] [PubMed]
- Zubair, H.; Azim, S.; Ahmad, A.; Khan, M.A.; Patel, G.K.; Singh, S.; Singh, A.P. Cancer Chemoprevention by Phytochemicals: Nature’s Healing Touch. Molecules 2017, 22, 395. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef]
- Gallus, S.; Talamini, R.; Giacosa, A.; Montella, M.; Ramazzotti, V.; Franceschi, S.; Negri, E.; La Vecchia, C. Does an apple a day keep the oncologist away? Ann. Oncol. 2005, 16, 1841–1844. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.V.; Thilakarathna, S.; Nair, S. Polyphenols of Apples and Their Potential Health Benefits. In Polyphenols: Chemistry, Dietary Sources and Health Benefits; Nova Science Publishers: Hauppauge, NY, USA, 2013; pp. 333–368. [Google Scholar]
- Rana, S.; Bhushan, S. Apple phenolics as nutraceuticals: Assessment, analysis and application. J. Food Sci. Technol. 2016, 53, 1727–1738. [Google Scholar] [CrossRef] [PubMed]
- Konopacka, D.; Jesionkowska, K.; Kruczyńska, D.; Stehr, R.; Schoorl, F.; Buehler, A.; Egger, S.; Codarin, S.; Hilaire, C.; Höller, I.; et al. Apple and peach consumption habits across European countries. Appetite 2010, 55, 478–483. [Google Scholar] [CrossRef]
- Kidoń, M.; Grabowska, J. Bioactive compounds, antioxidant activity, and sensory qualities of red-fleshed apples dried by different methods. LWT 2020, 136, 110302. [Google Scholar] [CrossRef]
- Dashbaldan, S.; Pączkowski, C.; Szakiel, A. Variations in triterpenoid deposition in cuticular Waxes during development and maturation of selected fruits of Rosaceae family. Int. J. Mol. Sci. 2020, 21, 9762. [Google Scholar] [CrossRef] [PubMed]
- Sut, S.; Poloniato, G.; Malagoli, M.; Dall’Acqua, S. Fragmentation of the main triterpene acids of apple by LC-APCI-MSn. J. Mass Spectrom. 2018, 53, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.A.; Bynum, J.P.; Sirovich, B.E. Association between apple consumption and physician visits: Appealing the conventional wisdom that an apple a day keeps the doctor away. JAMA Intern. Med. 2015, 175, 777–783. [Google Scholar] [CrossRef]
- Vinson, J.A.; Su, X.; Zubik, L.; Bose, P. Phenol Antioxidant Quantity and Quality in Foods: Fruits. J. Agric. Food Chem. 2001, 49, 5315–5321. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chu, Y.-F.; Wu, X.; Liu, R.H. Antioxidant and Antiproliferative Activities of Common Fruits. J. Agric. Food Chem. 2002, 50, 7449–7454. [Google Scholar] [CrossRef]
- McCann, M.; Gill, C.; Brien, G.O.; Rao, J.; McRoberts, W.; Hughes, P.; McEntee, R.; Rowland, I. Anti-cancer properties of phenolics from apple waste on colon carcinogenesis in vitro. Food Chem. Toxicol. 2007, 45, 1224–1230. [Google Scholar] [CrossRef]
- Kalinowska, M.; Bielawska, A.; Lewandowska-Siwkiewicz, H.; Priebe, W.; Lewandowski, W. Apples: Content of phenolic compounds vs. variety, part of apple and cultivation model, extraction of phenolic compounds, biological properties. Plant Physiol. Biochem. 2014, 84, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Imeh, U.; Khokhar, S. Distribution of conjugated and free phenols in fruits: Antioxidant activity and cultivar variations. J. Agric. Food Chem. 2002, 50, 6301–6306. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.P.; Gião, M.S.; Pintado, M.; Gomes, M.H. Bioactive phytochemicals in apple cultivars from the Portuguese protected geographical indication “Maçã de Alcobaça:” Basis for market segmentation. Int. J. Food Prop. 2017, 20, 2206–2214. [Google Scholar] [CrossRef]
- McGhie, T.K.; Hunt, M.; Barnett, L.E. Cultivar and growing region determine the antioxidant polyphenolic concentration and composition of apples grown in New Zealand. J. Agric. Food Chem. 2005, 53, 3065–3070. [Google Scholar] [CrossRef]
- Honda, C.; Moriya, S. Anthocyanin Biosynthesis in Apple Fruit. Hortic. J. 2018, 87, 305–314. [Google Scholar] [CrossRef]
- De Paepe, D.; Valkenborg, D.; Noten, B.; Servaes, K.; Diels, L.; De Loose, M.; Van Droogenbroeck, B.; Voorspoels, S. Variability of the phenolic profiles in the fruits from old, recent and new apple cultivars cultivated in Belgium. Metabolomics 2015, 11, 739–752. [Google Scholar] [CrossRef]
- Kruger, M.J.; Davies, N.; Myburgh, K.H.; Lecour, S. Proanthocyanidins, anthocyanins and cardiovascular diseases. Food Res. Int. 2014, 59, 41–52. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Liang, D.; Zou, Y.; Li, P.; Ma, F. Phenolic compounds and antioxidant activity in red-fleshed apples. J. Funct. Foods 2015, 18, 1086–1094. [Google Scholar] [CrossRef]
- Rana, S.; Rana, A.; Gupta, S.; Bhushan, S. Varietal influence on phenolic constituents and nutritive characteristics of pomace obtained from apples grown in western Himalayas. J. Food Sci. Technol. 2020, 58, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, O.; Kuldjärv, R.; Paalme, T.; Virkki, M.; Yang, B. Impact of apple cultivar, ripening stage, fermentation type and yeast strain on phenolic composition of apple ciders. Food Chem. 2017, 233, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Alberti, A.; Zielinski, A.; Couto, M.; Judacewski, P.; Igarashi-Mafra, L.; Nogueira, A. Distribution of phenolic compounds and antioxidant capacity in apples tissues during ripening. J. Food Sci. Technol. 2017, 54, 1511–1518. [Google Scholar] [CrossRef]
- Van der Sluis, A.A.; Dekker, M.; de Jager, A.; Jongen, W.M. Activity and concentration of polyphenolic antioxidants in apple: Effect of cultivar, harvest year, and storage conditions. J. Agric. Food Chem. 2001, 49, 3606–3613. [Google Scholar] [CrossRef]
- Łata, B.; Trampczynska, A.; Paczesna, J. Cultivar variation in apple peel and whole fruit phenolic composition. Sci. Hortic. 2009, 121, 176–181. [Google Scholar] [CrossRef]
- Kalinowska, M.; Gryko, K.; Wróblewska, A.M.; Jabłońska-Trypuć, A.; Karpowicz, D. Phenolic content, chemical composition and anti-/pro-oxidant activity of Gold Milenium and Papierowka apple peel extracts. Sci. Rep. 2020, 10, 14951. [Google Scholar] [CrossRef]
- Kschonsek, J.; Wolfram, T.; Stöckl, A.; Böhm, V. Polyphenolic Compounds Analysis of Old and New Apple Cultivars and Contribution of Polyphenolic Profile to the In Vitro Antioxidant Capacity. Antioxidants 2018, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Shehzadi, K.; Rubab, Q.; Asad, L.; Ishfaq, M.; Shafique, B.; Modassar, M.; Ranjha, A.N.; Mahmood, S.; Mueen-Ud-Din, G.; Javaid, T. A critical review on presence of polyphenols in commercial varieties of apple peel, their extraction and Health benefits. Open Access J. Biog. Sci. Res. 2020, 6, 18. [Google Scholar]
- Veberic, R.; Trobec, M.; Herbinger, K.; Hofer, M.; Grill, D.; Stampar, F. Phenolic compounds in some apple (Malus domestica Borkh) cultivars of organic and integrated production. J. Sci. Food Agric. 2005, 85, 1687–1694. [Google Scholar] [CrossRef]
- Francini, A.; Sebastiani, L. Phenolic compounds in apple (Malus × domestica Borkh): Compounds characterization and stability during postharvest and after processing. Antioxidants 2013, 2, 181–193. [Google Scholar] [CrossRef]
- Raudone, L.; Raudonis, R.; Liaudanskas, M.; Janulis, V.; Viskelis, P. Phenolic antioxidant profiles in the whole fruit, flesh and peel of apple cultivars grown in Lithuania. Sci. Hortic. 2017, 216, 186–192. [Google Scholar] [CrossRef]
- Tsao, R.; Yang, R.; Young, A.J.C.; Zhu, H. Polyphenolic Profiles in Eight Apple Cultivars Using High-Performance Liquid Chromatography (HPLC). J. Agric. Food Chem. 2003, 51, 6347–6353. [Google Scholar] [CrossRef] [PubMed]
- Łata, B. Relationship between Apple Peel and the Whole Fruit Antioxidant Content: Year and Cultivar Variation. J. Agric. Food Chem. 2007, 55, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Lewinska, A.; Adamczyk-Grochala, J.; Kwasniewicz, E.; Deregowska, A.; Wnuk, M. Ursolic acid-mediated changes in glycolytic pathway promote cytotoxic autophagy and apoptosis in phenotypically different breast cancer cells. Apoptosis 2017, 22, 800–815. [Google Scholar] [CrossRef] [PubMed]
- Le Bourvellec, C.; Bouzerzour, K.; Ginies, C.; Regis, S.; Plé, Y.; Renard, C.M. Phenolic and polysaccharidic composition of applesauce is close to that of apple flesh. J. Food Compos. Anal. 2011, 24, 537–547. [Google Scholar] [CrossRef]
- Selby-Pham, S.N.; Miller, R.B.; Howell, K.; Dunshea, F.; Bennett, L.E. Physicochemical properties of dietary phytochemicals can predict their passive absorption in the human small intestine. Sci. Rep. 2017, 7, 1931. [Google Scholar] [CrossRef]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. BioMed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Bondonno, C.P.; Ward, N.C.; Hodgson, J.M.; Croft, K.D. The cardiovascular health benefits of apples: Whole fruit vs. isolated compounds. Trends Food Sci. Technol. 2017, 69, 243–256. [Google Scholar] [CrossRef]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant phenolics: Bioavailability as a key determinant of their potential health-promoting applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef] [PubMed]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An overview on the role of dietary phenolics for the treatment of cancers. Nutr. J. 2016, 15, 1–16. [Google Scholar] [CrossRef]
- Aprikian, O.; Duclos, V.; Guyot, S.; Besson, C.; Manach, C.; Bernalier, A.; Morand, C.; Rémésy, C.; Demigné, C. Apple pectin and a polyphenol-rich apple concentrate are more effective together than separately on cecal fermentations and plasma lipids in rats. J. Nutr. 2003, 133, 1860–1865. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; Espín, J.C.; Tomas-Barberan, F. Interaction between Phenolics and Gut Microbiota: Role in Human Health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef] [PubMed]
- Stracke, B.A.; Rüfer, C.E.; Bub, A.; Seifert, S.; Weibel, F.P.; Kunz, C.; Watzl, B. No effect of the farming system (organic/conventional) on the bioavailability of apple (Malus domestica Bork., cultivar Golden Delicious) polyphenols in healthy men: A comparative study. Eur. J. Nutr. 2010, 49, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Wruss, J.; Lanzerstorfer, P.; Huemer, S.; Himmelsbach, M.; Mangge, H.; Höglinger, O.; Weghuber, D.; Weghuber, J. Differences in pharmacokinetics of apple polyphenols after standardized oral consumption of unprocessed apple juice. Nutr. J. 2015, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- DuPont, M.S.; Bennett, R.N.; Mellon, F.A.; Williamson, G. Polyphenols from alcoholic apple cider are absorbed, metabolized and excreted by humans. J. Nutr. 2002, 132, 172–175. [Google Scholar] [CrossRef]
- Koutsos, A.; Lima, M.; Conterno, L.; Gasperotti, M.; Bianchi, M.; Fava, F.; Vrhovsek, U.; Lovegrove, J.A.; Tuohy, K.M. Effects of Commercial Apple Varieties on Human Gut Microbiota Composition and Metabolic Output Using an In Vitro Colonic Model. Nutrients 2017, 9, 533. [Google Scholar] [CrossRef]
- Shinohara, K.; Ohashi, Y.; Kawasumi, K.; Terada, A.; Fujisawa, T. Effect of apple intake on fecal microbiota and metabolites in humans. Anaerobe 2010, 16, 510–515. [Google Scholar] [CrossRef]
- Williams, B.A.; Grant, L.J.; Gidley, M.J.; Mikkelsen, D. Gut fermentation of dietary fibres: Physico-chemistry of plant cell walls and implications for health. Int. J. Mol. Sci. 2017, 18, 2203. [Google Scholar] [CrossRef]
- Efimtseva, E.A.; Chelpanova, T.I. Apples as a Source of Soluble and Insoluble Dietary Fibers: Effect of Dietary Fibers on Appetite. Hum. Physiol. 2020, 46, 224–234. [Google Scholar] [CrossRef]
- Andoh, A.; Tsujikawa, T.; Fujiyama, Y. Role of dietary fiber and short-chain fatty acids in the colon. Curr. Pharm. Des. 2003, 9, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Starowicz, M.; Achrem-Achremowicz, B.; Piskula, M.; Zielinski, H. Phenolic compounds from apples: Reviewing their occurrence, absorption, bioavailability, processing, and antioxidant activity—A review. Pol. J. Food Nutr. Sci. 2020, 70, 321–336. [Google Scholar] [CrossRef]
- Zou, T.; Wang, B.; Li, S.; Liu, Y.; You, J. Dietary apple polyphenols promote fat browning in high-fat diet-induced obese mice through activation of adenosine monophosphate-activated protein kinase α. J. Sci. Food Agric. 2020, 100, 2389–2398. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-N.; Meng, X.; Li, Y.; Li, S.; Liu, Q.; Tang, G.-Y.; Li, H.-B. Fruits for Prevention and Treatment of Cardiovascular Diseases. Nutrients 2017, 9, 598. [Google Scholar] [CrossRef] [PubMed]
- Gayer, B.A.; Avendano, E.E.; Edelson, E.; Nirmala, N.; Johnson, E.J.; Raman, G. Effects of Intake of Apples, Pears, or Their Products on Cardiometabolic Risk Factors and Clinical Outcomes: A Systematic Review and Meta-Analysis. Curr. Dev. Nutr. 2019, 3, nzz109. [Google Scholar] [CrossRef]
- Hyun, T.K.; Jang, K.I. Apple as a source of dietary phytonutrients: An update on the potential health benefits of apple. EXCLI J. 2016, 15, 565. [Google Scholar]
- Mahmud, L.S.M.; Tisha, A.; Sagor, A.T. A comprehensive review on effective role of Apple polyphenols in the treatment of obesity, diabetes, and liver dysfunctions with some possible molecular mechanisms. Oxid. Antioxid. Med Sci. 2018, 7, 9–27. [Google Scholar]
- Guo, X.F.; Yang, B.; Tang, J.; Jiang, J.J.; Li, D. Apple and pear consumption and type 2 diabetes mellitus risk: A meta-analysis of prospective cohort studies. Food Funct. 2017, 8, 927–934. [Google Scholar] [CrossRef]
- Alam, M.N.; Almoyad, M.; Huq, F. Polyphenols in colorectal cancer: Current state of knowledge including clinical trials and molecular mechanism of action. BioMed Res. Int. 2018, 2018, 4154185. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, O.P.; Thompson-Bonilla, M.D.R.; Jaramillo-Flores, M.E. Association between obesity and breast cancer: Molecular bases and the effect of flavonoids in signaling pathways. Crit. Rev. Food Sci. Nutr. 2020, 60, 3770–3792. [Google Scholar] [CrossRef]
- Linseisen, J.; Rohrmann, S.; Miller, A.B.; Bueno-De-Mesquita, H.B.; Büchner, F.; Vineis, P.; Agudo, A.; Gram, I.T.; Janson, L.; Krogh, V.; et al. Fruit and vegetable consumption and lung cancer risk: Updated information from the European Prospective Investigation into Cancer and Nutrition (EPIC). Int. J. Cancer 2007, 121, 1103–1114. [Google Scholar] [CrossRef]
- Büchner, F.L.; Bueno-de-Mesquita, H.B.; Ros, M.M.; Kampman, E.; Egevad, L.; Overvad, K.; Raaschou-Nielsen, O.; Tjønneland, A.; Roswall, N.; Clavel-Chapelon, F.; et al. Consumption of vegetables and fruit and the risk of bladder cancer in the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer 2009, 125, 2643–2651. [Google Scholar] [CrossRef] [PubMed]
- Feskanich, D.; Ziegler, R.G.; Michaud, D.S.; Giovannucci, E.L.; Speizer, F.E.; Willett, W.C.; Colditz, G. Prospective Study of Fruit and Vegetable Consumption and Risk of Lung Cancer Among Men and Women. J. Natl. Cancer Inst. 2000, 92, 1812–1823. [Google Scholar] [CrossRef] [PubMed]
- Knekt, P.; Järvinen, R.; Seppänen, R.; Heliövaara, M.; Teppo, L.; Pukkala, E.; Aromaa, A. Dietary flavonoids and the risk of lung cancer and other malignant neoplasms. Am. J. Epidemiol. 1997, 146, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Le Marchand, L.; Murphy, S.P.; Hankin, J.H.; Wilkens, L.R.; Kolonel, L.N. Intake of flavonoids and lung cancer. J. Natl. Cancer Inst. 2000, 92, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Arts, I.C.; Hollman, P.C.; Bueno de Mesquita, H.B.; Feskens, E.J.; Kromhout, D. Dietary catechins and epithelial cancer incidence: The Zutphen elderly study. Int. J. Cancer 2001, 92, 298–302. [Google Scholar] [CrossRef]
- Farvid, M.S.; Chen, W.Y.; Rosner, B.A.; Tamimi, R.M.; Willett, W.C.; Eliassen, A.H. Fruit and vegetable consumption and breast cancer incidence: Repeated measures over 30 years of follow-up. Int. J. Cancer 2018, 144, 1496–1510. [Google Scholar] [CrossRef]
- Rossi, M.; Lugo, A.; Lagiou, P.; Zucchetto, A.; Polesel, J.; Serraino, D.; Negri, E.; Trichopoulos, D.; La Vecchia, C. Proanthocyanidins and other flavonoids in relation to pancreatic cancer: A case–control study in Italy. Ann. Oncol. 2012, 23, 1488–1493. [Google Scholar] [CrossRef]
- Smith-Warner, S.A.; Spiegelman, D.; Yaun, S.S.; Adami, H.O.; Beeson, W.L.; Van Den Brandt, P.A.; Folsom, A.R.; Fraser, G.E.; Freudenheim, J.L.; Goldbohm, R.A.; et al. Intake of fruits and vegetables and risk of breast cancer: A pooled analysis of cohort studies. JAMA 2001, 285, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Torres-Sánchez, L.; López-Carrillo, L.; Ló-Cervantes, M.; Rueda-Neria, C.; Wolff, M.S. Food Sources of Phytoestrogens and Breast Cancer Risk in Mexican Women. Nutr. Cancer 2000, 37, 134–139. [Google Scholar] [CrossRef]
- Jedrychowski, W.; Maugeri, U.; Popiela, T.; Kulig, J.; Sochacka-Tatara, E.; Pac, A.; Sowa, A.; Musial, A. Case–control study on beneficial effect of regular consumption of apples on colorectal cancer risk in a population with relatively low intake of fruits and vegetables. Eur. J. Cancer Prev. 2010, 19, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Kreimer, A.R.; Randi, G.; Herrero, R.; Castellsagué, X.; Vecchia, C.L.; Franceschi, S. Diet and body mass, and oral and oropharyngeal squamous cell carcinomas: Analysis from the IARC multinational case–control study. Int. J. Cancer 2006, 118, 2293–2297. [Google Scholar] [CrossRef] [PubMed]
- Rashidkhani, B.; Lindblad, P.; Wolk, A. Fruits, vegetables and risk of renal cell carcinoma: A prospective study of Swedish women. Int. J. Cancer 2004, 113, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Lindblad, P.; Wolk, A.; Bergström, R.; Adami, H.O. Diet and risk of renal cell cancer: A population-based case-control study. Cancer Epidemiol. Biomark. Prev. 1997, 6, 215–223. [Google Scholar]
- Askari, F.; Parizi, M.K.; Jessri, M.; Rashidkhani, B. Fruit and Vegetable Intake in Relation to Prostate Cancer in Iranian Men: A Case-Control Study. Asian Pac. J. Cancer Prev. 2014, 15, 5223–5227. [Google Scholar] [CrossRef] [PubMed]
- Malin, A.S.; Qi, D.; Shu, X.O.; Gao, Y.T.; Friedmann, J.M.; Jin, F.; Zheng, W. Intake of fruits, vegetables and selected micronutrients in relation to the risk of breast cancer. Int. J. Cancer 2003, 105, 413–418. [Google Scholar] [CrossRef]
- Fabiani, R.; Minelli, L.; Rosignoli, P. Apple intake and cancer risk: A systematic review and meta-analysis of observational studies. Public Health Nutr. 2016, 19, 2603–2617. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Farias, M.; Carrasco-Pozo, C. The Anti-Cancer Effect of Quercetin: Molecular Implications in Cancer Metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef]
- Li, C.X.; Zhao, X.H.; Zuo, W.F.; Zhang, T.L.; Zhang, Z.Y.; Chen, X.S. Phytochemical profiles, antioxidant, and antiproliferative activities of four red-fleshed apple varieties in China. J. Food Sci. 2020, 85, 718–726. [Google Scholar] [CrossRef]
- Sun, J.; Liu, R.H. Apple Phytochemical Extracts Inhibit Proliferation of Estrogen-Dependent and Estrogen-Independent Human Breast Cancer Cells through Cell Cycle Modulation. J. Agric. Food Chem. 2008, 56, 11661–11667. [Google Scholar] [CrossRef] [PubMed]
- Schiavano, G.F.; De Santi, M.; Brandi, G.; Fanelli, M.; Bucchini, A.; Giamperi, L.; Giomaro, G.M. Inhibition of Breast Cancer Cell Proliferation and In Vitro Tumorigenesis by a New Red Apple Cultivar. PLoS ONE 2015, 10, e0135840. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Eggert, D.; Mukhtar, H.; Ahmad, N. Antiproliferative Effects of Apple Peel Extract Against Cancer Cells. Nutr. Cancer 2010, 62, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Quagliuolo, L.; D’Angelo, S. Annurca Apple Biophenols’ Effects in Combination with Cisplatin on A549 Cells. Curr. Nutr. Food Sci. 2020, 17, 111–120. [Google Scholar] [CrossRef]
- Rana, S.; Kumar, S.; Rana, A.; Padwad, Y.; Bhushan, S. Biological activity of phenolics enriched extracts from industrial apple pomace. Ind. Crop. Prod. 2020, 160, 113158. [Google Scholar] [CrossRef]
- Miura, D.; Miura, Y.; Yagasaki, K. Effect of apple polyphenol extract on hepatoma proliferation and invasion in culture and on tumor growth, metastasis, and abnormal lipoprotein profiles in hepatoma-bearing rats. Biosci. Biotechnol. Biochem. 2007, 71, 2743–2750. [Google Scholar] [CrossRef]
- Almatroodi, S.; Alsahli, M.; Almatroudi, A.; Verma, A.; Aloliqi, A.; Allemailem, K.; Khan, A.; Rahmani, A. Potential Therapeutic Targets of Quercetin, a Plant Flavonol, and Its Role in the Therapy of Various Types of Cancer through the Modulation of Various Cell Signaling Pathways. Molecules 2021, 26, 1315. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.-H.; Chen, L.-C.; Ho, Y.-S. An apple a day to prevent cancer formation: Reducing cancer risk with flavonoids. J. Food Drug Anal. 2016, 25, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.-C.; Ho, C.-T.; Pan, M.-H. Recent advances in cancer chemoprevention with phytochemicals. J. Food Drug Anal. 2019, 28, 14–37. [Google Scholar] [CrossRef]
- Bars-Cortina, D.; Martínez-Bardají, A.; Macià, A.; Motilva, M.J.; Piñol-Felis, C. Consumption evaluation of one apple flesh a day in the initial phases prior to adenoma/adenocarcinoma in an azoxymethane rat colon carcinogenesis model. J. Nutr. Biochem. 2020, 83, 108418. [Google Scholar] [CrossRef]
- Liu, R.H.; Liu, J.; Chen, B. Apples prevent mammary tumors in rats. J. Agric. Food Chem. 2005, 53, 2341–2343. [Google Scholar] [CrossRef] [PubMed]
- Veeriah, S.; Kautenburger, T.; Habermann, N.; Sauer, J.; Dietrich, H.; Will, F.; Pool-Zobel, B.L. Apple flavonoids inhibit growth of HT29 human colon cancer cells and modulate expression of genes involved in the biotransformation of xenobiotics. Mol. Carcinog. 2006, 45, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Leng, E.; Xiao, Y.; Mo, Z.; Li, Y.; Zhang, Y.; Deng, X.; Li, W. Synergistic effect of phytochemicals on cholesterol metabolism and lipid accumulation in HepG2 cells. BMC Complementary Altern. Med. 2018, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Virgous, C.; Si, H. Synergistic anti-inflammatory effects and mechanisms of combined phytochemicals. J. Nutr. Biochem. 2019, 69, 19–30. [Google Scholar] [CrossRef]
- Yang, J.; Liu, R.H. Synergistic effect of apple extracts and quercetin 3-β-d-glucoside combination on antiproliferative activity in MCF-7 human breast cancer cells in vitro. J. Agric. Food Chem. 2009, 57, 8581–8586. [Google Scholar] [CrossRef]
- Moskovitz, J.; Yim, M.B.; Chock, P.B. Free radicals and disease. Arch. Biochem. Biophys. 2002, 397, 354–359. [Google Scholar] [CrossRef]
- Adom, K.K.; Liu, R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef]
- Lee, K.W.; Kim, Y.J.; Kim, D.O.; Lee, H.J.; Lee, C.Y. Major phenolics in apple and their contribution to the total antioxidant capacity. J. Agric. Food Chem. 2003, 51, 6516–6520. [Google Scholar] [CrossRef]
- Wandjou, J.G.N.; Lancioni, L.; Barbalace, M.C.; Hrelia, S.; Papa, F.; Sagratini, G.; Vittori, S.; Dall’Acqua, S.; Caprioli, G.; Beghelli, D.; et al. Comprehensive characterization of phytochemicals and biological activities of the Italian ancient apple ‘Mela Rosa dei Monti Sibillini’. Food Res. Int. 2020, 137, 109422. [Google Scholar] [CrossRef]
- Sies, H. Total Antioxidant Capacity: Appraisal of a Concept. J. Nutr. 2007, 137, 1493–1495. [Google Scholar] [CrossRef]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant Activity of Apple Peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H.; Sun, J. Antiproliferative Activity of Apples Is Not Due to Phenolic-Induced Hydrogen Peroxide Formation. J. Agric. Food Chem. 2003, 51, 1718–1723. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, M.V.; Lee, C.Y.; Liu, R.H. Antioxidant activity of fresh apples. Nat. Cell Biol. 2000, 405, 903–904. [Google Scholar] [CrossRef] [PubMed]
- Martino, E.; Vuoso, D.C.; D’Angelo, S.; Mele, L.; D’Onofrio, N.; Porcelli, M.; Cacciapuoti, G. Annurca apple polyphenol extract selectively kills MDA-MB-231 cells through ROS generation, sustained JNK activation and cell growth and survival inhibition. Scientific reports 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- León-González, A.J.; Auger, C.; Schini-Kerth, V.B. Pro-oxidant activity of polyphenols and its implication on cancer chemoprevention and chemotherapy. Biochem. Pharmacol. 2015, 98, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.A. Phenolics: Prooxidants or antioxidants? Nutr. Rev. 1997, 55, 396–398. [Google Scholar] [CrossRef]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative stress, prooxidants, and antioxidants: The interplay. BioMed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef]
- Sotler, R.; Poljšak, B.; Dahmane, R.; Jukić, T.; Jukić, D.P.; Rotim, C.; Trebše, P.; Starc, A. Prooxidant Activities of Antioxidants and Their Impact on Health. Acta Clin. Croat. 2019, 58, 726–736. [Google Scholar] [CrossRef]
- Azmi, A.S.; Sarkar, F.H.; Hadi, S.M. Pro-oxidant activity of dietary chemopreventive agents: An under-appreciated anti-cancer property. F1000Research 2013, 2, 135. [Google Scholar] [CrossRef][Green Version]
- Fernando, W.; Rupasinghe, H.V.; Hoskin, D.W. Dietary phytochemicals with anti-oxidant and pro-oxidant activities: A double-edged sword in relation to adjuvant chemotherapy and radiotherapy? Cancer Lett. 2019, 452, 168–177. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant activity of polyphenols, flavonoids, anthocyanins and carotenoids: Updated review of mechanisms and catalyzing metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, B.; Hosu, A.; David, L.; Cimpoiu, C. Total phenolics, anthocyanins, antioxidant and pro-oxidant activity of some red fruits teas. Acta Chim. Slov. 2016, 63, 213–219. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yordi, E.G.; Pérez, E.M.; Matos, M.J.; Villares, E.U. Antioxidant and pro-oxidant effects of polyphenolic compounds and structure-activity relationship evidence. Nutr. Well-Being Health 2012, 2, 23–48. [Google Scholar]
- Mendoza-Wilson, A.M.; Castro-Arredondo, S.I.; Espinosa-Plascencia, A.; del Refugio Robles-Burgueño, M.; Balandrán-Quintana, R.R.; del Carmen Bermúdez-Almada, M. Chemical composition and antioxidant-prooxidant potential of a polyphenolic extract and a proanthocyanidin-rich fraction of apple skin. Heliyon 2016, 2, e00073. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-T.; Ha, J.; Park, I.-J.; Lee, S.-K.; Baik, H.W.; Kim, Y.M.; Park, O.J. Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Lett. 2007, 247, 115–121. [Google Scholar] [CrossRef]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef]
- Kocyigit, A.; Guler, E.M.; Dikilitas, M. Role of Antioxidant Phytochemicals in Prevention, Formation and Treatment of Cancer. In Reactive Oxygen Species (ROS) in Living Cells; InterchOpen: London, UK, 2018; pp. 21–45. [Google Scholar]
- Almeida, A.F.; Borge, G.I.A.; Piskula, M.; Tudose, A.; Tudoreanu, L.; Valentová, K.; Williamson, G.; Santos, C.N. Bioavailability of Quercetin in Humans with a Focus on Interindividual Variation. Compr. Rev. Food Sci. Food Saf. 2018, 17, 714–731. [Google Scholar] [CrossRef]
- Karakaya, S. Bioavailability of phenolic compounds. Crit. Rev. Food Sci. Nutr. 2004, 44, 453–464. [Google Scholar] [CrossRef]
- Petersen, B.; Egert, S.; Bosy-Westphal, A.; Müller, M.J.; Wolffram, S.; Hubbermann, E.M.; Rimbach, G.; Schwarz, K. Bioavailability of quercetin in humans and the influence of food matrix comparing quercetin capsules and different apple sources. Food Res. Int. 2016, 88, 159–165. [Google Scholar] [CrossRef]
- Hollman, P.C.; Tijburg, L.B.; Yang, C.S. Bioavailability of flavonoids from tea. Crit. Rev. Food Sci. Nutr. 1997, 37, 719–738. [Google Scholar] [CrossRef]
- Hollman, P.C.; Van Trijp, J.; Buysman, M.N.; Gaag, M.S.V.; Mengelers, M.J.; De Vries, J.H.; Katan, M.B. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett. 1997, 418, 152–156. [Google Scholar] [CrossRef]
- Biedrzycka, E.; Amarowicz, R. Diet and Health: Apple Polyphenols as Antioxidants. Food Rev. Int. 2008, 24, 235–251. [Google Scholar] [CrossRef]
- Nishijima, T.; Iwai, K.; Saito, Y.; Takida, Y.; Matsue, H. Chronic Ingestion of Apple Pectin Can Enhance the Absorption of Quercetin. J. Agric. Food Chem. 2009, 57, 2583–2587. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Turco, I.; Bacchetti, T. Apple as a source of dietary phytonutrients: Bioavailability and evidence of protective effects against human cardiovascular disease. Food Nutr. Sci. 2014, 2014, 13. [Google Scholar] [CrossRef]
- Graefe, E.U.; Wittig, J.; Mueller, S.; Riethling, A.K.; Uehleke, B.; Drewelow, B.; Pforte, H.; Jacobasch, G.; Derendorf, H.; Veit, M. Pharmacokinetics and bioavailability of quercetin glycosides in humans. J. Clin. Pharmacol. 2001, 41, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Erlund, I.; Freese, R.; Marniemi, J.; Hakala, P.; Alfthan, G. Bioavailability of Quercetin from Berries and the Diet. Nutr. Cancer 2006, 54, 13–17. [Google Scholar] [CrossRef]
- Manach, C.; Morand, C.; Texier, O.; Favier, M.L.; Agullo, G.; Demigné, C.; Régérat, F.; Rémésy, C. Quercetin metabolites in plasma of rats fed diets containing rutin or quercetin. J. Nutr. 1995, 125, 1911–1922. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Khan, I.A.; Ur-Rehman, M.; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer potential of quercetin: A comprehensive review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef]
- Jeong, J.-H.; An, J.Y.; Kwon, Y.T.; Rhee, J.G.; Lee, Y.J. Effects of low dose quercetin: Cancer cell-specific inhibition of cell cycle progression. J. Cell. Biochem. 2008, 106, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Ezzati, M.; Yousefi, B.; Velaei, K.; Safa, A. A review on anti-cancer properties of Quercetin in breast cancer. Life Sci. 2020, 248, 117463. [Google Scholar] [CrossRef]
- Tao, S.-F.; He, H.-F.; Chen, Q. Quercetin inhibits proliferation and invasion acts by up-regulating miR-146a in human breast cancer cells. Mol. Cell. Biochem. 2015, 402, 93–100. [Google Scholar] [CrossRef]
- Deng, X.-H.; Song, H.-Y.; Zhou, Y.-F.; Yuan, G.-Y.; Zheng, F.-J. Effects of quercetin on the proliferation of breast cancer cells and expression of survivin in vitro. Exp. Ther. Med. 2013, 6, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.X.; Deng, X.H.; Ai, F.; Yuan, G.Y.; Song, H.Y. Effect of quercetin on the proliferation of the human ovarian cancer cell line SKOV-3 in vitro. Exp. Ther. Med. 2015, 10, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, R.; Mohammad, T.; Roy, S.; Anwar, S.; Gupta, P.; Haque, A.; Khan, P.; Kazim, S.N.; Islam, A.; Ahmad, F.; et al. Investigation of inhibitory potential of quercetin to the pyruvate dehydrogenase kinase 3: Towards implications in anticancer therapy. Int. J. Biol. Macromol. 2019, 136, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ding, H.; Tang, X.; Liang, M.; Li, S.; Zhang, J.; Cao, J. Quercetin induces pro-apoptotic autophagy via SIRT1 / AMPK signaling pathway in human lung cancer cell lines A549 and H1299 in vitro. Thorac. Cancer 2021, 12, 1415–1422. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Lee, Y.H.; Sharma, A.R.; Park, J.B.; Jagga, S.; Sharma, G.; Lee, S.S.; Nam, J.S. Quercetin induces apoptosis and cell cycle arrest in triple-negative breast cancer cells through modulation of Foxo3a activity. Korean J. Physiol. Pharmacol. 2017, 21, 205–213. [Google Scholar] [CrossRef]
- Gong, C.; Yang, Z.; Zhang, L.; Wang, Y.; Gong, W.; Liu, Y. Quercetin suppresses DNA double-strand break repair and enhances the radiosensitivity of human ovarian cancer cells via p53-dependent endoplasmic reticulum stress pathway. OncoTargets Ther. 2018, 11, 17–27. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, S.-K.; Kim, B.-S.; Lee, S.-H.; Park, Y.-S.; Park, B.-K.; Kim, S.-J.; Kim, J.; Choi, C.; Kim, J.-S.; et al. Apoptotic Effect of Quercetin on HT-29 Colon Cancer Cells via the AMPK Signaling Pathway. J. Agric. Food Chem. 2010, 58, 8643–8650. [Google Scholar] [CrossRef]
- Glick, D.; Barth, S.; MacLeod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef]
- Jia, L.; Huang, S.; Yin, X.; Zan, Y.; Guo, Y.; Han, L. Quercetin suppresses the mobility of breast cancer by suppressing glycolysis through Akt-mTOR pathway mediated autophagy induction. Life Sci. 2018, 208, 123–130. [Google Scholar] [CrossRef]
- Song, Y.; Han, M.; Zhang, X. Quercetin suppresses the migration and invasion in human colon cancer Caco-2 cells through regulating toll-like receptor 4/nuclear factor-kappa B pathway. Pharmacogn. Mag. 2016, 12, S237. [Google Scholar] [CrossRef]
- Oh, S.J.; Kim, O.; Lee, J.S.; Kim, J.-A.; Kim, M.R.; Choi, H.S.; Shim, J.-H.; Kang, K.W.; Kim, Y.C. Inhibition of angiogenesis by quercetin in tamoxifen-resistant breast cancer cells. Food Chem. Toxicol. 2010, 48, 3227–3234. [Google Scholar] [CrossRef] [PubMed]
- Pratheeshkumar, P.; Budhraja, A.; Son, Y.-O.; Wang, X.; Zhang, Z.; Ding, S.; Wang, L.; Hitron, A.; Lee, J.-C.; Xu, M.; et al. Quercetin Inhibits Angiogenesis Mediated Human Prostate Tumor Growth by Targeting VEGFR- 2 Regulated AKT/mTOR/P70S6K Signaling Pathways. PLoS ONE 2012, 7, e47516. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhao, X.; Xu, J.; Zhang, H. Quercetin inhibits angiogenesis-mediated human retinoblastoma growth by targeting vascular endothelial growth factor receptor. Oncol. Lett. 2017, 14, 3343–3348. [Google Scholar] [CrossRef]
- Monge, P.; Solheim, E.; Scheline, R.R. Dihydrochalcone metabolism in the rat: Phloretin. Xenobiotica 1984, 14, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Crespy, V.; Aprikian, O.; Morand, C.; Besson, C.; Manach, C.; Demigné, C.; Rémésy, C. Bioavailability of Phloretin and Phloridzin in Rats. J. Nutr. 2001, 131, 3227–3230. [Google Scholar] [CrossRef]
- Marks, S.C.; Mullen, W.; Borges, G.; Crozier, A. Absorption, Metabolism, and Excretion of Cider Dihydrochalcones in Healthy Humans and Subjects with an Ileostomy. J. Agric. Food Chem. 2009, 57, 2009–2015. [Google Scholar] [CrossRef] [PubMed]
- Kahle, K.; Huemmer, W.; Kempf, M.; Scheppach, W.; Erk, T.; Richling, E. Polyphenols Are Intensively Metabolized in the Human Gastrointestinal Tract after Apple Juice Consumption. J. Agric. Food Chem. 2007, 55, 10605–10614. [Google Scholar] [CrossRef]
- Wu, K.-H.; Ho, C.-T.; Chen, Z.-F.; Chen, L.-C.; Whang-Peng, J.; Lin, T.-N.; Ho, Y.-S. The apple polyphenol phloretin inhibits breast cancer cell migration and proliferation via inhibition of signals by type 2 glucose transporter. J. Food Drug Anal. 2017, 26, 221–231. [Google Scholar] [CrossRef]
- Lin, S.-T.; Tu, S.-H.; Yang, P.-S.; Hsu, S.-P.; Lee, W.H.; Ho, C.-T.; Wu, C.-H.; Lai, Y.-H.; Chen, M.-Y.; Chen, L.-C. Apple Polyphenol Phloretin Inhibits Colorectal Cancer Cell Growth via Inhibition of the Type 2 Glucose Transporter and Activation of p53-Mediated Signaling. J. Agric. Food Chem. 2016, 64, 6826–6837. [Google Scholar] [CrossRef]
- Wu, C.H.; Ho, Y.S.; Tsai, C.Y.; Wang, Y.J.; Tseng, H.; Wei, P.L.; Lee, C.H.; Liu, R.S.; Lin, S.Y. In vitro and in vivo study of phloretin—Induced apoptosis in human liver cancer cells involving inhibition of type II glucose transporter. Int. J. Cancer 2009, 124, 2210–2219. [Google Scholar] [CrossRef]
- Xu, M.; Gu, W.; Shen, Z.; Wang, F. Anticancer activity of phloretin against human gastric cancer cell lines involves apoptosis, cell cycle arrest, and inhibition of cell invasion and JNK signalling pathway. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 6551. [Google Scholar] [CrossRef]
- Min, J.; Li, X.; Huang, K.; Tang, H.; Ding, X.; Qi, C.; Qin, X.; Xu, Z. Phloretin induces apoptosis of non-small cell lung carcinoma A549 cells via JNK1/2 and p38 MAPK pathways. Oncol. Rep. 2015, 34, 2871–2879. [Google Scholar] [CrossRef]
- Kim, U.; Kim, C.Y.; Lee, J.M.; Oh, H.; Ryu, B.; Kim, J.; Park, J.H. Phloretin inhibits the human prostate cancer cells through the generation of reactive oxygen species. Pathol. Oncol. Res. 2020, 26, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Saraswati, S.; Alhaider, A.; Abdelgadir, A.M.; Tanwer, P.; Korashy, H.M. Phloretin attenuates STAT-3 activity and overcomes sorafenib resistance targeting SHP-1–mediated inhibition of STAT3 and Akt/VEGFR2 pathway in hepatocellular carcinoma. Cell Commun. Signal. 2019, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fan, C.; Pu, L.; Wei, C.; Jin, H.; Teng, Y.; Zhao, M.; Yu, A.C.H.; Jiang, F.; Shu, J.; et al. Phloretin induces cell cycle arrest and apoptosis of human glioblastoma cells through the generation of reactive oxygen species. J. Neuro-Oncol. 2016, 128, 217–223. [Google Scholar] [CrossRef]
- Duan, H.; Wang, R.; Yan, X.; Liu, H.; Zhang, Y.; Mu, D.; Han, J.; Li, X. Phloretin induces apoptosis of human esophageal cancer via a mitochondria-dependent pathway. Oncol. Lett. 2017, 14, 6763–6768. [Google Scholar] [CrossRef]
- Chen, M.; Gowd, V.; Wang, M.; Chen, F.; Cheng, K.-W. The apple dihydrochalcone phloretin suppresses growth and improves chemosensitivity of breast cancer cells via inhibition of cytoprotective autophagy. Food Funct. 2020, 12, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, R.; Nan, Y.; Li, W.; Wang, Q.; Jin, F. Phloretin exhibits an anticancer effect and enhances the anticancer ability of cisplatin on non-small cell lung cancer cell lines by regulating expression of apoptotic pathways and matrix metalloproteinases. Int. J. Oncol. 2015, 48, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, E.J.; Shin, H.-K.; Kwon, D.Y.; Kim, M.S.; Surh, Y.-J.; Park, J.H.Y. Induction of Apoptosis in HT-29 Colon Cancer Cells by Phloretin. J. Med. Food 2007, 10, 581–586. [Google Scholar] [CrossRef]
- Zielinska, D.; Laparra-Llopis, J.M.; Zielinski, H.; Szawara-Nowak, D.; Giménez-Bastida, J.A. Role of apple phytochemicals, phloretin and phloridzin, in modulating processes related to intestinal inflammation. Nutrients 2019, 11, 1173. [Google Scholar] [CrossRef]
- Yang, K.-C.; Tsai, C.-Y.; Wang, Y.-J.; Wei, P.-L.; Lee, C.-H.; Chen, J.-H.; Wu, C.-H.; Ho, Y.-S. Apple polyphenol phloretin potentiates the anticancer actions of paclitaxel through induction of apoptosis in human hep G2 cells. Mol. Carcinog. 2008, 48, 420–431. [Google Scholar] [CrossRef]
- Azuma, K.; Ippoushi, K.; Nakayama, M.; Ito, H.; Higashio, H.; Terao, J. Absorption of Chlorogenic Acid and Caffeic Acid in Rats after Oral Administration. J. Agric. Food Chem. 2000, 48, 5496–5500. [Google Scholar] [CrossRef] [PubMed]
- Gonthier, M.-P.; Verny, M.-A.; Besson, C.; Rémésy, C.; Scalbert, A. Chlorogenic Acid Bioavailability Largely Depends on Its Metabolism by the Gut Microflora in Rats. J. Nutr. 2003, 133, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Olthof, M.R.; Hollman, P.C.; Katan, M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001, 131, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Nardini, M.; Cirillo, E.; Natella, F.; Scaccini, C. Absorption of Phenolic Acids in Humans after Coffee Consumption. J. Agric. Food Chem. 2002, 50, 5735–5741. [Google Scholar] [CrossRef]
- Olthof, M.R.; Hollman, P.C.H.; Buijsman, M.N.; Van Amelsvoort, J.M.M.; Katan, M.B. Chlorogenic Acid, Quercetin-3-Rutinoside and Black Tea Phenols Are Extensively Metabolized in Humans. J. Nutr. 2003, 133, 1806–1814. [Google Scholar] [CrossRef]
- Huang, S.; Wang, L.-L.; Xue, N.-N.; Li, C.; Guo, H.-H.; Ren, T.-K.; Zhan, Y.; Li, W.-B.; Zhang, J.; Chen, X.-G.; et al. Chlorogenic acid effectively treats cancers through induction of cancer cell differentiation. Theranostics 2019, 9, 6745–6763. [Google Scholar] [CrossRef]
- Yamagata, K.; Izawa, Y.; Onodera, D.; Tagami, M. Chlorogenic acid regulates apoptosis and stem cell marker-related gene expression in A549 human lung cancer cells. Mol. Cell. Biochem. 2017, 441, 9–19. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, N.; Hou, N.; Dong, L.; Li, J. Chlorogenic acid inhibits hepatocellular carcinoma in vitro and in vivo. J. Nutr. Biochem. 2017, 46, 68–73. [Google Scholar] [CrossRef]
- Hou, N.; Liu, N.; Han, J.; Yan, Y.; Li, J. Chlorogenic acid induces reactive oxygen species generation and inhibits the viability of human colon cancer cells. Anti-Cancer Drugs 2017, 28, 59–65. [Google Scholar] [CrossRef]
- Sapio, L.; Salzillo, A.; Illiano, M.; Ragone, A.; Spina, A.; Chiosi, E.; Pacifico, S.; Catauro, M.; Naviglio, S. Chlorogenic acid activates ERK1/2 and inhibits proliferation of osteosarcoma cells. J. Cell. Physiol. 2020, 235, 3741–3752. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi Ekbatan, S.; Li, X.Q.; Ghorbani, M.; Azadi, B.; Kubow, S. Chlorogenic acid and its microbial metabolites exert anti-proliferative effects, S-phase cell-cycle arrest and apoptosis in human colon cancer Caco-2 cells. Int. J. Mol. Sci. 2018, 19, 723. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Du, H.; Chen, P. Chlorogenic acid inhibits the proliferation of human lung cancer A549 cell lines by targeting annexin A2 in vitro and in vivo. Biomed. Pharmacother. 2020, 131, 110673. [Google Scholar] [CrossRef]
- Zeng, A.; Liang, X.; Zhu, S.; Liu, C.; Wang, S.; Zhang, Q.; Zhao, J.; Song, L. Chlorogenic acid induces apoptosis, inhibits metastasis and improves antitumor immunity in breast cancer via the NF-κB signaling pathway. Oncol. Rep. 2021, 45, 717–727. [Google Scholar] [CrossRef]
- Cai, Z.-Y.; Li, X.-M.; Liang, J.-P.; Xiang, L.-P.; Wang, K.-R.; Shi, Y.-L.; Yang, R.; Shi, M.; Ye, J.-H.; Lu, J.-L.; et al. Bioavailability of Tea Catechins and Its Improvement. Molecules 2018, 23, 2346. [Google Scholar] [CrossRef] [PubMed]
- Warden, B.A.; Smith, L.S.; Beecher, G.R.; Balentine, D.A.; Clevidence, B.A. Catechins Are Bioavailable in Men and Women Drinking Black Tea throughout the Day. J. Nutr. 2001, 131, 1731–1737. [Google Scholar] [CrossRef]
- Lin, L.-C.; Wang, M.-N.; Tseng, T.-Y.; Sung, A.J.-S.; Tsai, T.-H. Pharmacokinetics of (−)-Epigallocatechin-3-gallate in Conscious and Freely Moving Rats and Its Brain Regional Distribution. J. Agric. Food Chem. 2007, 55, 1517–1524. [Google Scholar] [CrossRef]
- Catterall, F.; King, L.J.; Clifford, M.N.; Ioannides, C. Bioavailability of dietary doses of3H-labelled tea antioxidants (+)-catechin and (−)-epicatechin in rat. Xenobiotica 2003, 33, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Cao, J.; Cai, Z.; An, H.; Li, Y.; Peng, Y.; Chen, N.; Luo, A.; Tao, H.; Li, K. Epigallocatechin gallate induces chemopreventive effects on rats with diethylnitrosamine-induced liver cancer via inhibition of cell division cycle 25A. Mol. Med. Rep. 2020, 22, 3873–3885. [Google Scholar] [CrossRef]
- Rathore, K.; Choudhary, S.; Odoi, A.; Wang, H.-C.R. Green tea catechin intervention of reactive oxygen species-mediated ERK pathway activation and chronically induced breast cell carcinogenesis. Carcinogenesis 2011, 33, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Siddique, H.R.; Liao, D.J.; Mishra, S.K.; Schuster, T.; Wang, L.; Matter, B.; Campbell, P.M.; Villalta, P.; Nanda, S.; Deng, Y.; et al. Epicatechin-rich cocoa polyphenol inhibits Kras-activated pancreatic ductal carcinoma cell growth in vitro and in a mouse model. Int. J. Cancer 2011, 131, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Mayr, C.; Wagner, A.; Neureiter, D.; Pichler, M.; Jakab, M.; Illig, R.; Berr, F.; Kiesslich, T. The green tea catechin epigallocatechin gallate induces cell cycle arrest and shows potential synergism with cisplatin in biliary tract cancer cells. BMC Complement. Altern. Med. 2015, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Dong, J. (-)-Epicatechin acts as a potent agonist of the membrane androgen receptor, ZIP9 (SLC39A9), to promote apoptosis of breast and prostate cancer cells. J. Steroid Biochem. Mol. Biol. 2021, 211, 105906. [Google Scholar] [CrossRef]
- Nifli, A.P.; Bosson-Kouamé, A.; Papadopoulou, N.; Kogia, C.; Kampa, M.; Castagnino, C.; Stournaras, C.; Vercauteren, J.; Castanas, E. Monomeric and oligomeric flavanols are agonists of membrane androgen receptors. Exp. Cell Res. 2005, 309, 329–339. [Google Scholar] [CrossRef]
- Pereyra-Vergara, F.; Olivares-Corichi, I.M.; Perez-Ruiz, A.G.; Luna-Arias, J.P.; García-Sánchez, J.R. Apoptosis induced by (−)-epicatechin in human breast cancer cells is mediated by reactive oxygen species. Molecules 2020, 25, 1020. [Google Scholar] [CrossRef]
- Ju, J.; Hong, J.; Zhou, J.N.; Pan, Z.; Bose, M.; Liao, J.; Yang, G.Y.; Liu, Y.Y.; Hou, Z.; Lin, Y.; et al. Inhibition of intestinal tumorigenesis in Apcmin/+ mice by (−)-epigallocatechin-3-gallate, the major catechin in green tea. Cancer Res. 2005, 65, 10623–10631. [Google Scholar] [CrossRef]
- Holt, R.R.; Lazarus, S.A.; Sullards, M.C.; Zhu, Q.Y.; Schramm, D.D.; Hammerstone, J.F.; Fraga, C.G.; Schmitz, H.H.; Keen, C.L. Procyanidin dimer B2 [epicatechin-(4β-8)-epicatechin] in human plasma after the consumption of a flavanol-rich cocoa. Am. J. Clin. Nutr. 2002, 76, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Sano, A.; Yamakoshi, J.; Tokutake, S.; Tobe, K.; Kubota, Y.; Kikuchi, M. Procyanidin B1 Is Detected in Human Serum after Intake of Proanthocyanidin-rich Grape Seed Extract. Biosci. Biotechnol. Biochem. 2003, 67, 1140–1143. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.L.; Lee, A.; Manach, C.; Rios, L.; Morand, C.; Scalbert, A.; Remesy, C. Procyanidins are not bioavailable in rats fed a single meal containing a grapeseed extract or the procyanidin dimer B3. Br. J. Nutr. 2002, 87, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Deprez, S.; Mila, I.; Huneau, J.-F.; Tome, D.; Scalbert, A. Transport of Proanthocyanidin Dimer, Trimer, and Polymer Across Monolayers of Human Intestinal Epithelial Caco-2 Cells. Antioxidants Redox Signal. 2001, 3, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Gossé, F.; Guyot, S.; Roussi, S.; Lobstein, A.; Fischer, B.; Seiler, N.; Raul, F. Chemopreventive properties of apple procyanidins on human colon cancer-derived metastatic SW620 cells and in a rat model of colon carcinogenesis. Carcinogenesis 2005, 26, 1291–1295. [Google Scholar] [CrossRef]
- Miura, T.; Chiba, M.; Kasai, K.; Nozaka, H.; Nakamura, T.; Shoji, T.; Kanda, T.; Ohtake, Y.; Sato, T. Apple procyanidins induce tumor cell apoptosis through mitochondrial pathway activation of caspase-3. Carcinogenesis 2007, 29, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Li, M.C.; Wang, F.R.; Mackenzie, G.G.; Oteiza, P.I. The inhibitory effect of ECG and EGCG dimeric procyanidins on colorectal cancer cells growth is associated with their actions at lipid rafts and the inhibition of the epidermal growth factor receptor signaling. Biochem. Pharmacol. 2020, 175, 113923. [Google Scholar] [CrossRef]
- Daveri, E.; Adamo, A.M.; Alfine, E.; Zhu, W.; Oteiza, P.I. Hexameric procyanidins inhibit colorectal cancer cell growth through both redox and non-redox regulation of the epidermal growth factor signaling pathway. Redox Biol. 2020, 38, 101830. [Google Scholar] [CrossRef]
- Na, W.; Ma, B.; Shi, S.; Chen, Y.; Zhang, H.; Zhan, Y.; An, H. Procyanidin B1, a novel and specific inhibitor of Kv10. 1 channel, suppresses the evolution of hepatoma. Biochem. Pharmacol. 2020, 178, 114089. [Google Scholar] [CrossRef]
- Shilpi, A.; Parbin, S.; Sengupta, D.; Kar, S.; Deb, M.; Rath, S.K.; Pradhan, N.; Rakshit, M.; Patra, S.K. Mechanisms of DNA methyltransferase–inhibitor interactions: Procyanidin B2 shows new promise for therapeutic intervention of cancer. Chem.-Biol. Interact. 2015, 233, 122–138. [Google Scholar] [CrossRef]
- Gouvêa, C.C.P.; Avelar, M. Procyanidin B2 cytotoxicity to MCF-7 human breast adenocarcinoma cells. Indian J. Pharm. Sci. 2012, 74, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Deep, G.; Wempe, M.F.; Surek, J.; Kumar, A.; Agarwal, R.; Agarwal, C. Procyanidin B2 3, 3 ″-di-O-gallate induces oxidative stress-mediated cell death in prostate cancer cells via inhibiting MAP kinase phosphatase activity and activating ERK1/2 and AMPK. Mol. Carcinog. 2018, 57, 57–69. [Google Scholar] [CrossRef]
- Maldonado-Celis, M.E.; Bousserouel, S.; Gossé, F.; Minker, C.; Lobstein, A.; Raul, F. Differential induction of apoptosis by apple procyanidins in TRAIL-sensitive human colon tumor cells and derived TRAIL-resistant metastatic cells. J. Cancer Mol. 2009, 5, 21–30. [Google Scholar]
- Moyle, C.W.A.; Cerezo, A.B.; Winterbone, M.S.; Hollands, W.J.; Alexeev, Y.; Needs, P.W.; Kroon, P.A. Potent inhibition of VEGFR-2 activation by tight binding of green tea epigallocatechin gallate and apple procyanidins to VEGF: Relevance to angiogenesis. Mol. Nutr. Food Res. 2014, 59, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Furtado, N.A.J.C.; Pirson, L.; Edelberg, H.; Miranda, L.M.; Loira-Pastoriza, C.; Preat, V.; Larondelle, Y.; André, C.M. Pentacyclic Triterpene Bioavailability: An Overview of In Vitro and In Vivo Studies. Molecules 2017, 22, 400. [Google Scholar] [CrossRef] [PubMed]
- Patlolla, J.M.R.; Rao, C.V. Triterpenoids for cancer prevention and treatment: Current status and future prospects. Curr. Pharm. Biotechnol. 2012, 13, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, G.J.; Wang, S.J.; Li, X.T.; Xu, Y.P.; Wang, S.P.; De Xiang, J.; Pan, S.R.; Cao, G.X.; Ye, W.C. Quantitative analysis of 23-hydroxybetulinic acid in mouse plasma using electrospray liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 1619–1623. [Google Scholar] [CrossRef]
- Jeong, D.W.; Kim, Y.H.; Kim, H.H.; Ji, H.Y.; Yoo, S.D.; Choi, W.R.; Lee, S.M.; Han, C.K.; Lee, H.S. Dose-linear pharmacokinetics of oleanolic acid after intravenous and oral administration in rats. Biopharm. Drug Dispos. 2007, 28, 51–57. [Google Scholar] [CrossRef]
- Kanellos, P.T.; Kaliora, A.C.; Gioxari, A.; Christopoulou, G.O.; Kalogeropoulos, N.; Karathanos, V.T. Absorption and Bioavailability of Antioxidant Phytochemicals and Increase of Serum Oxidation Resistance in Healthy Subjects Following Supplementation with Raisins. Plant Foods Hum. Nutr. 2013, 68, 411–415. [Google Scholar] [CrossRef]
- Zhu, Z.; Du, S.; Ding, F.; Guo, S.; Ying, G.; Yan, Z. Ursolic acid attenuates temozolomide resistance in glioblastoma cells by downregulating O6-methylguanine-DNA methyltransferase (MGMT) expression. Am. J. Transl. Res. 2016, 8, 3299–3308. [Google Scholar]
- Qiao, A.; Wang, Y.; Xiang, L.; Wang, C.; He, X. A novel triterpenoid isolated from apple functions as an anti-mammary tumor agent via a mitochondrial and caspase-independent apoptosis pathway. J. Agric. Food Chem. 2015, 63, 185–191. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, S.; Zheng, Y.; Wang, N.; Yang, B.; Wang, D.; Yang, D.; Mei, W.; Zhao, Z.; Wang, Z. Betulinic acid suppresses breast cancer aerobic glycolysis via caveolin-1/NF-κB/c-Myc pathway. Biochem. Pharmacol. 2019, 161, 149–162. [Google Scholar] [CrossRef]
- Zeng, A.-Q.; Yu, Y.; Yao, Y.-Q.; Yang, F.-F.; Liao, M.; Song, L.-J.; Li, Y.-L.; Li, Y.-J.; Deng, Y.-L.; Yang, S.-P.; et al. Betulinic acid impairs metastasis and reduces immunosuppressive cells in breast cancer models. Oncotarget 2017, 9, 3794–3804. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, P.; Wang, N.; Wang, S.; Yang, B.; Li, M.; Chen, J.; Situ, H.; Xie, M.; Lin, Y.; et al. Betulinic Acid Suppresses Breast Cancer Metastasis by Targeting GRP78-Mediated Glycolysis and ER Stress Apoptotic Pathway. Oxidative Med. Cell. Longev. 2019, 2019, 8781690. [Google Scholar] [CrossRef]
- Luo, J.; Hu, Y.L.; Wang, H. Ursolic acid inhibits breast cancer growth by inhibiting proliferation, inducing autophagy and apoptosis, and suppressing inflammatory responses via the PI3K/AKT and NF-κB signaling pathways in vitro. Exp. Ther. Med. 2017, 14, 3623–3631. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Lin, Z.-M.; Ge, N.; Zhang, D.-L.; Huang, J.; Kong, F. Ursolic Acid Induces Apoptosis of Prostate Cancer Cells via the PI3K/Akt/mTOR Pathway. Am. J. Chin. Med. 2015, 43, 1471–1486. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, R.H. Triterpenoids isolated from apple peels have potent antiproliferative activity and may be partially responsible for apple’s anticancer activity. J. Agric. Food Chem. 2007, 55, 4366–4370. [Google Scholar] [CrossRef]
- Qiu, B.; Simon, M.C. Oncogenes strike a balance between cellular growth and homeostasis. Semin. Cell Dev. Biol. 2015, 43, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Pang, Q.; Wang, Y.; Yan, X. Betulinic acid induces apoptosis by regulating PI3K/Akt signaling and mitochondrial pathways in human cervical cancer cells. Int. J. Mol. Med. 2017, 40, 1669–1678. [Google Scholar] [CrossRef]
- Shankar, E.; Zhang, A.; Franco, D.; Gupta, S. Betulinic acid-mediated apoptosis in human prostate cancer cells involves p53 and nuclear factor-kappa B (NF-κB) pathways. Molecules 2017, 22, 264. [Google Scholar] [CrossRef]
- Lee, D.; Lee, S.R.; Kang, K.S.; Ko, Y.; Pang, C.; Yamabe, N.; Kim, K.H. Betulinic Acid Suppresses Ovarian Cancer Cell Proliferation through Induction of Apoptosis. Biomolecules 2019, 9, 257. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.; Li, Q.J.; Feng, Y.L.; Pan, F. Betulinic acid promotes TRAIL function on liver cancer progression inhibition through p53/Caspase-3 signaling activation. Biomed. Pharmacother. 2017, 88, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Chen, S.Y.; Lu, C.C.; Lin, J.A.; Yen, G.C. Ursolic acid promotes apoptosis, autophagy, and chemosensitivity in gemcitabine-resistant human pancreatic cancer cells. Phytother. Res. 2020, 34, 2053–2066. [Google Scholar] [CrossRef]
- Li, H.-F.; Wang, X.-A.; Xiang, S.-S.; Hu, Y.-P.; Jiang, L.; Shu, Y.-J.; Li, M.-L.; Wu, X.-S.; Zhang, F.; Ye, Y.-Y.; et al. Oleanolic acid induces mitochondrial-dependent apoptosis and G0/G1 phase arrest in gallbladder cancer cells. Drug Des. Dev. Ther. 2015, 9, 3017–3030. [Google Scholar] [CrossRef]
- Shi, Y.; Song, Q.; Hu, D.; Zhuang, X.; Yu, S.; Teng, D. Oleanolic acid induced autophagic cell death in hepatocellular carcinoma cells via PI3K/Akt/mTOR and ROS-dependent pathway. Korean J. Physiol. Pharmacol. 2016, 20, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shu, B.; Tian, Y.; Wang, G.; Wang, Y.; Wang, J.; Dong, Y. Oleanolic acid induces osteosarcoma cell apoptosis by inhibition of Notch signaling. Mol. Carcinog. 2018, 57, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Lúcio, K.A.; Rocha, G.D.G.; Monção-Ribeiro, L.C.; Fernandes, J.; Takiya, C.M.; Gattass, C.R. Oleanolic Acid Initiates Apoptosis in Non-Small Cell Lung Cancer Cell Lines and Reduces Metastasis of a B16F10 Melanoma Model In Vivo. PLoS ONE 2011, 6, e28596. [Google Scholar] [CrossRef]
- Liao, L.; Liu, C.; Xie, X.; Zhou, J. Betulinic acid induces apoptosis and impairs migration and invasion in a mouse model of ovarian cancer. J. Food Biochem. 2020, 44, e13278. [Google Scholar] [CrossRef]
- Xiang, L.; Chi, T.; Tang, Q.; Yang, X.; Ou, M.; Chen, X.; Yu, X.; Chen, J.; Ho, R.J.; Shao, J.; et al. A pentacyclic triterpene natural product, ursolic acid and its prodrug US597 inhibit targets within cell adhesion pathway and prevent cancer metastasis. Oncotarget 2015, 6, 9295–9312. [Google Scholar] [CrossRef] [PubMed]
- Conway, G.; Zizyte, D.; Mondala, J.; He, Z.; Lynam, L.; Lecourt, M.; Barcia, C.; Howe, O.; Curtin, J. Ursolic Acid Inhibits Collective Cell Migration and Promotes JNK-Dependent Lysosomal Associated Cell Death in Glioblastoma Multiforme Cells. Pharmaceuticals 2021, 14, 91. [Google Scholar] [CrossRef]
- Koteswari, L.L.; Kumari, S.; Kumar, A.B.; Malla, R.R. A comparative anticancer study on procyanidin C1 against receptor positive and receptor negative breast cancer. Nat. Prod. Res. 2019, 34, 3267–3274. [Google Scholar] [CrossRef]
- Da Silva, M.; Jaggers, G.K.; Verstraeten, S.V.; Erlejman, A.G.; Fraga, C.G.; Oteiza, P.I. Large procyanidins prevent bile-acid-induced oxidant production and membrane-initiated ERK1/2, p38, and Akt activation in Caco-2 cells. Free. Radic. Biol. 2012, 52, 151–159. [Google Scholar] [CrossRef]
- Choy, Y.Y.; Fraga, M.; Mackenzie, G.G.; Waterhouse, A.L.; Cremonini, E.; Oteiza, P.I. The PI3K/Akt pathway is involved in procyanidin-mediated suppression of human colorectal cancer cell growth. Mol. Carcinog. 2016, 55, 2196–2209. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).