Effect of Daily Oral Lactobacillus plantarum PS128 on Exercise Capacity Recovery after a Half-Marathon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lactic Acid Bacteria
2.2. Participants
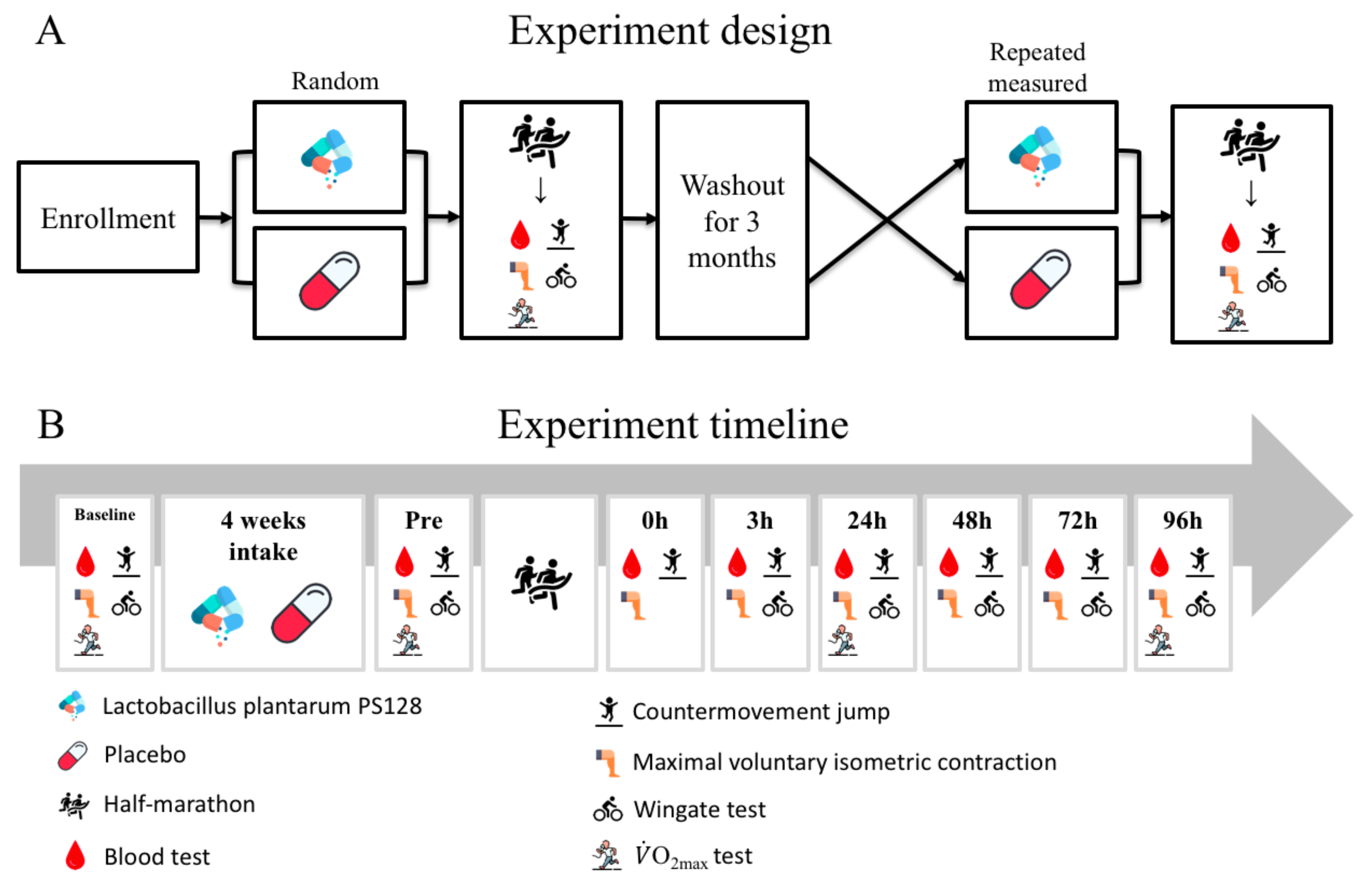
2.3. Experimental Design
2.4. Half-Marathon
2.5. Lower Extremity Muscle Strength Test
2.6. Anaerobic Power Test
2.7. Lower Extremity Explosive Force Test
2.8. Aerobic Capacity Test
2.9. Blood Sampling
2.10. Biochemical Variables
2.11. Statistical Analysis
3. Results
3.1. Test of Homogeneity
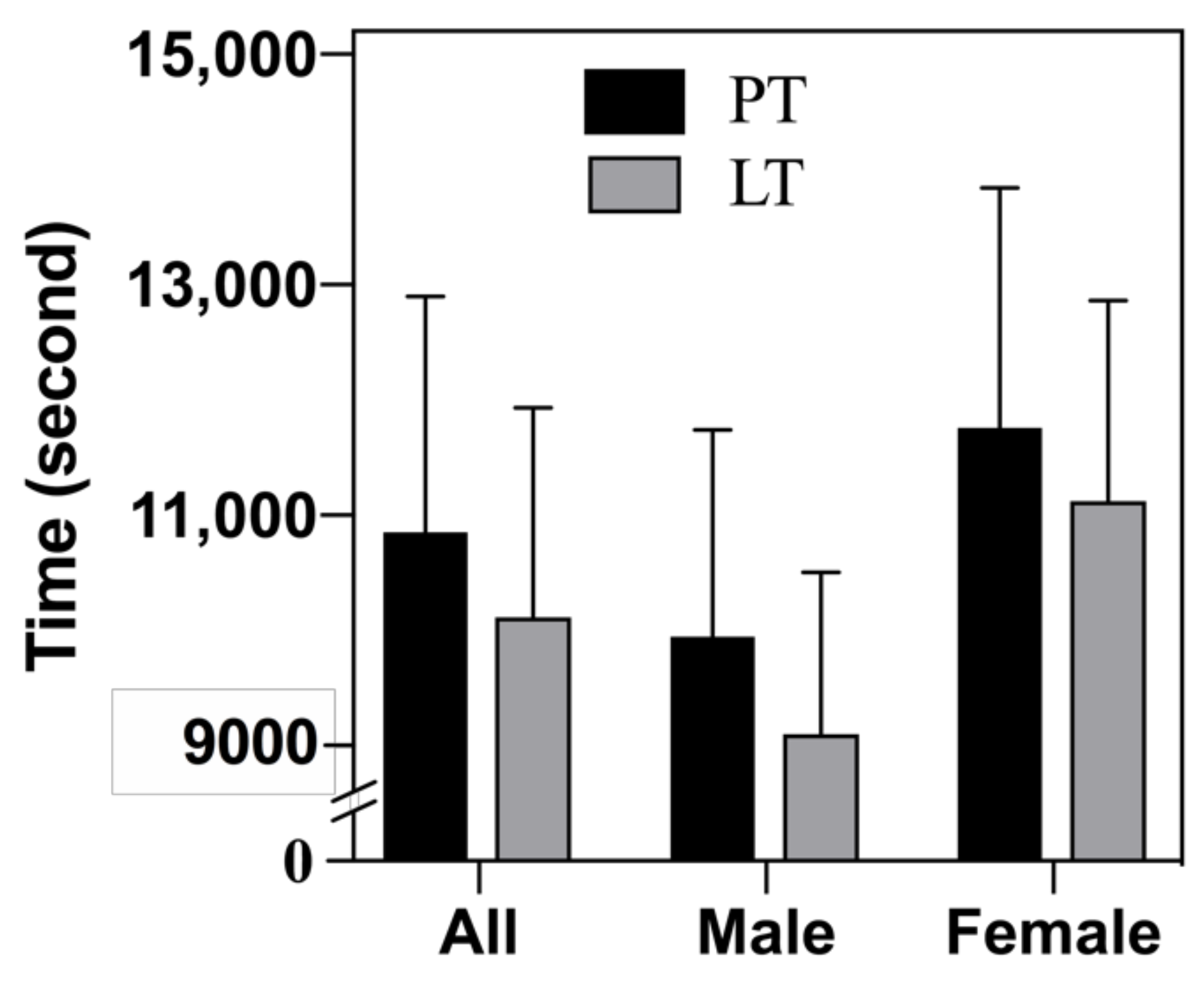
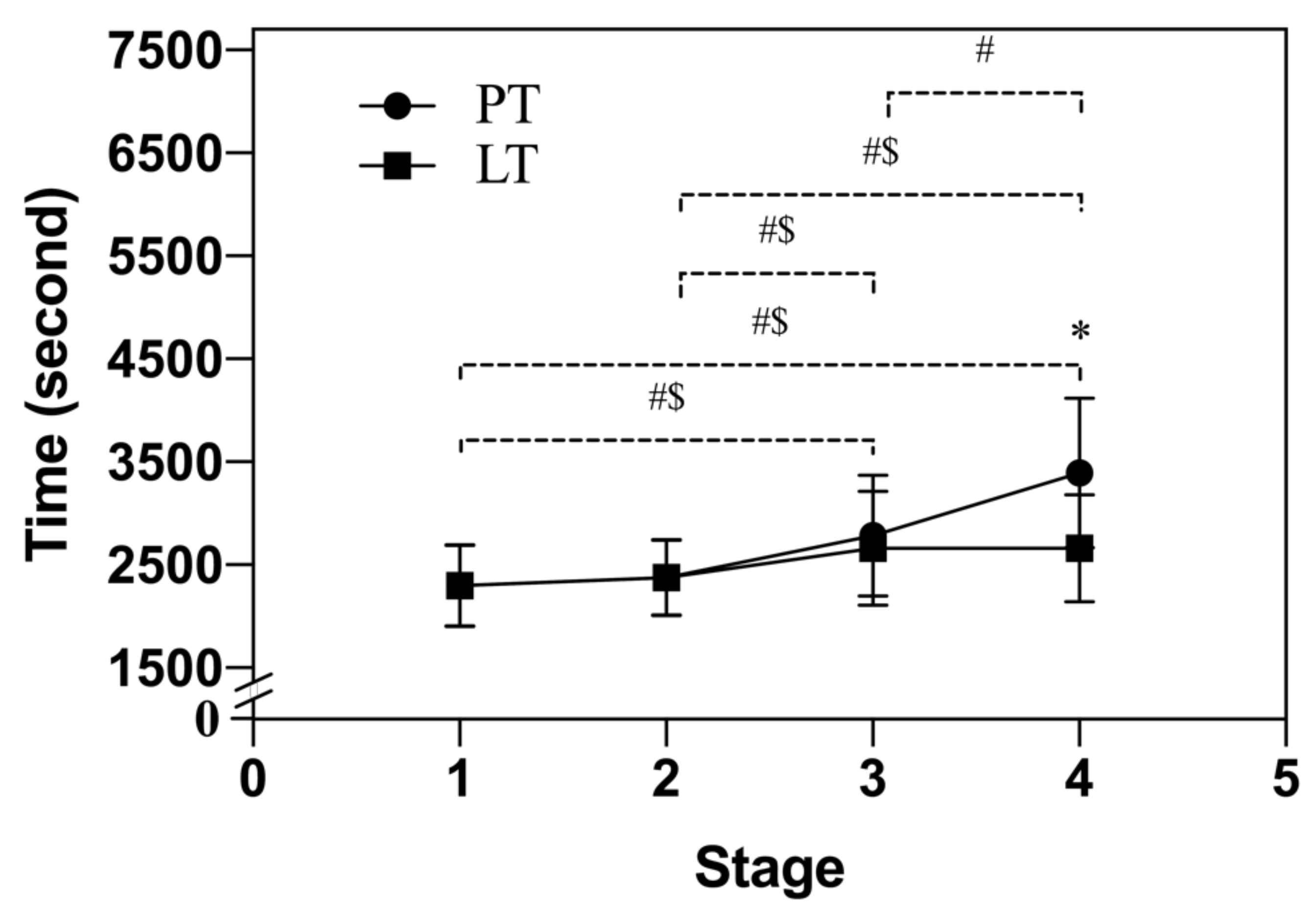
3.2. Half-Marathon
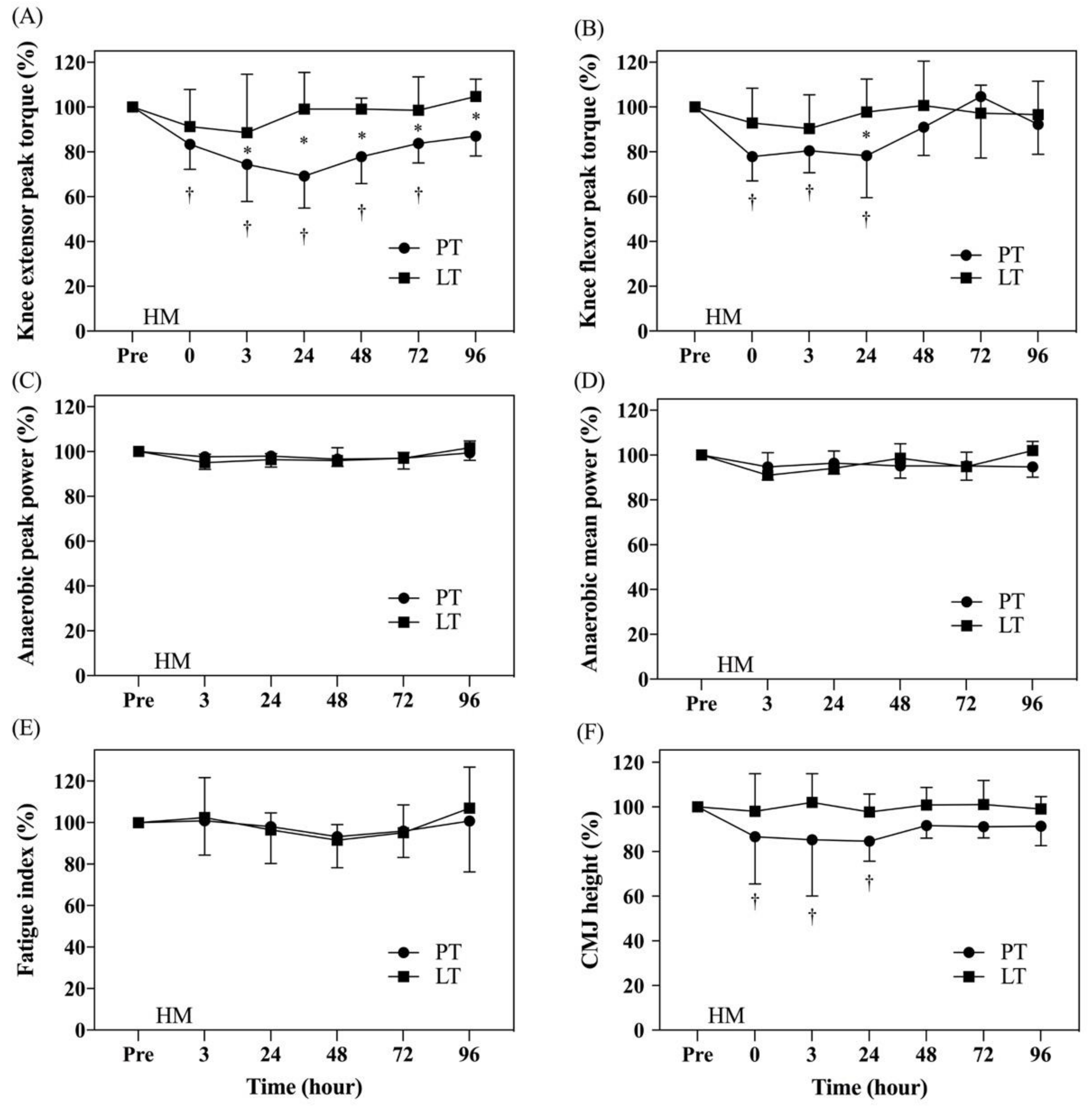
3.3. Analysis of Lower Extremity Muscle Strength
3.4. Analysis of Anaerobic Power
3.5. Analysis of Lower Extremity Muscle Explosive Force
3.6. Analysis of Aerobic Capacity and Body Composition
3.7. Analysis of Blood Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clifford, T.; Allerton, D.M.; Brown, M.A.; Harper, L.; Horsburgh, S.; Keane, K.M.; Stevenson, E.J.; Howatson, G. Minimal muscle damage after a marathon and no influence of beetroot juice on inflammation and recovery. Appl. Physiol. Nutr. Metab. 2017, 42, 263–270. [Google Scholar] [CrossRef]
- Takayama, F.; Aoyagi, A.; Shimazu, W.; Nabekura, Y. Effects of Marathon Running on Aerobic Fitness and Performance in Recreational Runners One Week after a Race. J Sports Med (Hindawi Publ Corp). 2017, 2017, 9402386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junker, D.; Stöggl, T. The Training Effects of Foam Rolling on Core Strength Endurance, Balance, Muscle Performance and Range of Motion: A Randomized Controlled Trial. Sports Sci. Med. 2019, 18, 229–238. [Google Scholar]
- Brentano, M.A.; Martins Kruel, L.F. A review on strength exercise-induced muscle damage: Applications, adaptation mechanisms and limitations. J. Sports Med. Phys. Fitness 2011, 51, 1–10. [Google Scholar] [PubMed]
- Rubio-Arias, J.Á.; Ávila-Gandía, V.; López-Román, F.J.; Soto-Méndez, F.; Alcaraz, P.E.; Ramos-Campo, D.J. Muscle damage and inflammation biomarkers after two ultra-endurance mountain races of different distances: 54 km vs 111 km. Physiol. Behav. 2019, 205, 51–57. [Google Scholar] [CrossRef]
- Lynn, A.; Garner, S.; Nelson, N.; Simper, T.N.; Hall, A.C.; Ranchordas, M.K. Effect of bilberry juice on indices of muscle damage and inflammation in runners completing a half-marathon: A randomised, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2018, 15, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hooijmans, M.T.; Monte, J.R.C.; Froeling, M.; van den Berg-Faay, S.; Aengevaeren, V.L.; Hemke, R.; Smithuis, F.F.; Eijsvogels, T.M.H.; Bakermans, A.J.; Maas, M.; et al. Quantitative MRI Reveals Microstructural Changes in the Upper Leg Muscles After Running a Marathon. J. Magn. Reson. Imaging 2020, 52, 407–417. [Google Scholar] [CrossRef]
- Wan, B.; Shan, G. Biomechanical modeling as a practical tool for predicting injury risk related to repetitive muscle lengthening during learning and training of human complex motor skills. SpringerPlus 2016, 5, 441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorski, T.; Cadore, E.L.; Pinto, S.S.; da Silva, E.M.; Correa, C.S.; Beltrami, F.G.; Kruel, L.F.M. Use of NSAIDs in triathletes: Prevalence, level of awareness and reasons for use. Br. J. Sports Med. 2011, 45, 85. [Google Scholar] [CrossRef] [PubMed]
- Howatson, G.; van Someren, K.A. The Prevention and Treatment of Exercise-Induced Muscle Damage. Sports Med. 2008, 38, 483–503. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef] [PubMed]
- Vaghef-Mehrabany, E.; Alipour, B.; Homayouni-Rad, A.; Sharif, S.-K.; Asghari-Jafarabadi, M.; Zavvari, S. Probiotic supplementation improves inflammatory status in patients with rheumatoid arthritis. Nutrition 2014, 30, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Phavichitr, N.; Puwdee, P.; Tantibhaedhyangkul, R. Cost-benefit analysis of the probiotic treatment of children hospitalized for acute diarrhea in Bangkok, Thailand. Southeast. Asian J. Trop. Med. Public Health 2013, 44, 1065–1071. [Google Scholar]
- Ciccarelli, S.; Stolfi, I.; Caramia, G. Management strategies in the treatment of neonatal and pediatric gastroenteritis. Infect. Drug Resist. 2013, 6, 133–161. [Google Scholar]
- Pyne, D.; West, N.; Cox, A.; Cripps, A. Probiotics supplementation for athletes–Clinical and physiological effects. Eur. J. Sport Sci. 2014, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Purpura, M.; Stone, J.; Turner, S.; Anzalone, A.; Eimerbrink, M.; Pane, M.; Amoruso, A.; Rowlands, D.; Oliver, J. Probiotic Streptococcus thermophilus FP4 and Bifidobacterium breve BR03 Supplementation Attenuates Performance and Range-of-Motion Decrements Following Muscle Damaging Exercise. Nutrients 2016, 8, 642. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Hsu, Y.-J.; Li, H.; Kan, N.-W.; Chen, Y.-M.; Lin, J.-S.; Hsu, T.-K.; Tsai, T.-Y.; Chiu, Y.-S.; Huang, C.-C. Effect of Lactobacillus Plantarum TWK10 on Improving Endurance Performance in Humans. Chin. J. Physiol. 2018, 61, 163–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-M.; Wei, L.; Chiu, Y.-S.; Hsu, Y.-J.; Tsai, T.-Y.; Wang, M.-F.; Huang, C.-C. Lactobacillus plantarum TWK10 Supplementation Improves Exercise Performance and Increases Muscle Mass in Mice. Nutrients 2016, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Komano, Y.; Shimada, K.; Naito, H.; Fukao, K.; Ishihara, Y.; Fujii, T.; Kokubo, T.; Daida, H. Efficacy of heat-killed Lactococcus lactis JCM 5805 on immunity and fatigue during consecutive high intensity exercise in male athletes: A randomized, placebo-controlled, double-blinded trial. J. Int. Soc. Sports Nutr. 2018, 15, 39. [Google Scholar] [CrossRef] [Green Version]
- Martarelli, D.; Verdenelli, M.C.; Scuri, S.; Cocchioni, M.; Silvi, S.; Cecchini, C.; Pompei, P. Effect of a Probiotic Intake on Oxidant and Antioxidant Parameters in Plasma of Athletes During Intense Exercise Training. Curr. Microbiol. 2011, 62, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Purpura, M.; Farmer, S.; Cash, H.A.; Keller, D. Probiotic Bacillus coagulans GBI-30, 6086 Improves Protein Absorption and Utilization. Probiotics Antimicrob. Proteins 2018, 10, 611–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkema, W.; Boekhorst, J.; Wels, M.; van Hijum, S.A.F.T. Microbial bioinformatics for food safety and production. Brief. Bioinform. 2016, 17, 283–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, M.-C.; Tsai, W.-H.; Jheng, Y.-P.; Su, S.-L.; Wang, S.-Y.; Lin, C.-C.; Chen, Y.-H.; Chang, W.-W. The beneficial effects of Lactobacillus reuteri ADR-1 or ADR-3 consumption on type 2 diabetes mellitus: A randomized, double-blinded, placebo-controlled trial. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Toral, M.; Romero, M.; Rodríguez-Nogales, A.; Jiménez, R.; Robles-Vera, I.; Algieri, F.; Chueca-Porcuna, N.; Sánchez, M.; de la Visitación, N.; Olivares, M.; et al. Lactobacillus fermentum Improves Tacrolimus-Induced Hypertension by Restoring Vascular Redox State and Improving eNOS Coupling. Mol. Nutr. Food Res. 2018, 62, 1800033. [Google Scholar] [CrossRef]
- Oh, N.S.; Joung, J.Y.; Lee, J.Y.; Kim, Y. Probiotic and anti-inflammatory potential of Lactobacillus rhamnosus 4B15 and Lactobacillus gasseri 4M13 isolated from infant feces. PLoS ONE 2018, 13, e0192021. [Google Scholar] [CrossRef] [PubMed]
- Son, S.H.; Yang, S.J.; Jeon, H.L.; Yu, H.S.; Lee, N.K.; Park, Y.S.; Paik, H.D. Antioxidant and immunostimulatory effect of potential probiotic Lactobacillus paraplantarum SC61 isolated from Korean traditional fermented food, jangajji. Microb. Pathog. 2018, 125, 486–492. [Google Scholar] [CrossRef]
- Chao, S.H.; Wu, R.J.; Watanabe, K.; Tsai, Y.C. Diversity of lactic acid bacteria in suan-tsai and fu-tsai, traditional fermented mustard products of Taiwan. Int. J. Food Microbiol. 2009, 135, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-W.; Liong, M.T.; Chung, Y.-C.E.; Huang, H.-Y.; Peng, W.-S.; Cheng, Y.-F.; Lin, Y.-S.; Wu, Y.-Y.; Tsai, Y.-C. Effects of Lactobacillus plantarum PS128 on Children with Autism Spectrum Disorder in Taiwan: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2019, 11, 820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.-H.; Yang, C.-H.; Lin, C.-T.; Li, S.-W.; Cheng, W.-S.; Jiang, Y.-P.; Wu, C.-C.; Chang, C.-H.; Tsai, Y.-C. Genome architecture of Lactobacillus plantarum PS128, a probiotic strain with potential immunomodulatory activity. Gut Pathog. 2015, 7, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.-H.; Chuang, H.-L.; Huang, Y.-T.; Wu, C.-C.; Chou, G.-T.; Wang, S.; Tsai, Y.-C. Alteration of behavior and monoamine levels attributable to Lactobacillus plantarum PS128 in germ-free mice. Behav. Brain Res. 2016, 298, 202–209. [Google Scholar] [CrossRef]
- Huang, W.-C.; Wei, C.-C.; Huang, C.-C.; Chen, W.-L.; Huang, H.-Y. The Beneficial Effects of Lactobacillus plantarum PS128 on High-Intensity, Exercise-Induced Oxidative Stress, Inflammation, and Performance in Triathletes. Nutrients 2019, 11, 353. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.-C.; Pan, C.-H.; Wei, C.-C.; Huang, H.-Y. Lactobacillus plantarum PS128 Improves Physiological Adaptation and Performance in Triathletes through Gut Microbiota Modulation. Nutrients 2020, 12, 2315. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Chen, H.L.; Lin, M.J.; Wu, C.J.; Nosaka, K. Muscle damage responses of the elbow flexors to four maximal eccentric exercise bouts performed every 4 weeks. Eur. J. Appl. Physiol. 2009, 106, 267–275. [Google Scholar] [CrossRef]
- Bar-Or, O. The Wingate anaerobic test. An update on methodology, reliability and validity. Sports Med. 1987, 4, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Moir, G.L. Three Different Methods of Calculating Vertical Jump Height from Force Platform Data in Men and Women. Meas. Phys. Educ. Exerc. Sci. 2008, 12, 207–218. [Google Scholar] [CrossRef]
- Nieman, D.C.; Austin, M.D.; Dew, D.; Utter, A.C. Validity of COSMED’s quark CPET mixing chamber system in evaluating energy metabolism during aerobic exercise in healthy male adults. Res. Sports Med. 2013, 21, 136–145. [Google Scholar] [CrossRef]
- Hall-Lopez, J.A.; Ochoa-Martinez, P.Y.; Moncada-Jimenez, J.; Ocampo Mendez, M.A.; Martinez Garcia, I.; Martinez Garcia, M.A. Reliability of the maximal oxygen uptake following two consecutive trials by indirect calorimetry. Nutr. Hosp. 2015, 31, 1726–1732. [Google Scholar] [PubMed]
- Mullaney, M.J.; Fukunaga, T. Current concepts and treatment of patellofemoral compressive issues. Int. J. Sports Phys. Ther. 2016, 11, 891–902. [Google Scholar]
- Dawson, L.G.; Dawson, K.A.; Tiidus, P.M. Evaluating the influence of massage on leg strength, swelling, and pain following a half-marathon. J. Sports Sci. Med. 2004, 3, 37–43. [Google Scholar] [PubMed]
- Suzuki, K.; Peake, J.; Nosaka, K.; Okutsu, M.; Abbiss, C.R.; Surriano, R.; Bishop, D.; Quod, M.J.; Lee, H.; Martin, D.T.; et al. Changes in markers of muscle damage, inflammation and HSP70 after an Ironman Triathlon race. Eur. J. Appl. Physiol. 2006, 98, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sánchez, A.; Ramos-Campo, D.J.; Fernández-Lobato, B.; Rubio-Arias, J.A.; Alacid, F.; Aguayo, E. Biochemical, physiological, and performance response of a functional watermelon juice enriched in L-citrulline during a half-marathon race. Food Nutr. Res. 2017, 61, 1330098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiewelhove, T.; Schneider, C.; Döweling, A.; Hanakam, F.; Rasche, C.; Meyer, T.; Kellmann, M.; Pfeiffer, M.; Ferrauti, A. Effects of different recovery strategies following a half-marathon on fatigue markers in recreational runners. PLoS ONE 2018, 13, e0207313. [Google Scholar] [CrossRef] [Green Version]
- Withee, E.D.; Tippens, K.M.; Dehen, R.; Tibbitts, D.; Hanes, D.; Zwickey, H. Effects of Methylsulfonylmethane (MSM) on exercise-induced oxidative stress, muscle damage, and pain following a half-marathon: A double-blind, randomized, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2017, 14, 24. [Google Scholar] [CrossRef] [Green Version]
- Clarkson, P.M.; Sayers, S.P. Etiology of exercise-induced muscle damage. J. Appl. Physiol. 1999, 24, 234–248. [Google Scholar] [CrossRef]
- Clarkson, P.M.; Hubal, M.J. Exercise-induced muscle damage in humans. Am. J. Phys. Med. Rehabil. 2002, 81, S52–S69. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.; Eston, R.G.; Edwards, R.H. Characteristics of isometric and dynamic strength loss following eccentric exercise-induced muscle damage. Scand J. Med. Sci. Sports 2001, 11, 134–140. [Google Scholar] [CrossRef]
- Boccia, G.; Dardanello, D.; Tarperi, C.; Rosso, V.; Festa, L.; La Torre, A.; Pellegrini, B.; Schena, F.; Rainoldi, A. Decrease of muscle fiber conduction velocity correlates with strength loss after an endurance run. Physiol. Meas. 2017, 38, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Boccia, G.; Dardanello, D.; Tarperi, C.; Festa, L.; La Torre, A.; Pellegrini, B.; Schena, F.; Rainoldi, A. Women show similar central and peripheral fatigue to men after half-marathon. J. Sport Sci. 2018, 18, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Harty, P.S.; Cottet, M.L.; Malloy, J.K.; Kerksick, C.M. Nutritional and Supplementation Strategies to Prevent and Attenuate Exercise-Induced Muscle Damage: A Brief Review. Sports Med. Open 2019, 5, 1. [Google Scholar] [CrossRef]
- McCullough, P.A.; Chinnaiyan, K.M.; Gallagher, M.J.; Colar, J.M.; Geddes, T.; Gold, J.M.; Trivax, J.E. Changes in renal markers and acute kidney injury after marathon running. Nephrology 2011, 16, 194–199. [Google Scholar] [CrossRef]

| Exercise Capacity and Blood Samples | Baseline PT (n = 8) | Baseline LT (n = 8) | p-Value |
|---|---|---|---|
| Lower extremity muscle strength | |||
| knee extensor peak torque (N-m/kg) | 3.21 ± 0.88 | 3.08 ± 0.84 | 0.38 |
| knee flexor peak torque (N-m/kg) | 1.23 ± 0.35 | 1.15 ± 0.31 | 0.33 |
| Anaerobic capacity | |||
| anaerobic peak power (w/kg) | 10.06 ± 1.66 | 10 ± 1.37 | 0.47 |
| anaerobic mean power (w/kg) | 7.05 ± 1.23 | 7.16 ± 1.47 | 0.44 |
| fatigue index | 14.95 ± 5.49 | 14.24 ± 3.32 | 0.38 |
| Explosive force of the lower extremities | |||
| CMJ height (cm) | 32 ± 7.86 | 27.38 ± 11.49 | 0.18 |
| Muscle fatigue | |||
| BCAA (nmol/ul) | 0.33 ± 0.07 | 0.34 ± 0.04 | 0.29 |
| NH3 (μmol/L) | 35.63 ± 20.56 | 28.25 ± 6.48 | 0.18 |
| Muscle damage | |||
| Myoglobin (ng/mL) | 23.79 ± 9.65 | 29.72 ± 30.73 | 0.31 |
| LDH (IU/L) | 132.25 ± 8.92 | 132 ± 21.47 | 0.49 |
| CPK (IU/L) | 168.25 ± 125.41 | 188.13 ± 233.09 | 0.42 |
| Renal injury | |||
| BUN (mg/dL) | 10.22 ± 3.14 | 10.79 ± 3.25 | 0.36 |
| Anti-oxidative capacity | |||
| SOD (U/mL) | 0.11 ± 0.09 | 0.16 ± 0.06 | 0.10 |
| CAT (nmol/min/mL) | 64.88 ± 28.24 | 67.8 ± 24.9 | 0.41 |
| Pre-PT (n = 8) | Pre-LT (n = 8) | p-Value | |
|---|---|---|---|
| Lower extremity muscle strength | |||
| knee extensor peak torque (N-m/kg) | 3.1 ± 0.82 | 3.07 ± 0.84 | 0.47 |
| knee flexor peak torque (N-m/kg) | 1.21 ± 0.36 | 1.14 ± 0.29 | 0.32 |
| Anaerobic capacity | |||
| anaerobic peak power (w/kg) | 10.04 ± 1.6 | 10.07 ± 1.5 | 0.48 |
| anaerobic mean power (w/kg) | 7.06 ± 1.3 | 7.04 ± 1.54 | 0.49 |
| fatigue index | 14.88 ± 5.38 | 14.61 ± 3.91 | 0.46 |
| Explosive force of the lower extremities | |||
| CMJ height (cm) | 31.86 ± 8.04 | 31.94 ± 8.07 | 0.49 |
| Muscle fatigue | |||
| BCAA (nmol/ul) | 0.33 ± 0.07 | 0.33 ± 0.04 | 0.49 |
| NH3 (μmol/L) | 35.25 ± 20.81 | 27.63 ± 6.55 | 0.18 |
| Muscle damage | |||
| Myoglobin (ng/mL) | 23.98 ± 10.04 | 29.76 ± 30.73 | 0.31 |
| LDH (IU/L) | 131.13 ± 10.53 | 131.25 ± 21.59 | 0.49 |
| CPK (IU/L) | 167.5 ± 125.62 | 187.38 ± 233.51 | 0.42 |
| Renal injury | |||
| BUN (mg/dL) | 10.24 ± 3.18 | 10.86 ± 3.32 | 0.35 |
| Anti-oxidative capacity | |||
| SOD (U/mL) | 0.13 ± 0.09 | 0.15 ± 0.05 | 0.29 |
| CAT (nmol/min/mL) | 62.48 ± 42.97 | 57.81 ± 33.9 | 0.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, S.-K.; Tseng, W.-C.; Tseng, K.-W.; Lai, C.-C.; Tsai, Y.-C.; Tai, H.-L.; Hsu, C.-C. Effect of Daily Oral Lactobacillus plantarum PS128 on Exercise Capacity Recovery after a Half-Marathon. Nutrients 2021, 13, 4023. https://doi.org/10.3390/nu13114023
Fu S-K, Tseng W-C, Tseng K-W, Lai C-C, Tsai Y-C, Tai H-L, Hsu C-C. Effect of Daily Oral Lactobacillus plantarum PS128 on Exercise Capacity Recovery after a Half-Marathon. Nutrients. 2021; 13(11):4023. https://doi.org/10.3390/nu13114023
Chicago/Turabian StyleFu, Szu-Kai, Wei-Chin Tseng, Kuo-Wei Tseng, Chang-Chi Lai, Ying-Chieh Tsai, Hsia-Ling Tai, and Chia-Chen Hsu. 2021. "Effect of Daily Oral Lactobacillus plantarum PS128 on Exercise Capacity Recovery after a Half-Marathon" Nutrients 13, no. 11: 4023. https://doi.org/10.3390/nu13114023
APA StyleFu, S.-K., Tseng, W.-C., Tseng, K.-W., Lai, C.-C., Tsai, Y.-C., Tai, H.-L., & Hsu, C.-C. (2021). Effect of Daily Oral Lactobacillus plantarum PS128 on Exercise Capacity Recovery after a Half-Marathon. Nutrients, 13(11), 4023. https://doi.org/10.3390/nu13114023






