Acute Paraxanthine Ingestion Improves Cognition and Short-Term Memory and Helps Sustain Attention in a Double-Blind, Placebo-Controlled, Crossover Trial
Abstract
:1. Introduction
2. Methods
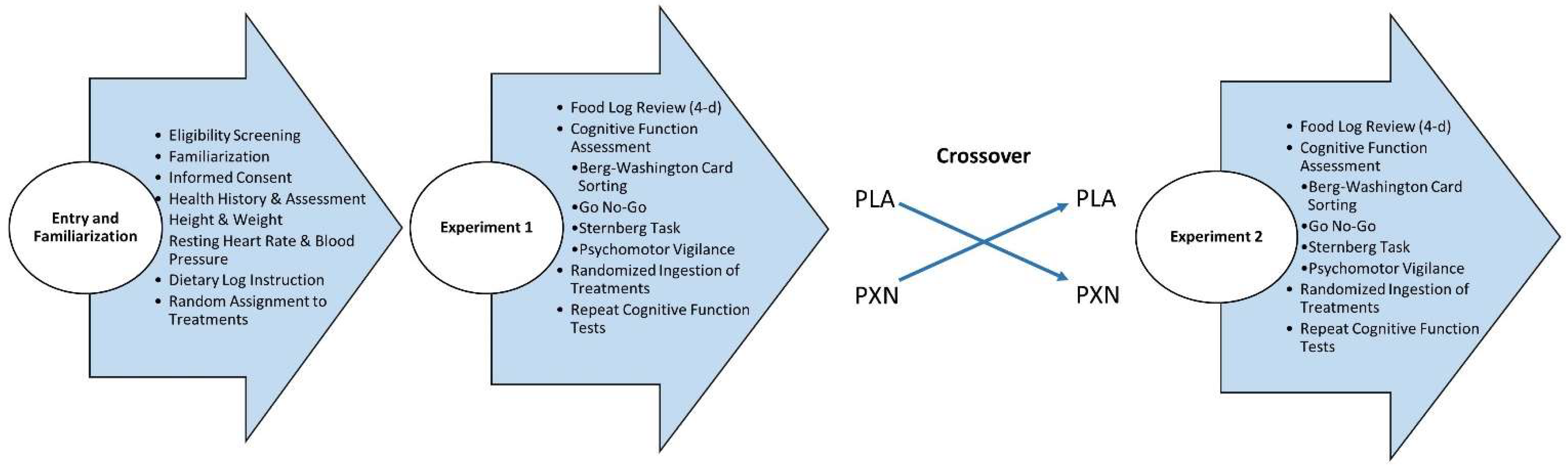
2.1. Experimental Design
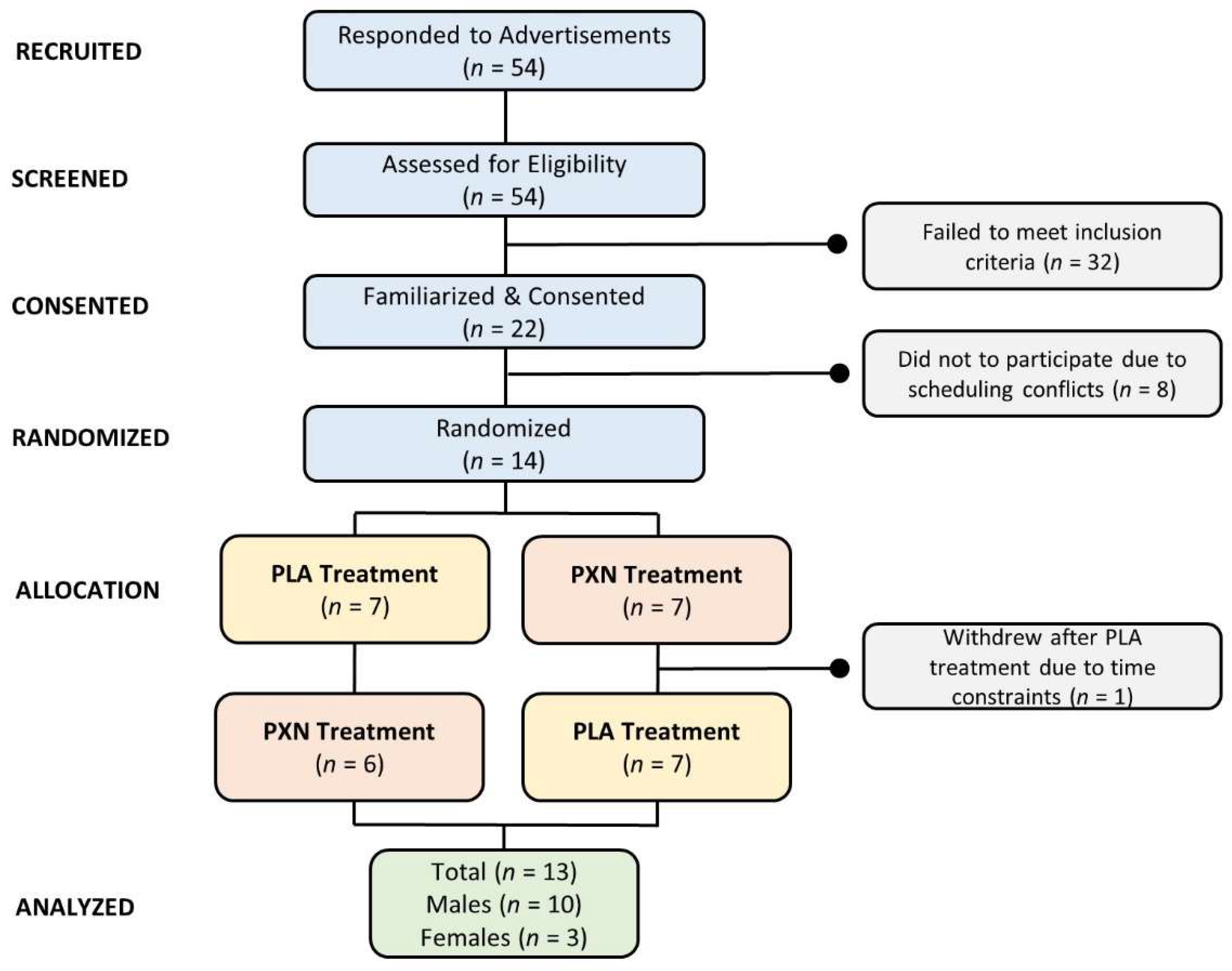
2.2. Participants
2.3. Testing Protocol
2.4. Supplementation Protocol
3. Procedures
3.1. Demographics
3.2. Dietary Assessment
3.3. PEBL Cognitive and Executive Function Assessment
3.4. Adverse Event Monitoring
3.5. Statistical Analysis
4. Results
4.1. Demographic Data
4.2. PEBL Cognitive Function Assessment
4.2.1. Berg Wisconsin Card Sorting Test
4.2.2. Go/No-Go Task Test
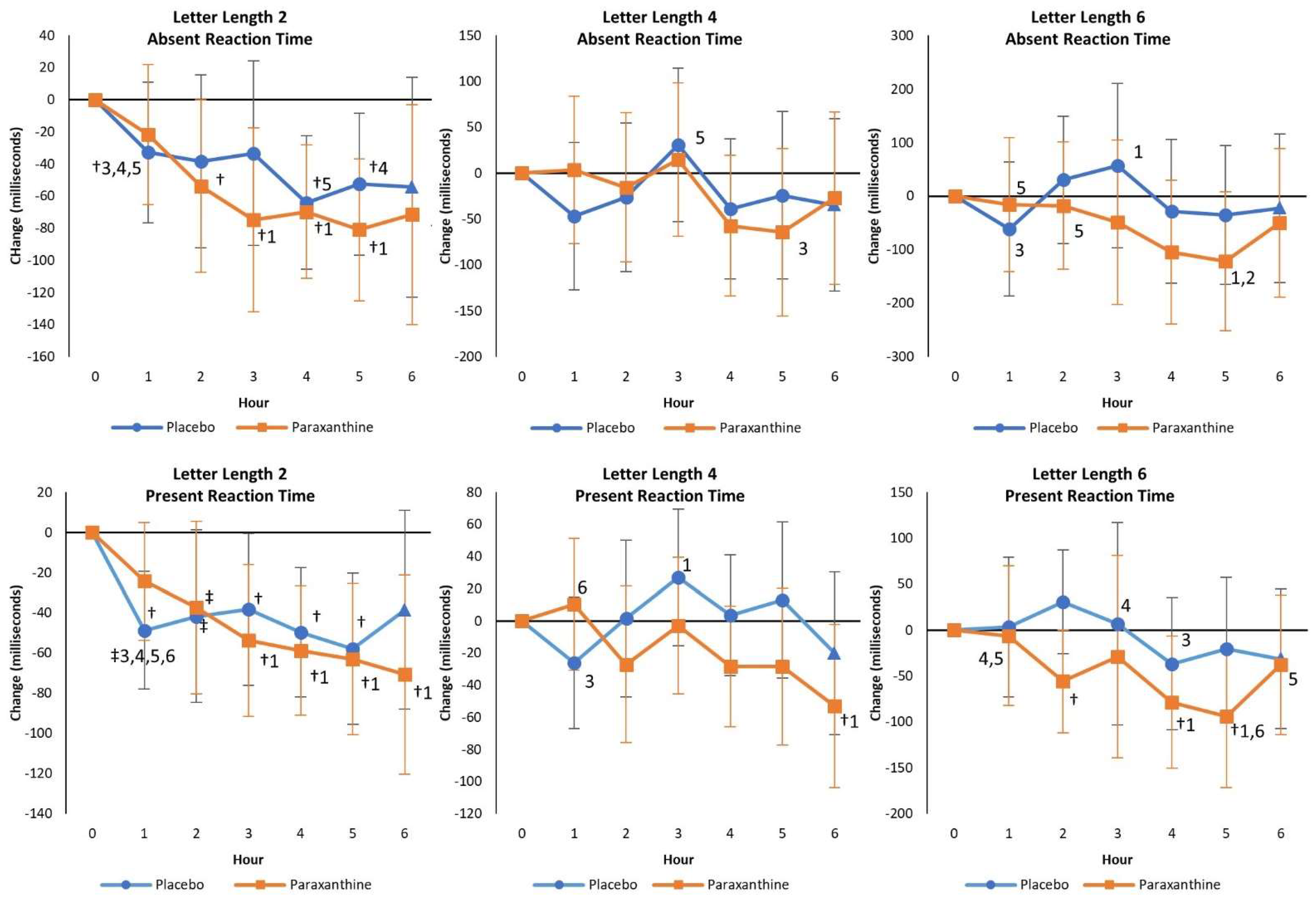
4.2.3. Sternberg Task Test
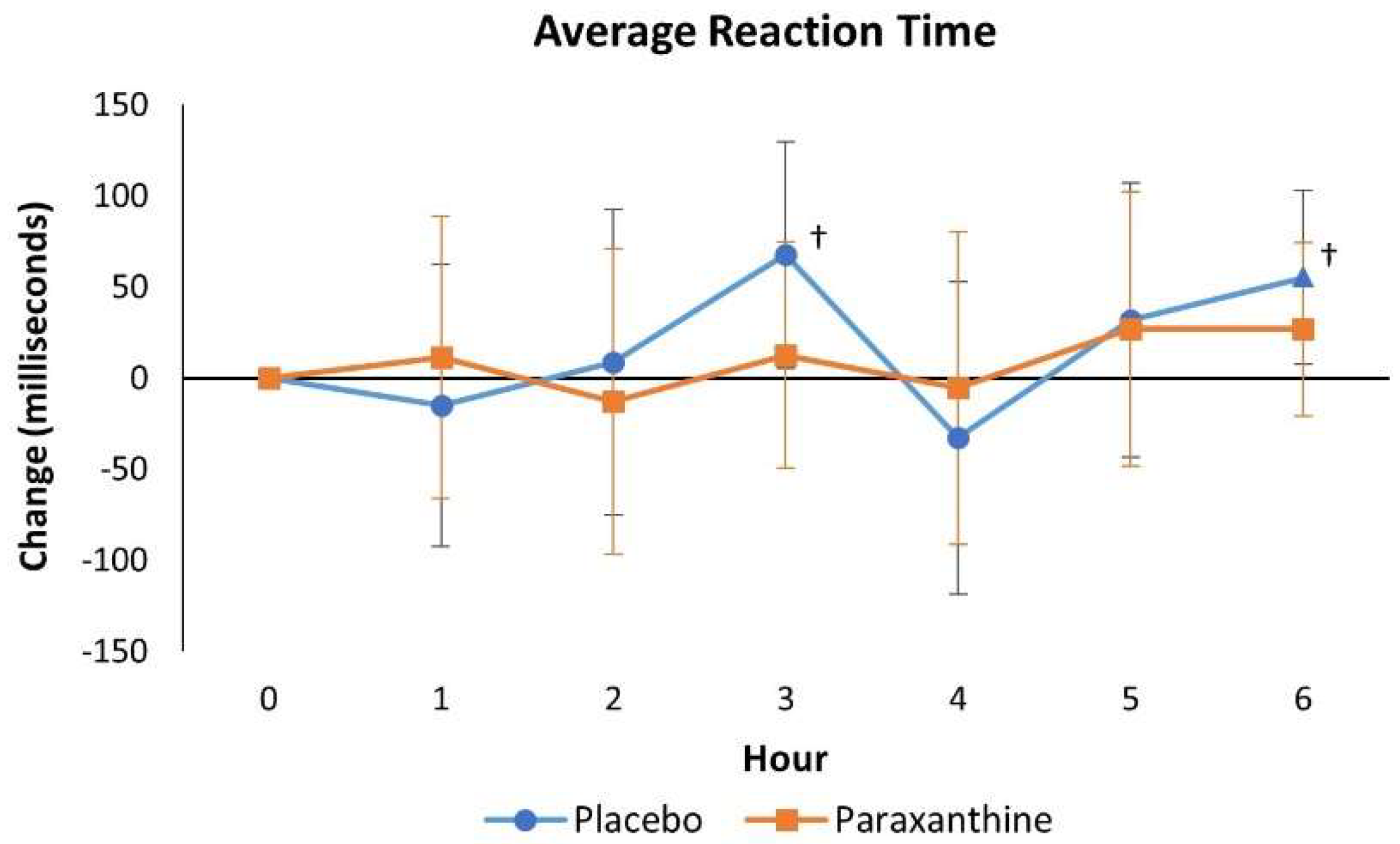
4.2.4. Psychomotor Vigilance Task Test
4.3. Safety Assessment
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stavric, B. Methylxanthines: Toxicity to humans. 3. Theobromine, paraxanthine and the combined effects of methylxanthines. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 1988, 26, 725–733. [Google Scholar] [CrossRef]
- Zheng, X.Q.; Koyama, Y.; Nagai, C.; Ashihara, H. Biosynthesis, accumulation and degradation of theobromine in developing Theobroma cacao fruits. J. Plant. Physiol. 2004, 161, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.H.; Waller, G.R. Possible allelopathic constituents of Coffea arabica. J. Chem. Ecol. 1980, 6, 643–653. [Google Scholar] [CrossRef]
- Jiang, M.; Kameda, K.; Han, L.-K.; Kimura, Y.; Okuda, H. Isolation of Lipolytic Substances Caffeine and 1,7-Dimethylxanthine from the Stem and Rhizome of Sinomenium actum. Planta Med. 1998, 64, 375–377. [Google Scholar] [CrossRef]
- Kretschmar, J.A.; Baumann, T.W. Caffeine in Citrus flowers. Phytochemistry 1999, 52, 19–23. [Google Scholar] [CrossRef]
- Lelo, A.; Birkett, D.J.; Robson, R.A.; Miners, J.O. Comparative pharmacokinetics of caffeine and its primary demethylated metabolites paraxanthine, theobromine and theophylline in man. Br. J. Clin. Pharm. 1986, 22, 177–182. [Google Scholar] [CrossRef]
- Purpura, M.; Jäger, R.; Falk, M. An assessment of mutagenicity, genotoxicity, acute-, subacute and subchronic oral toxicity of paraxanthine (1,7-dimethylxanthine). Food Chem. Toxicol. 2021, 158, 112579. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Jacob, P.; Mayan, H.; Denaro, C. Sympathomimetic effects of paraxanthine and caffeine in humans. Clin. Pharmacol. Ther. 1995, 58, 684–691. [Google Scholar] [CrossRef]
- Undem, B.J. Pharmacotherapy of asthma In Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 11th ed.; Brunton, L.L., Lazo, J.S., Parker, K.L., Eds.; McGraw-Hill Professional: New York, NY, USA, 2005. [Google Scholar]
- Chou, C.C.; Vickroy, T.W. Antagonism of adenosine receptors by caffeine and caffeine metabolites in equine forebrain tissues. Am. J. Vet. Res. 2003, 64, 216–224. [Google Scholar] [CrossRef]
- Orrú, M.; Guitart, X.; Karcz-Kubicha, M.; Solinas, M.; Justinova, Z.; Barodia, S.K.; Zanoveli, J.; Cortes, A.; Lluis, C.; Casado, V.; et al. Psychostimulant pharmacological profile of paraxanthine, the main metabolite of caffeine in humans. Neuropharmacology 2013, 67, 476–484. [Google Scholar] [CrossRef] [Green Version]
- Guerreiro, S.; Toulorge, D.; Hirsch, E.; Marien, M.; Sokoloff, P.; Michel, P.P. Paraxanthine, the primary metabolite of caffeine, provides protection against dopaminergic cell death via stimulation of ryanodine receptor channels. Mol. Pharm. 2008, 74, 980–989. [Google Scholar] [CrossRef] [Green Version]
- Okuro, M.; Fujiki, N.; Kotorii, N.; Ishimaru, Y.; Sokoloff, P.; Nishino, S. Effects of paraxanthine and caffeine on sleep, locomotor activity, and body temperature in orexin/ataxin-3 transgenic narcoleptic mice. Sleep 2010, 33, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Guest, N.S.; VanDusseldorp, T.A.; Nelson, M.T.; Grgic, J.; Schoenfeld, B.J.; Jenkins, N.D.M.; Arent, S.M.; Antonio, J.; Stout, J.R.; Trexler, E.T.; et al. International society of sports nutrition position stand: Caffeine and exercise performance. J. Int. Soc. Sports Nutr. 2021, 18, 1. [Google Scholar] [CrossRef]
- Collins, P.B.; Earnest, C.P.; Dalton, R.L.; Sowinski, R.J.; Grubic, T.J.; Favot, C.J.; Coletta, A.M.; Rasmussen, C.; Greenwood, M.; Kreider, R.B. Short-Term Effects of a Ready-to-Drink Pre-Workout Beverage on Exercise Performance and Recovery. Nutrients 2017, 9, 823. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.P.; Earnest, C.P.; Koozehchian, M.; Cho, M.; Barringer, N.; Walker, D.; Rasmussen, C.; Greenwood, M.; Murano, P.S.; Kreider, R.B. Effects of ingesting a pre-workout dietary supplement with and without synephrine for 8 weeks on training adaptations in resistance-trained males. J. Int. Soc. Sports Nutr. 2017, 14, 1. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.P.; Earnest, C.P.; Koozehchian, M.; Galvan, E.; Dalton, R.; Walker, D.; Rasmussen, C.; Murano, P.S.; Greenwood, M.; Kreider, R.B. Effects of acute ingestion of a pre-workout dietary supplement with and without p-synephrine on resting energy expenditure, cognitive function and exercise performance. J. Int. Soc. Sports Nutr. 2017, 14, 3. [Google Scholar] [CrossRef] [Green Version]
- Kreider, R.B. Current perspectives of caffeinated energy drinks on exercise performance and safety assessment. Nutr. Diet. Supp. 2018, 10, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Parker, A.G.; Gordon, J.; Thornton, A.; Byars, A.; Lubker, J.; Bartlett, M.; Byrd, M.; Oliver, J.; Simbo, S.; Rasmussen, C.; et al. The effects of IQPLUS Focus on cognitive function, mood and endocrine response before and following acute exercise. J. Int. Soc. Sports Nutr. 2011, 8, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carswell, A.T.; Howland, K.; Martinez-Gonzalez, B.; Baron, P.; Davison, G. The effect of caffeine on cognitive performance is influenced by CYP1A2 but not ADORA2A genotype, yet neither genotype affects exercise performance in healthy adults. Eur. J. Appl. Physiol. 2020, 120, 1495–1508. [Google Scholar] [CrossRef]
- Kalman, D.; Harvey, P.D.; Perez Ojalvo, S.; Komorowski, J. Randomized Prospective Double-Blind Studies to Evaluate the Cognitive Effects of Inositol-Stabilized Arginine Silicate in Healthy Physically Active Adults. Nutrients 2016, 8, 736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, C.J.; Rothschild, J.; Earnest, C.P.; Blaisdell, A. The Effects of Energy Drink Consumption on Cognitive and Physical Performance in Elite League of Legends Players. Sports 2019, 7, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.; Stein, H. A spreadsheet program for making a balanced Latin Square design. Rev. Colomb. Cienc. Pecu. 2009, 22, 591–596. [Google Scholar]
- Ferguson, B. ACSM’s Guidelines for Exercise Testing and Prescription 9th Ed. 2014. J. Can. Chiropr. Assoc. 2014, 58, 328. [Google Scholar]
- Bazzano, L.A.; He, J.; Ogden, L.G.; Loria, C.M.; Vupputuri, S.; Myers, L.; Whelton, P.K. Agreement on nutrient intake between the databases of the First National Health and Nutrition Examination Survey and the ESHA Food Processor. Am. J. Epidemiol. 2002, 156, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Mueller, S.T.; Piper, B.J. The Psychology Experiment Building Language (PEBL) and PEBL Test Battery. J. Neurosci Methods 2014, 222, 250–259. [Google Scholar] [CrossRef] [Green Version]
- Mueller, S.T. The Psychology Experiment Building Language. 2019. Available online: http://pebl.sourceforge.net/ (accessed on 4 April 2019).
- Berg, E.A. A simple objective technique for measuring flexibility in thinking. J. Gen. Psychol. 1948, 39, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, F.; Logan, G.D. Automatic and controlled response inhibition: Associative learning in the go/no-go and stop-signal paradigms. J. Exp. Psychol. Gen. 2008, 137, 649–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezdjian, S.; Baker, L.A.; Lozano, D.I.; Raine, A. Assessing inattention and impulsivity in children during the Go/NoGo task. Br. J. Dev. Psychol. 2009, 27, 365–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sternberg, S. High-Speed Scanning in Human Memory. Science 1966, 153, 652–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leark, R.A.; Greenberg, L.K.; Kindschi, C.L.; Dupuy, T.R.; Hughes, S.J. Test of Variables of Attention: Professional Manual; The TOVA Company: Langley, WA, USA, 2020. [Google Scholar]
- Greenberg, L.K.; Kindschi, C.L.; Dupuy, T.R.; Holder, C. Test of Variables of Attention: Clinical Manual; The TOVA Company: Langley, WA, USA, 2020. [Google Scholar]
- Piper, B.J.; Mueller, S.T.; Geerken, A.R.; Dixon, K.L.; Kroliczak, G.; Olsen, R.H.J.; Miller, J.K. Reliability and validity of neurobehavioral function on the Psychology Experimental Building Language test battery in young adults. PeerJ 2015, 3, e1460. [Google Scholar] [CrossRef] [Green Version]
- Page, P. Beyond statistical significance: Clinical interpretation of rehabilitation research literature. Int. J. Sports Phys. Ther. 2014, 9, 726–736. [Google Scholar] [PubMed]
- Earnest, C.P.; Roberts, B.M.; Harnish, C.R.; Kutz, J.L.; Cholewa, J.M.; Johannsen, N.M. Reporting Characteristics in Sports Nutrition. Sports 2018, 6, 139. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. Statistical Power Analysis for the Social Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Heckman, M.A.; Weil, J.; Gonzalez de Mejia, E. Caffeine (1, 3, 7-trimethylxanthine) in foods: A comprehensive review on consumption, functionality, safety, and regulatory matters. J. Food Sci. 2010, 75, R77–R87. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, P.; Jordan, S.; Eastwood, J.; Rotstein, J.; Hugenholtz, A.; Feeley, M. Effects of caffeine on human health. Food Addit. Contam. 2003, 20, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Kavouras, S.A. Caffeine use in sports, pharmacokinetics in man, and cellular mechanisms of action. Crit Rev. Food Sci. Nutr. 2005, 45, 535–562. [Google Scholar] [CrossRef]
- Chester, N.; Wojek, N. Caffeine consumption amongst British athletes following changes to the 2004 WADA prohibited list. Int J. Sports Med. 2008, 29, 524–528. [Google Scholar] [CrossRef]
- Brachtel, D.; Richter, E. Absolute bioavailability of caffeine from a tablet formulation. J. Hepatol. 1992, 16, 385. [Google Scholar] [CrossRef]
- Kaplan, G.B.; Greenblatt, D.J.; Ehrenberg, B.L.; Goddard, J.E.; Cotreau, M.M.; Harmatz, J.S.; Shader, R.I. Dose-dependent pharmacokinetics and psychomotor effects of caffeine in humans. J. Clin. Pharm. 1997, 37, 693–703. [Google Scholar] [CrossRef]
- Graham, T.E. Caffeine and exercise: Metabolism, endurance and performance. Sports Med. 2001, 31, 785–807. [Google Scholar] [CrossRef]
- Bell, D.G.; Jacobs, I.; McLellan, T.M.; Zamecnik, J. Reducing the dose of combined caffeine and ephedrine preserves the ergogenic effect. Aviat. Space Env. Med. 2000, 71, 415–419. [Google Scholar]
- Trexler, E.T.; Smith-Ryan, A.E. Creatine and Caffeine: Considerations for Concurrent Supplementation. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Temple, J.L.; Bernard, C.; Lipshultz, S.E.; Czachor, J.D.; Westphal, J.A.; Mestre, M.A. The Safety of Ingested Caffeine: A Comprehensive Review. Front. Psychiatry 2017, 8, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, A.; Palmer, A.A.; de Wit, H. Genetics of caffeine consumption and responses to caffeine. Psychopharmacol. 2010, 211, 245–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nehlig, A. Interindividual Differences in Caffeine Metabolism and Factors Driving Caffeine Consumption. Pharm. Rev. 2018, 70, 384–411. [Google Scholar] [CrossRef] [Green Version]
- Arnaud, M.J. Pharmacokinetics and metabolism of natural methylxanthines in animal and man. Handb. Exp. Pharm. 2011, 200, 33–91. [Google Scholar] [CrossRef]
- Labedzki, A.; Buters, J.; Jabrane, W.; Fuhr, U. Differences in caffeine and paraxanthine metabolism between human and murine CYP1A2. Biochem. Pharm. 2002, 63, 2159–2167. [Google Scholar] [CrossRef]
- Munoz, A.; Lopez-Samanes, A.; Aguilar-Navarro, M.; Varillas-Delgado, D.; Rivilla-Garcia, J.; Moreno-Perez, V.; Del Coso, J. Effects of CYP1A2 and ADORA2A Genotypes on the Ergogenic Response to Caffeine in Professional Handball Players. Genes 2020, 11, 933. [Google Scholar] [CrossRef]
- Abernethy, D.R.; Todd, E.L. Impairment of caffeine clearance by chronic use of low-dose oestrogen-containing oral contraceptives. Eur. J. Clin. Pharm. 1985, 28, 425–428. [Google Scholar] [CrossRef]
- Tanida, M.; Sakatani, K.; Tsujii, T. Relation between working memory performance and evoked cerebral blood oxygenation changes in the prefrontal cortex evaluated by quantitative time-resolved near-infrared spectroscopy. Neurol. Res. 2012, 34, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Sakatani, K.; Tanida, M.; Hirao, N.; Takemura, N. Ginkobiloba extract improves working memory performance in middle-aged women: Role of asymmetry of prefrontal cortex activity during a working memory task. Adv. Exp. Med. Biol. 2014, 812, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, R.; Marceglia, S.; Vergari, M.; Cogiamanian, F.; Mrakic-Sposta, S.; Mameli, F.; Zago, S.; Barbieri, S.; Priori, A. Cerebellar transcranial direct current stimulation impairs the practice-dependent proficiency increase in working memory. J. Cogn. Neurosci. 2008, 20, 1687–1697. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, C.; Xing, D.; Gonzalez, D.; Jenkins, V.; Nottingham, K.; Dickerson, B.; Leonard, M.; Ko, J.; Faries, M.; Kephart, W.; et al. Acute Paraxanthine Ingestion Improves Cognition and Short-Term Memory and Helps Sustain Attention in a Double-Blind, Placebo-Controlled, Crossover Trial. Nutrients 2021, 13, 3980. https://doi.org/10.3390/nu13113980
Yoo C, Xing D, Gonzalez D, Jenkins V, Nottingham K, Dickerson B, Leonard M, Ko J, Faries M, Kephart W, et al. Acute Paraxanthine Ingestion Improves Cognition and Short-Term Memory and Helps Sustain Attention in a Double-Blind, Placebo-Controlled, Crossover Trial. Nutrients. 2021; 13(11):3980. https://doi.org/10.3390/nu13113980
Chicago/Turabian StyleYoo, Choongsung, Dante Xing, Drew Gonzalez, Victoria Jenkins, Kay Nottingham, Broderick Dickerson, Megan Leonard, Joungbo Ko, Mark Faries, Wesley Kephart, and et al. 2021. "Acute Paraxanthine Ingestion Improves Cognition and Short-Term Memory and Helps Sustain Attention in a Double-Blind, Placebo-Controlled, Crossover Trial" Nutrients 13, no. 11: 3980. https://doi.org/10.3390/nu13113980
APA StyleYoo, C., Xing, D., Gonzalez, D., Jenkins, V., Nottingham, K., Dickerson, B., Leonard, M., Ko, J., Faries, M., Kephart, W., Purpura, M., Jäger, R., Wells, S. D., Sowinski, R., Rasmussen, C. J., & Kreider, R. B. (2021). Acute Paraxanthine Ingestion Improves Cognition and Short-Term Memory and Helps Sustain Attention in a Double-Blind, Placebo-Controlled, Crossover Trial. Nutrients, 13(11), 3980. https://doi.org/10.3390/nu13113980







