Dose-Response of Paraxanthine on Cognitive Function: A Double Blind, Placebo Controlled, Crossover Trial
Abstract
:1. Introduction
2. Methods
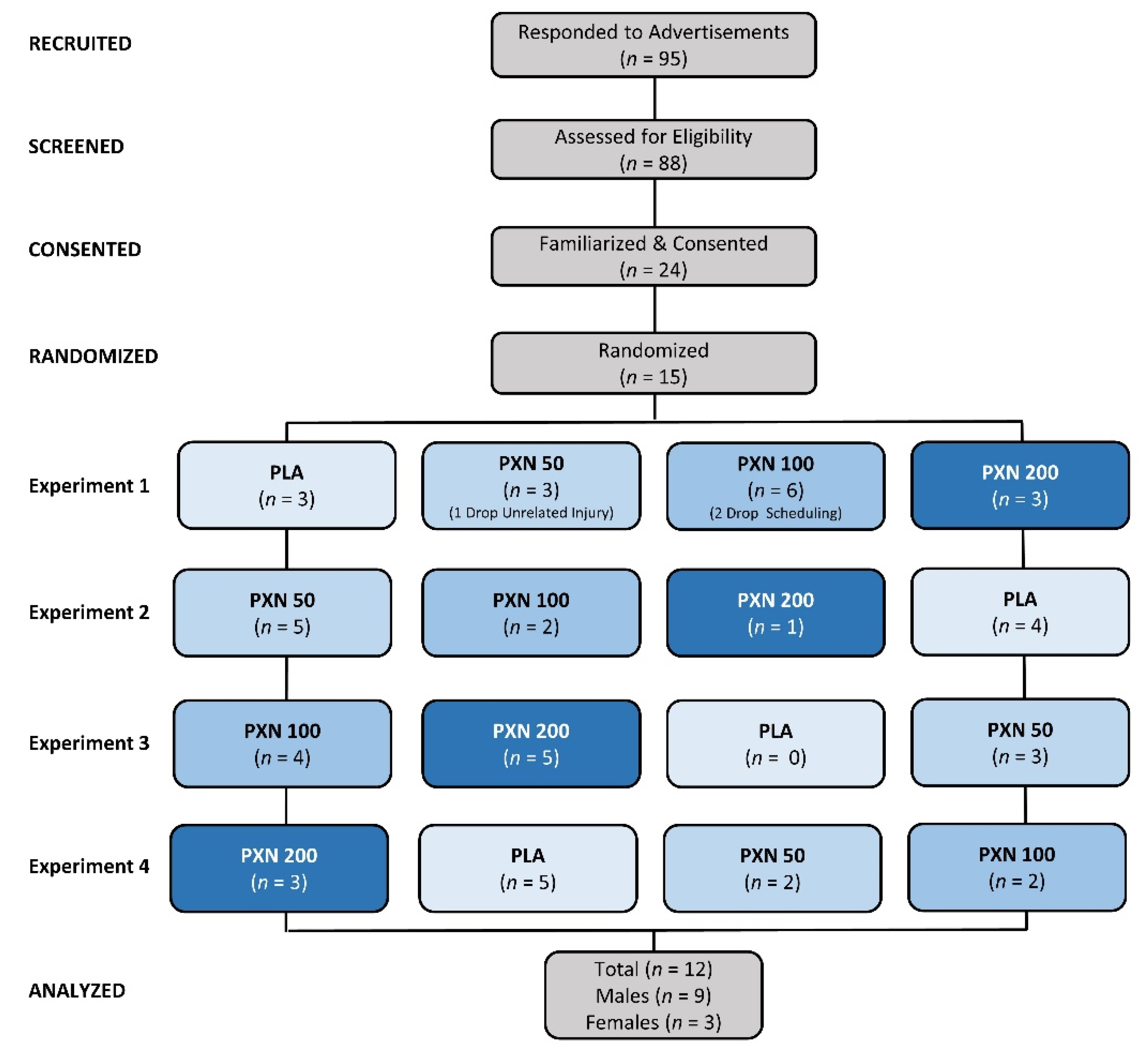
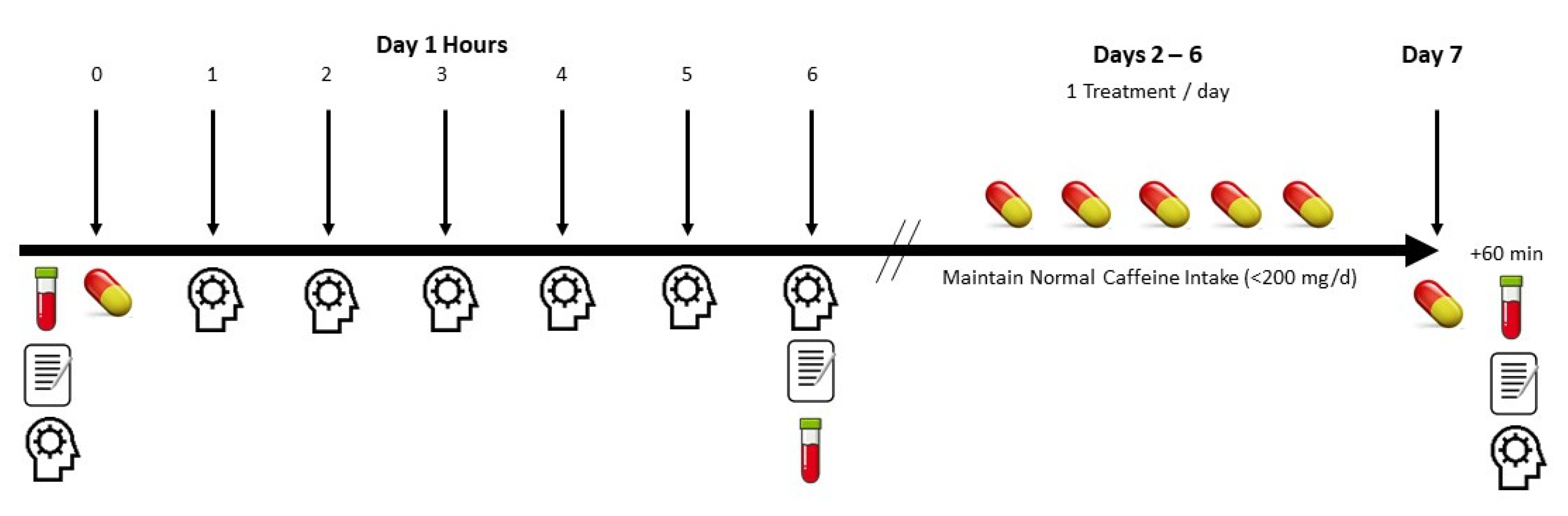
2.1. Experimental Design
2.2. Participants
2.3. Testing Protocol
2.4. Supplementation Protocol
3. Procedures
3.1. Demographics
3.2. Diet Control
3.3. PEBL Cognitive and Executive Function Assessment
3.4. Blood Colletion and Analysis
3.5. Side Effect Questionnaire
3.6. Statistical Analysis
4. Results
4.1. Demographic Data
4.2. PEBL Cognitive Function Assessment
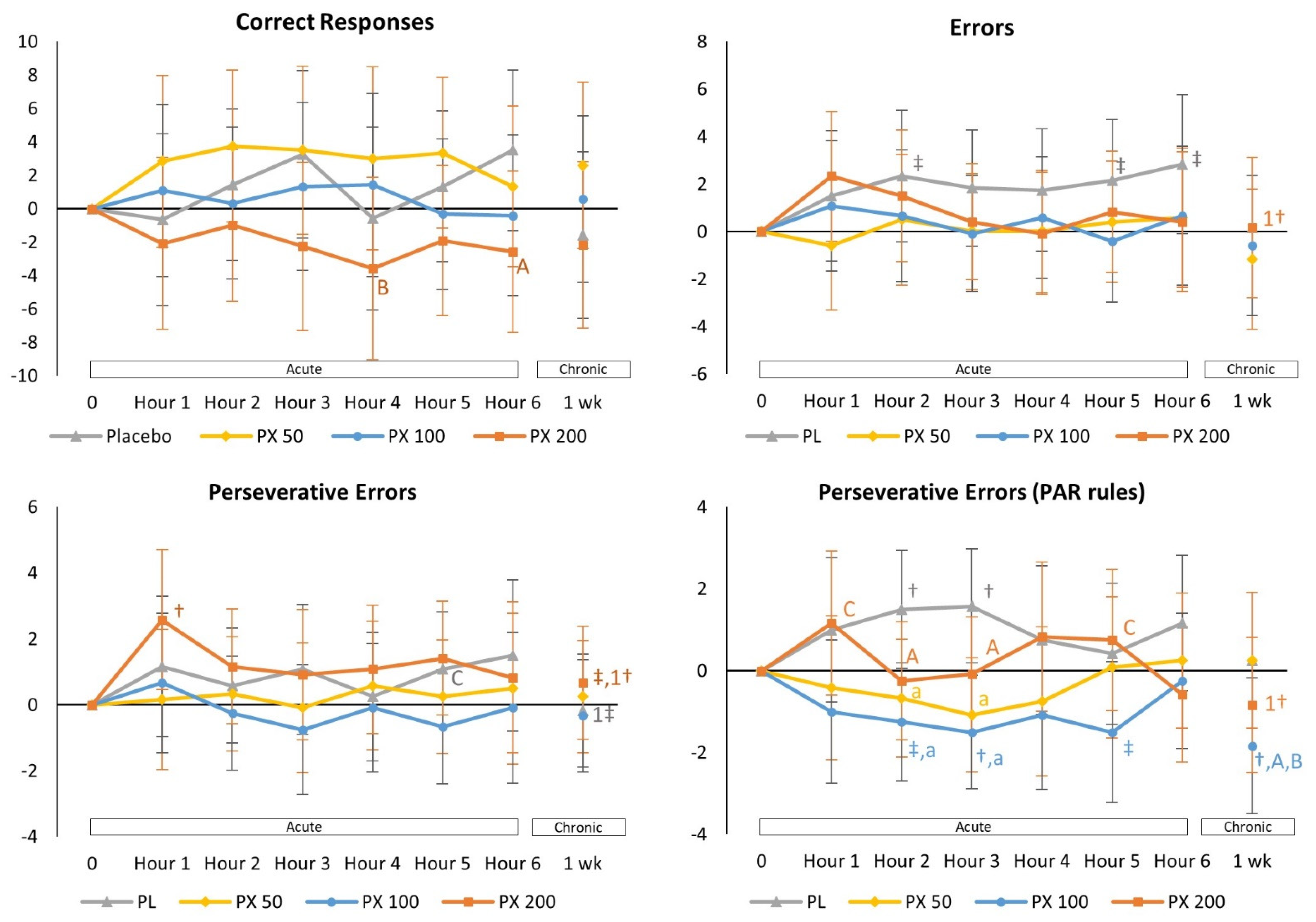
4.2.1. Berg-Wisconsin Card Sorting Task Test
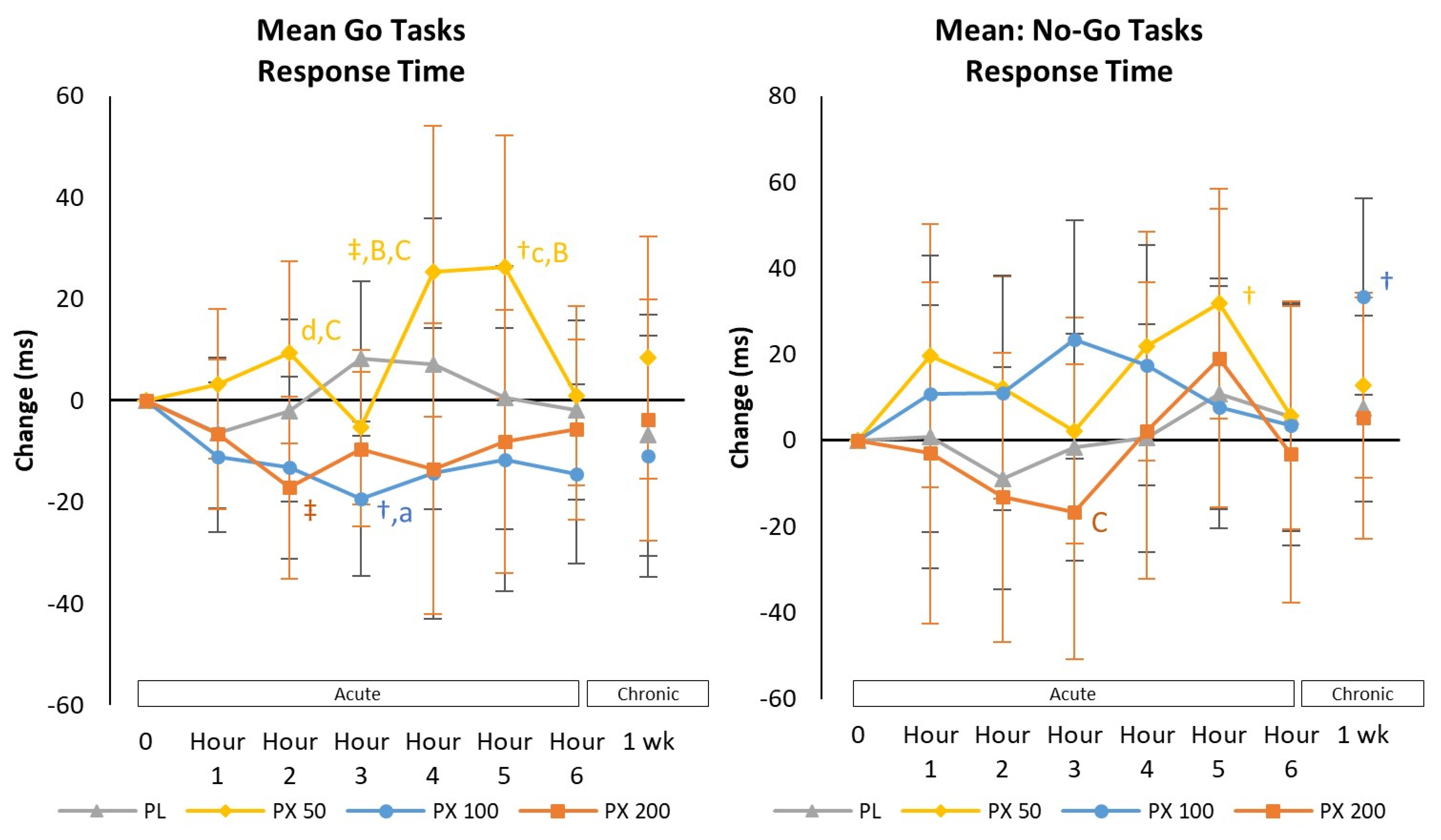
4.2.2. Go/No-Go Task Test
4.2.3. Sternberg Task Test
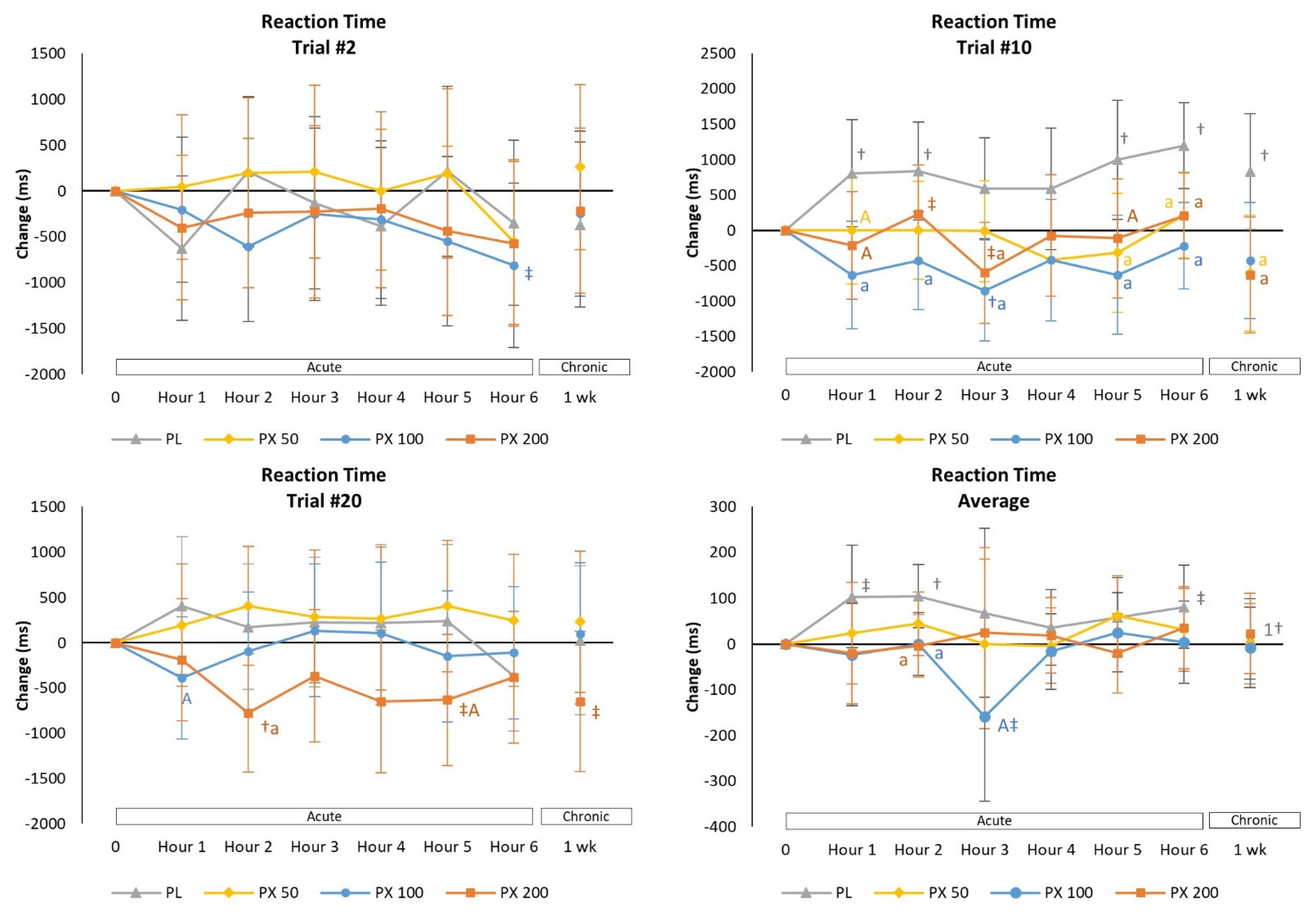
4.2.4. Psychomotor Vigilance Task Test
4.3. Safety Assessment
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nawrot, P.; Jordan, S.; Eastwood, J.; Rotstein, J.; Hugenholtz, A.; Feeley, M. Effects of caffeine on human health. Food Addit. Contam. 2003, 20, 1–30. [Google Scholar] [CrossRef]
- Magkos, F.; Kavouras, S.A. Caffeine use in sports, pharmacokinetics in man, and cellular mechanisms of action. Crit. Rev. Food Sci. Nutr. 2005, 45, 535–562. [Google Scholar] [CrossRef]
- Chester, N.; Wojek, N. Caffeine consumption amongst British athletes following changes to the 2004 WADA prohibited list. Int. J. Sports Med. 2008, 29, 524–528. [Google Scholar] [CrossRef]
- Lelo, A.; Birkett, D.J.; Robson, R.A.; Miners, J.O. Comparative pharmacokinetics of caffeine and its primary demethylated metabolites paraxanthine, theobromine and theophylline in man. Br. J. Clin. Pharmacol. 1986, 22, 177–182. [Google Scholar] [CrossRef]
- Brachtel, D.; Richter, E. Absolute bioavailability of caffeine from a tablet formulation. J. Hepatol. 1992, 16, 385. [Google Scholar] [CrossRef]
- Kaplan, G.B.; Greenblatt, D.J.; Ehrenberg, B.L.; Goddard, J.E.; Cotreau, M.M.; Harmatz, J.S.; Shader, R.I. Dose-dependent pharmacokinetics and psychomotor effects of caffeine in humans. J. Clin. Pharmacol. 1997, 37, 693–703. [Google Scholar] [CrossRef]
- Graham, T.E. Caffeine and exercise: Metabolism, endurance and performance. Sports Med. 2001, 31, 785–807. [Google Scholar] [CrossRef]
- Guest, N.S.; VanDusseldorp, T.A.; Nelson, M.T.; Grgic, J.; Schoenfeld, B.J.; Jenkins, N.D.M.; Arent, S.M.; Antonio, J.; Stout, J.R.; Trexler, E.T.; et al. International society of sports nutrition position stand: Caffeine and exercise performance. J. Int. Soc. Sports Nutr. 2021, 18, 1. [Google Scholar] [CrossRef]
- Grgic, J.; Pickering, C.; Del Coso, J.; Schoenfeld, B.J.; Mikulic, P. CYP1A2 genotype and acute ergogenic effects of caffeine intake on exercise performance: A systematic review. Eur. J. Nutr. 2021, 60, 1181–1195. [Google Scholar] [CrossRef]
- Palatini, P.; Benetti, E.; Mos, L.; Garavelli, G.; Mazzer, A.; Cozzio, S.; Fania, C.; Casiglia, E. Association of coffee consumption and CYP1A2 polymorphism with risk of impaired fasting glucose in hypertensive patients. Eur. J. Epidemiol. 2015, 30, 209–217. [Google Scholar] [CrossRef]
- Soares, R.N.; Schneider, A.; Valle, S.C.; Schenkel, P.C. The influence of CYP1A2 genotype in the blood pressure response to caffeine ingestion is affected by physical activity status and caffeine consumption level. Vasc. Pharmacol. 2018, 106, 67–73. [Google Scholar] [CrossRef]
- Cornelis, M.C.; El-Sohemy, A.; Kabagambe, E.K.; Campos, H. Coffee, CYP1A2 Genotype, and Risk of Myocardial Infarction. JAMA 2006, 295, 1135–1141. [Google Scholar] [CrossRef] [Green Version]
- Guest, N.; Corey, P.; Vescovi, J.; El-Sohemy, A. Caffeine, CYP1A2 Genotype, and Endurance Performance in Athletes. Med. Sci. Sports Exerc. 2018, 50, 1570–1578. [Google Scholar] [CrossRef]
- Womack, C.J.; Saunders, M.J.; Bechtel, M.K.; Bolton, D.J.; Martin, M.; Luden, N.D.; Dunham, W.; Hancock, M. The influence of a CYP1A2 polymorphism on the ergogenic effects of caffeine. J. Int. Soc. Sports Nutr. 2012, 9, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schubert, M.M.; Irwin, C.; Seay, R.F.; Clarke, H.E.; Allegro, D.; Desbrow, B. Caffeine, coffee, and appetite control: A review. Int. J. Food Sci. Nutr. 2017, 68, 901–912. [Google Scholar] [CrossRef] [Green Version]
- Van Schaik, L.; Kettle, C.; Green, R.; Irving, H.R.; Rathner, J.A. Effects of Caffeine on Brown Adipose Tissue Thermogenesis and Metabolic Homeostasis: A Review. Front. Neurosci. 2021, 15, 621356. [Google Scholar] [CrossRef]
- Stavric, B. Methylxanthines: Toxicity to humans. 3. Theobromine, paraxanthine and the combined effects of methylxanthines. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 1988, 26, 725–733. [Google Scholar] [CrossRef]
- Purpura, M.; Jäger, R.; Falk, M. An assessment of mutagenicity, genotoxicity, acute-, subacute and subchronic oral toxicity of paraxanthine (1,7-dimethylxanthine). Food Chem. Toxicol. 2021, 158, 112579. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L.; Jacob, P.; Mayan, H.; Denaro, C. Sympathomimetic effects of paraxanthine and caffeine in humans. Clin. Pharmacol. Ther. 1995, 58, 684–691. [Google Scholar] [CrossRef]
- Undem, B.J. Pharmacotherapy of asthma. In Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 11th ed.; Brunton, L.L., Lazo, J.S., Parker, K.L., Eds.; McGraw-Hill Professional: New York, NY, USA, 2005. [Google Scholar]
- Chou, C.C.; Vickroy, T.W. Antagonism of adenosine receptors by caffeine and caffeine metabolites in equine forebrain tissues. Am. J. Vet. Res. 2003, 64, 216–224. [Google Scholar] [CrossRef]
- Guerreiro, S.; Toulorge, D.; Hirsch, E.; Marien, M.; Sokoloff, P.; Michel, P.P. Paraxanthine, the primary metabolite of caffeine, provides protection against dopaminergic cell death via stimulation of ryanodine receptor channels. Mol. Pharmacol. 2008, 74, 980–989. [Google Scholar] [CrossRef] [Green Version]
- Okuro, M.; Fujiki, N.; Kotorii, N.; Ishimaru, Y.; Sokoloff, P.; Nishino, S. Effects of paraxanthine and caffeine on sleep, locomotor activity, and body temperature in orexin/ataxin-3 transgenic narcoleptic mice. Sleep 2010, 33, 930–942. [Google Scholar] [CrossRef]
- Orrú, M.; Guitart, X.; Karcz-Kubicha, M.; Solinas, M.; Justinova, Z.; Barodia, S.K.; Zanoveli, J.; Cortes, A.; Lluis, C.; Casado, V.; et al. Psychostimulant pharmacological profile of paraxanthine, the main metabolite of caffeine in humans. Neuropharmacology 2013, 67, 476–484. [Google Scholar] [CrossRef] [Green Version]
- Yoo, C.; Xing, D.; Gonzalez, D.; Jenkins, V.; Nottingham, K.; Dickerson, B.; Leonard, M.; Ko, J.; Faries, M.; Kephart, W.; et al. Acute Paraxanthine Ingestion Improves Cognition and Short-Term Memory and Helps Sustain Attention in a Double-Blind, Placebo-Controlled, Crossover Trial. Nutrients 2021, 13, 3980. [Google Scholar] [CrossRef]
- Ferguson, B. ACSM’s Guidelines for Exercise Testing and Prescription 9th ed. 2014. J. Can Chiropr. Assoc. 2014, 58, 328. [Google Scholar]
- Bazzano, L.A.; He, J.; Ogden, L.G.; Loria, C.M.; Vupputuri, S.; Myers, L.; Whelton, P.K. Agreement on nutrient intake between the databases of the First National Health and Nutrition Examination Survey and the ESHA Food Processor. Am. J. Epidemiol. 2002, 156, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Piper, B.J.; Mueller, S.T.; Geerken, A.R.; Dixon, K.L.; Kroliczak, G.; Olsen, R.H.; Miller, J.K. Reliability and validity of neurobehavioral function on the Psychology Experimental Building Language test battery in young adults. PeerJ 2015, 3, e1460. [Google Scholar] [CrossRef] [Green Version]
- Mueller, S.T.; Piper, B.J. The Psychology Experiment Building Language (PEBL) and PEBL Test Battery. J. Neurosci. Methods 2014, 222, 250–259. [Google Scholar] [CrossRef] [Green Version]
- Mueller, S.T. The Psychology Experiment Building Language. Available online: http://pebl.sourceforge.net (accessed on 19 June 2019).
- Berg, E.A. A simple objective technique for measuring flexibility in thinking. J. Gen. Psychol. 1948, 39, 15–22. [Google Scholar] [CrossRef]
- Verbruggen, F.; Logan, G.D. Automatic and controlled response inhibition: Associative learning in the go/no-go and stop-signal paradigms. J. Exp. Psychol. Gen. 2008, 137, 649–672. [Google Scholar] [CrossRef] [Green Version]
- Bezdjian, S.; Baker, L.A.; Lozano, D.I.; Raine, A. Assessing inattention and impulsivity in children during the Go/NoGo task. Br. J. Dev. Psychol. 2009, 27, 365–383. [Google Scholar] [CrossRef] [Green Version]
- Sternberg, S. High-Speed Scanning in Human Memory. Science 1966, 153, 652–654. [Google Scholar] [CrossRef] [Green Version]
- Leark, R.A.; Greenberg, L.K.; Kindschi, C.L.; Dupuy, T.R.; Hughes, S.J. Test of Variables of Attention: Professional Manual; The TOVA Company: Langley, WA, USA, 2020; Available online: https://www.amazon.com/Variables-Attention-Professional-Clinical-Volumes/dp/B01HWNNGOO (accessed on 8 December 2021).
- Greenberg, L.K.; Kindschi, C.L.; Dupuy, T.R.; Holder, C. Test of Variables of Attention: Clinical Manual; The TOVA Company: Langley, WA, USA, 2020; Available online: https://files.tovatest.com/documentation/9/Clinical%20Manual.pdf (accessed on 8 December 2021).
- Bowling, J.L.; Katayev, A. An evaluation of the Roche Cobas c 111. Lab. Med. 2010, 41, 398–402. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [CrossRef] [Green Version]
- Bush, V.J.; Janu, M.R.; Bathur, F.; Wells, A.; Dasgupta, A. Comparison of BD Vacutainer SST Plus Tubes with BD SST II Plus Tubes for common analytes. Clin. Chim. Acta 2001, 306, 139–143. [Google Scholar] [CrossRef]
- Grubic, T.J.; Sowinski, R.J.; Nevares, B.E.; Jenkins, V.M.; Williamson, S.L.; Reyes, A.G.; Rasmussen, C.; Greenwood, M.; Murano, P.S.; Earnest, C.P.; et al. Comparison of ingesting a food bar containing whey protein and isomalto-oligosaccharides to carbohydrate on performance and recovery from an acute bout of resistance-exercise and sprint conditioning: An open label, randomized, counterbalanced, crossover pilot study. J. Int. Soc. Sports Nutr. 2019, 16, 34. [Google Scholar] [CrossRef] [Green Version]
- Sowinski, R.; Gonzalez, D.; Xing, D.; Yoo, C.; Jenkins, V.; Nottingham, K.; Dickerson, B.; Humphries, M.; Leonard, M.; Ko, J.; et al. Effects of Inositol-Enhanced Bonded Arginine Silicate Ingestion on Cognitive and Executive Function in Gamers. Nutrients 2021, 13, 3758. [Google Scholar] [CrossRef]
- Jung, Y.P.; Earnest, C.P.; Koozehchian, M.; Galvan, E.; Dalton, R.; Walker, D.; Rasmussen, C.; Murano, P.S.; Greenwood, M.; Kreider, R.B. Effects of acute ingestion of a pre-workout dietary supplement with and without p-synephrine on resting energy expenditure, cognitive function and exercise performance. J. Int. Soc. Sports Nutr. 2017, 14, 3. [Google Scholar] [CrossRef] [Green Version]
- Collins, P.B.; Earnest, C.P.; Dalton, R.L.; Sowinski, R.J.; Grubic, T.J.; Favot, C.J.; Coletta, A.M.; Rasmussen, C.; Greenwood, M.; Kreider, R.B. Short-Term Effects of a Ready-to-Drink Pre-Workout Beverage on Exercise Performance and Recovery. Nutrients 2017, 9, 823. [Google Scholar] [CrossRef] [Green Version]
- Dalton, R.L.; Sowinski, R.J.; Grubic, T.J.; Collins, P.B.; Coletta, A.M.; Reyes, A.G.; Sanchez, B.; Koozehchian, M.; Jung, Y.P.; Rasmussen, C.; et al. Hematological and Hemodynamic Responses to Acute and Short-Term Creatine Nitrate Supplementation. Nutrients 2017, 9, 1359. [Google Scholar] [CrossRef] [Green Version]
- Galvan, E.; Walker, D.K.; Simbo, S.Y.; Dalton, R.; Levers, K.; O’Connor, A.; Goodenough, C.; Barringer, N.D.; Greenwood, M.; Rasmussen, C.; et al. Acute and chronic safety and efficacy of dose dependent creatine nitrate supplementation and exercise performance. J. Int. Soc. Sports Nutr. 2016, 13, 12. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.; Stein, H. A spreadsheet program for making a balanced Latin Square design. Rev. Colomb. D Cienc. Pecu. 2009, 22, 591–596. [Google Scholar]
- Page, P. Beyond statistical significance: Clinical interpretation of rehabilitation research literature. Int. J. Sports Phys. Ther. 2014, 9, 726–736. [Google Scholar] [PubMed]
- Cohen, J. Statistical Power Analysis for the Social Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Landry, O.; Mitchell, P. An examination of perseverative errors and cognitive flexibility in autism. PLoS ONE 2021, 16, e0223160. [Google Scholar] [CrossRef] [PubMed]
- Tanida, M.; Sakatani, K.; Tsujii, T. Relation between working memory performance and evoked cerebral blood oxygenation changes in the prefrontal cortex evaluated by quantitative time-resolved near-infrared spectroscopy. Neurol. Res. 2012, 34, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Sakatani, K.; Tanida, M.; Hirao, N.; Takemura, N. Ginkobiloba extract improves working memory performance in middle-aged women: Role of asymmetry of prefrontal cortex activity during a working memory task. Adv. Exp. Med. Biol. 2014, 812, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, R.; Marceglia, S.; Vergari, M.; Cogiamanian, F.; Mrakic-Sposta, S.; Mameli, F.; Zago, S.; Barbieri, S.; Priori, A. Cerebellar transcranial direct current stimulation impairs the practice-dependent proficiency increase in working memory. J. Cogn. Neurosci. 2008, 20, 1687–1697. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.G.; Jacobs, I.; McLellan, T.M.; Zamecnik, J. Reducing the dose of combined caffeine and ephedrine preserves the ergogenic effect. Aviat. Space Environ. Med. 2000, 71, 415–419. [Google Scholar]
- Trexler, E.T.; Smith-Ryan, A.E. Creatine and Caffeine: Considerations for Concurrent Supplementation. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, R.; Cordery, P.; Funnell, M.; Mears, S.; James, L.; Watson, P. Chronic ingestion of a low dose of caffeine induces tolerance to the performance benefits of caffeine. J. Sports Sci. 2017, 35, 1920–1927. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, D.; Yoo, C.; Gonzalez, D.; Jenkins, V.; Nottingham, K.; Dickerson, B.; Leonard, M.; Ko, J.; Faries, M.; Kephart, W.; et al. Dose-Response of Paraxanthine on Cognitive Function: A Double Blind, Placebo Controlled, Crossover Trial. Nutrients 2021, 13, 4478. https://doi.org/10.3390/nu13124478
Xing D, Yoo C, Gonzalez D, Jenkins V, Nottingham K, Dickerson B, Leonard M, Ko J, Faries M, Kephart W, et al. Dose-Response of Paraxanthine on Cognitive Function: A Double Blind, Placebo Controlled, Crossover Trial. Nutrients. 2021; 13(12):4478. https://doi.org/10.3390/nu13124478
Chicago/Turabian StyleXing, Dante, Choongsung Yoo, Drew Gonzalez, Victoria Jenkins, Kay Nottingham, Broderick Dickerson, Megan Leonard, Joungbo Ko, Mark Faries, Wesley Kephart, and et al. 2021. "Dose-Response of Paraxanthine on Cognitive Function: A Double Blind, Placebo Controlled, Crossover Trial" Nutrients 13, no. 12: 4478. https://doi.org/10.3390/nu13124478
APA StyleXing, D., Yoo, C., Gonzalez, D., Jenkins, V., Nottingham, K., Dickerson, B., Leonard, M., Ko, J., Faries, M., Kephart, W., Purpura, M., Jäger, R., Wells, S. D., Sowinski, R., Rasmussen, C. J., & Kreider, R. B. (2021). Dose-Response of Paraxanthine on Cognitive Function: A Double Blind, Placebo Controlled, Crossover Trial. Nutrients, 13(12), 4478. https://doi.org/10.3390/nu13124478