Length of Nutritional Transition Associates Negatively with Postnatal Growth in Very Low Birthweight Infants
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Study Design and Population
2.2. Clinical Characteristics
2.3. Nutrition Data
2.4. Growth Data
2.5. Nutrition Practices
2.6. Statistical Analysis
3. Results
3.1. Clinical Characteristics
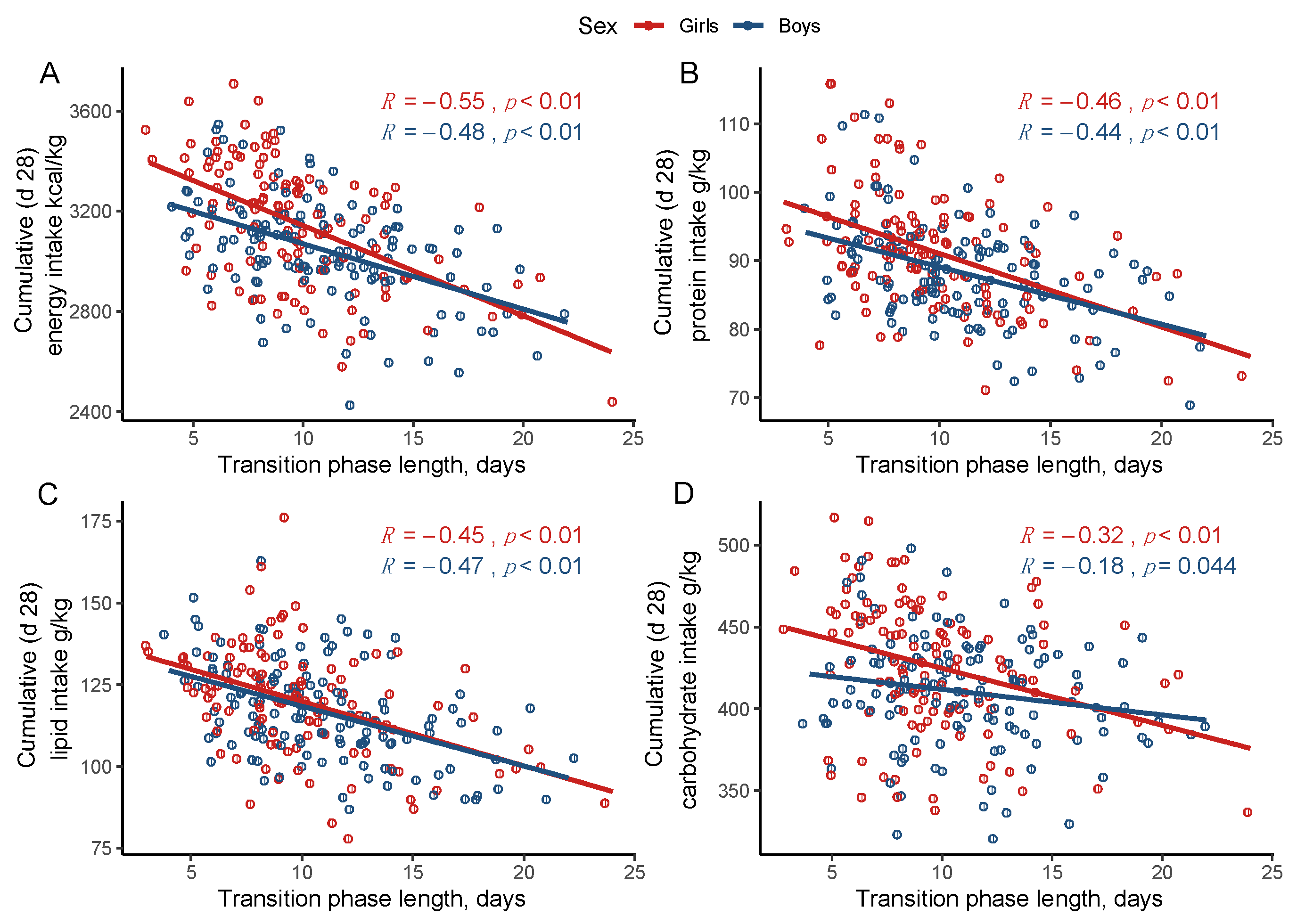
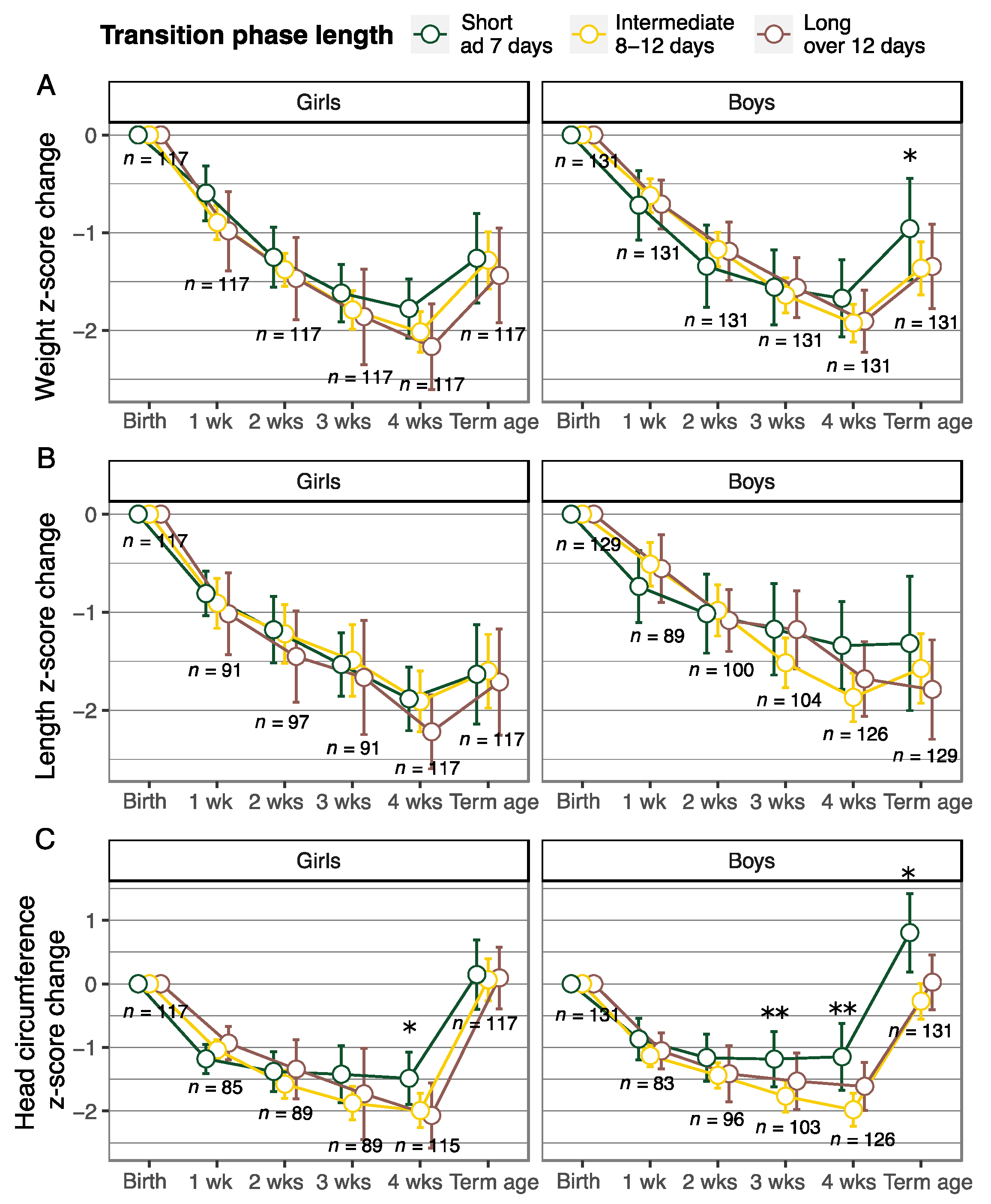
3.2. Cumulative Nutrient Intake and Longitudinal Growth According to the Transition Phase Length
3.3. Primary and Secondary Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ronald, E.K.; Frank, R.G. (Eds.) Pediatric Nutrition, 8th ed.; American Academy of Pediatrics: Itasca, IL, USA, 2019; ISBN 978-1-61002-361-0. [Google Scholar]
- Mihatsch, W.A.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; Darmaun, D.; Desci, T.; Domellöf, M.; Embleton, N.; et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition. Clin. Nutr. 2018, 37, 2303–2305. [Google Scholar] [CrossRef] [Green Version]
- Agostoni, C.; Buonocore, G.; Carnielli, V.P.; De Curtis, M.; Darmaun, D.; Decsi, T.; Domellöf, M.; Embleton, N.D.; Fusch, C.; Genzel-Boroviczeny, O.; et al. Enteral nutrient supply for preterm infants: Commentary from the european society of paediatric gastroenterology, hepatology and nutrition committee on nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 85–91. [Google Scholar] [CrossRef]
- Griffin, I.J.; Tancredi, D.J.; Bertino, E.; Lee, H.C.; Profit, J. Postnatal growth failure in very low birthweight infants born between 2005 and 2012. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, F50–F55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephens, B.E.; Walden, R.V.; Gargus, R.A.; Tucker, R.; McKinley, L.; Mance, M.; Nye, J.; Vohr, B.R. First-Week Protein and Energy Intakes Are Associated With 18-Month Developmental Outcomes in Extremely Low Birth Weight Infants. Pediatrics 2009, 123, 1337–1343. [Google Scholar] [CrossRef]
- Coviello, C.; Keunen, K.; Kersbergen, K.J.; Groenendaal, F.; Leemans, A.; Peels, B.; Isgum, I.; Viergever, M.A.; De Vries, L.S.; Buonocore, G.; et al. Effects of early nutrition and growth on brain volumes, white matter microstructure, and neurodevelopmental outcome in preterm newborns. Pediatr. Res. 2018, 83, 102–110. [Google Scholar] [CrossRef] [Green Version]
- Sjöström, E.S.; Lundgren, P.; Öhlund, I.; Holmström, G.; Hellström, A.; Domellöf, M. Low energy intake during the first 4 weeks of life increases the risk for severe retinopathy of prematurity in extremely preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, F108–F113. [Google Scholar] [CrossRef] [Green Version]
- Immeli, L.; Mäkelä, P.M.; Leskinen, M.; Rinta-Koski, O.P.; Sund, R.; Andersson, S.; Luukkainen, P. A triple-chamber parenteral nutrition solution was associated with improved protein intake in very low birthweight infants. Acta Paediatr. Int. J. Paediatr. 2020, 109, 1588–1594. [Google Scholar] [CrossRef]
- Medical Birth Register—THL. Available online: https://thl.fi/en/web/thlfi-en/statistics/information-on-statistics/register-descriptions/newborns (accessed on 31 August 2021).
- Sankilampi, U.; Hannila, M.L.; Saari, A.; Gissler, M.; Dunkel, L. New population-based references for birth weight, length, and head circumference in singletons and twins from 23 to 43 gestation weeks. Ann. Med. 2013, 45, 446–454. [Google Scholar] [CrossRef]
- Marshall, R.; Barton, L.; Brotherton, T.; Ascp, M. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann. Surg. 1978, 187, 1–7. [Google Scholar]
- Papile, L.A.; Burstein, J.; Burstein, R.; Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1500 gm. J. Pediatr. 1978, 92, 529–534. [Google Scholar] [CrossRef]
- Mathers, N.J.; Pohlandt, F. Diagnostic audit of C-reactive protein in neonatal infection. Eur. J. Pediatr. 1987, 146, 147–151. [Google Scholar] [CrossRef]
- Gidrewicz, D.A.; Fenton, T.R. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr. 2014, 14, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koletzko, B.; Goulet, O.; Hunt, J.; Krohn, K.; Shamir, R. Guidelines on Paediatric Parenteral Nutrition of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), Supported by the European Society of Paedia. J. Pediatr. Gastroenterol. Nutr. 2005, 41 (Suppl. 2), S1–S87. [Google Scholar] [CrossRef] [Green Version]
- Brennan, A.-M.; Fenton, S.; Murphy, B.P.; Kiely, M.E. Transition Phase Nutrition Recommendations. J. Parenter. Enter. Nutr. 2018, 42, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Falciglia, G.H.; Murthy, K.; Holl, J.L.; Palac, H.L.; Oumarbaeva, Y.; Woods, D.M.; Robinson, D.T. Energy and Protein Intake During the Transition from Parenteral to Enteral Nutrition in Infants of Very Low Birth Weight. J. Pediatr. 2018, 202, 38–43.e1. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.; Vaidya, R.; Rastogi, D.; Bhutada, A.; Rastogi, S. From parenteral to enteral nutrition: A nutrition-based approach for evaluating postnatal growth failure in preterm infants. J. Parenter. Enter. Nutr. 2014, 38, 489–497. [Google Scholar] [CrossRef]
- Miller, M.; Donda, K.; Bhutada, A.; Rastogi, D.; Rastogi, S. Transitioning Preterm Infants From Parenteral Nutrition: A Comparison of 2 Protocols. J. Parenter. Enter. Nutr. 2017, 41, 1371–1379. [Google Scholar] [CrossRef] [Green Version]
- Liotto, N.; Amato, O.; Piemontese, P.; Menis, C.; Orsi, A.; Corti, M.G.; Colnaghi, M.; Cecchetti, V.; Pugni, L.; Mosca, F.; et al. Protein intakes during weaning from parenteral nutrition drive growth gain and body composition in very low birth weight preterm infants. Nutrients 2020, 12, 1298. [Google Scholar] [CrossRef] [PubMed]
- Mackay, M.W.; Cash, J.; Farr, F.; Bspharm, M.H.; Jones, K. Clinical Investigation Improving Pediatric Outcomes through Intravenous and Oral Medication Standardization. J. Pediatr. Pharmacol. Ther. 2009, 14, 226–235. [Google Scholar]
- Späth, C.; Zamir, I.; Sjöström, E.S.; Domellöf, M. Use of Concentrated Parenteral Nutrition Solutions Is Associated With Improved Nutrient Intakes and Postnatal Growth in Very Low-Birth-Weight Infants. J. Parenter. Enter. Nutr. 2020, 44, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Brennan, A.M.; Kiely, M.E.; Fenton, S.; Murphy, B.P. Standardized parenteral nutrition for the transition phase in preterm infants: A bag that fits. Nutrients 2018, 10, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riskin, A.; Picaud, J.; Shamir, R.; Espr, E.; Rousse, C.; Lyon, H.C.D.; Inserm, U.; Inra, U.; Bernard, C. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Standard versus individualized parenteral nutrition. Clin. Nutr. 2018, 37, 2409–2417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorling, J.; Abbott, J.; Berrington, J.; Bosiak, B.; Bowler, U.; Boyle, E.; Embleton, N.; Hewer, O.; Johnson, S.; Juszczak, E.; et al. Controlled Trial of Two Incremental Milk-Feeding Rates in Preterm Infants. N. Engl. J. Med. 2019, 381, 1434–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christmann, V.; Roeleveld, N.; Visser, R.; Janssen, A.J.W.M.; Reuser, J.J.C.M.; van Goudoever, J.B.; van Heijst, A.F.J. The early postnatal nutritional intake of preterm infants affected neurodevelopmental outcomes differently in boys and girls at 24 months. Acta Paediatr. Int. J. Paediatr. 2017, 106, 242–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Den Akker, C.H.P.; Te Braake, F.W.J.; Weisglas-Kuperus, N.; Van Goudoever, J.B. Observational outcome results following a randomized controlled trial of early amino acid administration in preterm infants. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Alur, P.; Kalikkot Thekkeveedu, R.; Meeks, M.; Hart, K.C.; Desai, J.; Johnson, M.; Presley, S.M.; Hussain, N. Calorie intake is associated with weight gain during transition phase of nutrition in female extremely low birth weight infants. Biol. Sex. Differ. 2020, 11, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volpe, J.J. Cerebellum of the premature infant: Rapidly developing, vulnerable, clinically important. J. Child. Neurol. 2009, 24, 1085–1104. [Google Scholar] [CrossRef] [Green Version]
- Beauport, L.; Schneider, J.; Faouzi, M.; Hagmann, P.; Hüppi, P.S.; Tolsa, J.F.; Truttmann, A.C.; Fischer Fumeaux, C.J. Impact of Early Nutritional Intake on Preterm Brain: A Magnetic Resonance Imaging Study. J. Pediatr. 2017, 181, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Newgard, C.D.; Zive, D.; Jui, J.; Weathers, C.; Daya, M. Electronic versus manual data processing: Evaluating the use of electronic health records in out-of-hospital clinical research. Acad. Emerg. Med. 2012, 19, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Cuestas, E.; Aguilera, B.; Cerutti, M.; Rizzotti, A. Sustained Neonatal Inflammation Is Associated with Poor Growth in Infants Born Very Preterm during the First Year of Life. J. Pediatr. 2019, 205, 91–97. [Google Scholar] [CrossRef]
- González, S.; Selma-Royo, M.; Arboleya, S.; Martínez-Costa, C.; Solís, G.; Suárez, M.; Fernández, N.; de los Reyes-Gavilán, C.G.; Díaz-Coto, S.; Martínez-Camblor, P.; et al. Levels of predominant intestinal microorganisms in 1 month-old full-term babies and weight gain during the first year of life. Nutrients 2021, 13, 2412. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, F.; Catellani, C.; Sartori, C.; Lazzeroni, P.; Street, M.E. The Role of MicroRNAs in Influencing Body Growth and Development. Horm. Res. Paediatr. 2020, 93, 7–15. [Google Scholar] [CrossRef] [PubMed]
| Total | Girls | Boys | p-Value | |
|---|---|---|---|---|
| Number of infants, n (%) | 248 | 117 (47.2) | 131 (52.8) | 0.37 a |
| Gestational age, weeks, mean (SD) | 26.5 (1.6) | 26.4 (1.5) | 26.6 (1.8) | 0.60 b |
| Birthweight, g, mean (SD) | 845 (199) | 798 (170) | 887 (213) | 0.0003 b,* |
| Birthweight < 1000 g, n (%) | 198 (79.8) | 104 (88.9) | 94 (71.8) | 0.001 a,* |
| Small for gestational age, n (%) | 51 (20.6) | 28 (23.9) | 23 (17.6) | 0.28 a |
| Birthweight, z-score, median (IQR) | −0.2 (−1.8–0.9) | −0.4 (−2.0–0.7) | 0.0 (−1.8–0.9) | 0.12 c |
| Length at birth, z-score, median (IQR) | −0.6 (−1.9–0.6) | −0.6 (−1.7–0.4) | −0.4 (−2–0.7) | 0.6 c |
| HC at birth, z-score, mean (SD) | −0.5 (1.3) | −0.7 (1.3) | −0.4 (1.3) | 0.04 b |
| Multiple gestation (twins), n (%) | 53 (21.4) | 24 (20.5) | 29 (22.1) | 0.88 a |
| Duration of invasive mechanical ventilation, days, median (IQR) | 15 (5–33) | 13 (5–30) | 17 (5–34.5) | 0.18 c |
| Respiratory distress syndrome, n (%) | 168 (67.7) | 79 (67.5) | 89 (67.9) | 1 a |
| Bronchopulmonary dysplasia, n (%) | 127 (51.2) | 52 (44.4) | 75 (57.3) | 0.059 a |
| Patent ductus arteriosus, n (%) | 165 (66.5) | 80 (68.4) | 85 (64.9) | 0.66 a |
| Sepsis, n (%) | 59 (23.8) | 23 (19.7) | 36 (27.5) | 0.2 a |
| Severe IVH (grade III or IV), n (%) | 27 (10.9) | 13 (11.1) | 14 (10.7) | 1 a |
| Discharged before 42 weeks of PMA, n (%) | 184 (74.2) | 88 (75.2) | 96 (73.3) | 0.62 a |
| Parenteral phase length, d, median (IQR) | 5 (3–7) | 5 (2–7) | 5 (3–7.5) | 0.7 c |
| Transition phase length, d, median (IQR) | 10 (8–12.3) | 9 (7–11) | 10 (8–13) | 0.007 c,* |
| Enteral phase length, d, median (IQR) | 13 (9–16) | 14 (10–17) | 12 (8–15) | 0.02 c,* |
| Girls (n = 117) | Boys (n = 131) | |||||||
|---|---|---|---|---|---|---|---|---|
| Short Transition ad 7 Days (n = 31) | Intermediate Transition 8–12 Days (n = 64) | Long Transition over 12 Days (n = 22) | p-Value | Short Transition ad 7 Days (n = 23) | Intermediate Transition 8–12 Days (n = 68) | Long Transition over 12 Days (n = 40) | p-Value | |
| Cumulative nutrient intake at 28 days of age, mean (SD) | ||||||||
| energy kcal/kg | 3299 (223) | 3142 (230) | 2971 (217) | <0.05 b,* | 3214 (188) | 3058 (187) | 2930 (181) | <0.05 b,* |
| protein g/kg | 95.26 (9.9) | 91.4 (7.4) | 85.5 (7.9) | <0.05 b,** | 94.7 (8.1) | 88.5 (5.6) | 84.5 (6.9) | <0.05 b,* |
| lipid g/kg | 126.6 (9.5) | 121.5 (17.9) | 108.4 (14.2) | <0.05 b,** | 126.1 (13.0) | 118.6 (14.0) | 108.3 (13.3) | <0.05 b,** |
| Carbohydrate g/kg | 444.7 (45.6) | 420.8 (36.1) | 413.4 (39.7) | <0.05 b,*** | 425.1 (33.9) | 409.0 (34.6) | 404.5 (30.6) | 0.06 b |
| Clinical characteristics | ||||||||
| Gestational age, wk, mean (SD) | 26.4 (1.5) | 25.6 (1.4) | 26.6 (1.6) | <0.05 b,^ | 26.0 (1.8) | 26.0 (1.6) | 26.4 (2.1) | 0.5 b |
| Birthweight, g, mean (SD) | 805 (143) | 764 (161) | 886 (203) | <0.05 b,^ | 865 (263) | 897 (201) | 883 (205) | 0.8 b |
| Birthweight < 1000 g, n (%) | 28 (90.3) | 59 (92.2) | 17 (77.3) | 0.15 a | 19 (82.6) | 47 (69.1) | 28 (70.0) | 0.44 a |
| Small for GA, n (%) | 8 (25.8) | 14 (21.9) | 6 (27.3) | 0.8 a | 7 (30.4) | 9 (13.2) | 7 (17.5) | 0.2 a |
| Duration of invasive mechanical ventilation, days median (IQR) | 7 (3.5–16) | 22 (8–35.3) | 10 (3–27.3) | <0.05 c,**** | 20 (4–36) | 17.5 (6.5–34.3) | 17 (5.8–32.8) | 0.98 c |
| Change in z-Score from Birth to Term Equivalent Age ^ | Change in z-Score from Birth to 28 Days of Age | |||||
|---|---|---|---|---|---|---|
| Weight | Length | Head Circumference | Weight | Length | Head Circumference | |
| Coefficient (95% CI) | Coefficient (95% CI) | |||||
| Intermediate transition (8–12 d) | −0.13 (−0.35, 0.09) | 0.03 (−0.32, 0.39) | −0.43 (−0.74, −0.11) * | −0.12 (−0.27, 0.03) | −0.18 (−0.45, 0.093) | −0.49 (−0.79, −0.20) * |
| Long transition (over 12 d) | −0.3 (−0.56, −0.04) * | −0.25 (−0.67, 0.16) | −0.44 (−0.81, −0.07) * | −0.22 (−0.4, −0.044) * | −0.23 (−0.54, 0.086) | −0.34 (−0.69, 0.011) |
| Gestational age (week) | 0.03 (−0.03, 0.09) | 0.19 (0.10, 0.28) * | 0.082 (−0.0009, 0.17) | −0.06 (−0.10, −0.02) * | 0.004 (−0.064, 0.073) | 0.10 (0.022, 0.18) * |
| Weight/length/head circumference at birth (z-score) | −0.53 (−0.59, −0.47) * | −0.45 (−0.53, −0.37) * | −0.58 (−0.68, −0.47) * | −0.47 (−0.51, −0.43) * | −0.35 (−0.41, −0.29) * | −0.38 (−0.48, −0.28) * |
| Sex (boys) | 0.11 (−0.07, 0.29) | −0.09 (−0.37, 0.19) | 0.093 (−0.16, 0.34) | 0.26 (0.14, 0.38) * | 0.26 (0.04, 0.47) * | 0.30 (0.065, 0.54) * |
| PMA (week) at the time of the measurement ^ | 0.16 (0.11, 0.21) * | 0.16 (0.087, 0.24) * | 0.14 (0.077, 0.21) * | – | – | – |
| Severe IVH | – | – | 0.55 (0.14, 0.95) * | – | – | 0.80 (0.41, 1.18) * |
| R2 | 0.67 | 0.49 | 0.45 | 0.70 | 0.38 | 0.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Immeli, L.; Sankilampi, U.; Mäkelä, P.M.; Leskinen, M.; Sund, R.; Andersson, S.; Luukkainen, P. Length of Nutritional Transition Associates Negatively with Postnatal Growth in Very Low Birthweight Infants. Nutrients 2021, 13, 3961. https://doi.org/10.3390/nu13113961
Immeli L, Sankilampi U, Mäkelä PM, Leskinen M, Sund R, Andersson S, Luukkainen P. Length of Nutritional Transition Associates Negatively with Postnatal Growth in Very Low Birthweight Infants. Nutrients. 2021; 13(11):3961. https://doi.org/10.3390/nu13113961
Chicago/Turabian StyleImmeli, Lotta, Ulla Sankilampi, Pauliina M. Mäkelä, Markus Leskinen, Reijo Sund, Sture Andersson, and Päivi Luukkainen. 2021. "Length of Nutritional Transition Associates Negatively with Postnatal Growth in Very Low Birthweight Infants" Nutrients 13, no. 11: 3961. https://doi.org/10.3390/nu13113961
APA StyleImmeli, L., Sankilampi, U., Mäkelä, P. M., Leskinen, M., Sund, R., Andersson, S., & Luukkainen, P. (2021). Length of Nutritional Transition Associates Negatively with Postnatal Growth in Very Low Birthweight Infants. Nutrients, 13(11), 3961. https://doi.org/10.3390/nu13113961






