The Effects of TRX Suspension Training Combined with Taurine Supplementation on Body Composition, Glycemic and Lipid Markers in Women with Type 2 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Anthropometry and Body Composition
2.3. Blood Sampling and Laboratory Analysis
2.4. TRX Suspension Training Protocol
2.5. Taurine and Placebo Supplementation
2.6. Diet
2.7. Statistical Analysis
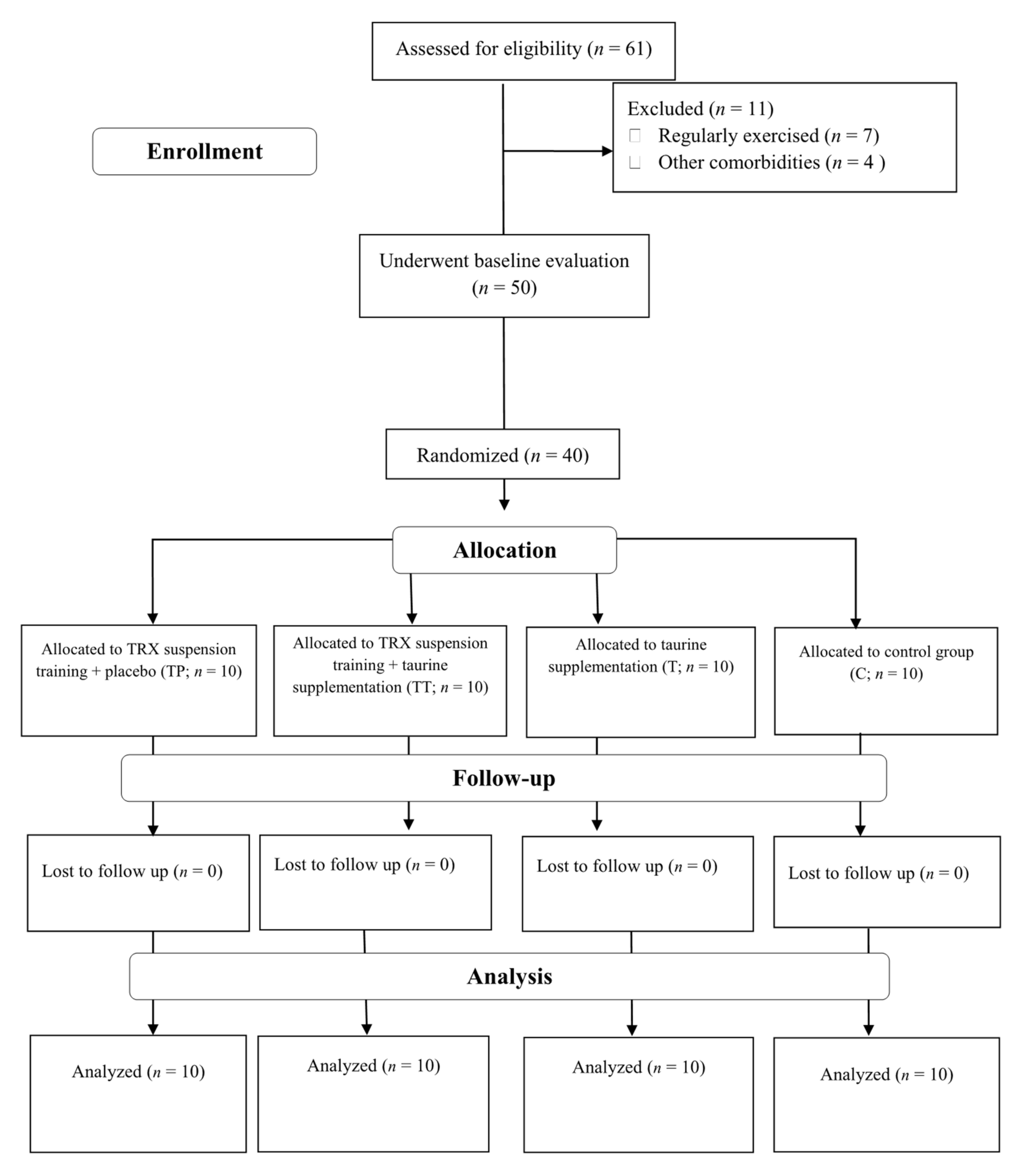
3. Results
3.1. Study Compliance, Diet, and Body Composition
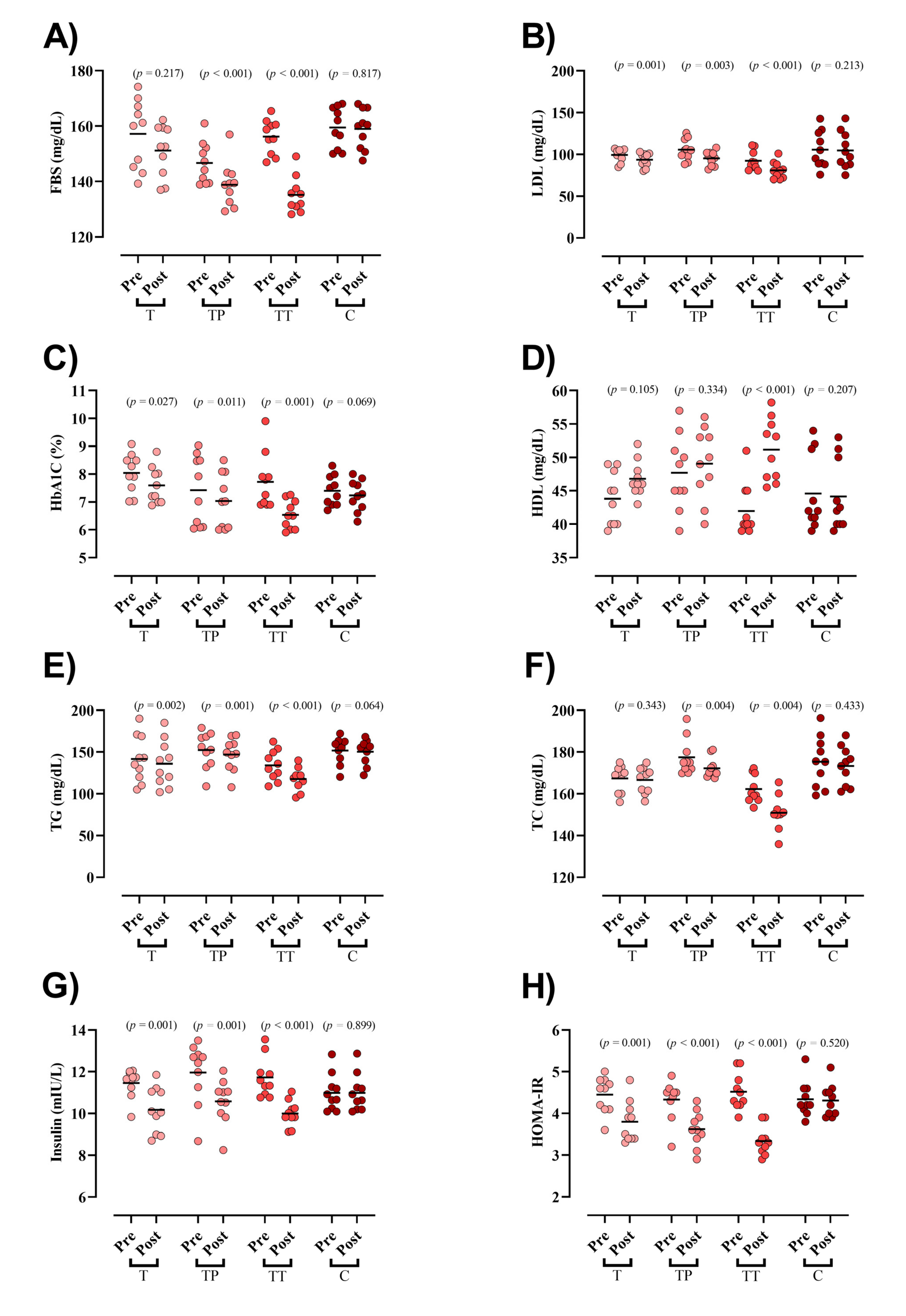
3.2. Blood Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Long, A.N.; Dagogo-Jack, S. Comorbidities of diabetes and hypertension: Mechanisms and approach to target organ protection. J. Clin. Hypertens. 2011, 13, 244–251. [Google Scholar]
- Asbaghi, O.; Fouladvand, F.; Ashtary-Larky, D.; Bagheri, R.; Choghakhori, R.; Wong, A.; Baker, J.S.; Abbasnezhad, A. Effects of green tea supplementation on serum concentrations of adiponectin in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Arch. Physiol. Biochem. 2020, 1–8. [Google Scholar] [CrossRef]
- Roglic, G. Diabetes in women: The global perspective. Int. J. Gynecol. Obstet. 2009, 104, S11–S13. [Google Scholar] [CrossRef] [PubMed]
- Haghighat, N.; Ashtary-Larky, D.; Bagheri, R.; Mahmoodi, M.; Rajaei, M.; Alipour, M.; Kooti, W.; Aghamohammdi, V.; Wong, A. The effect of 12 weeks of euenergetic high-protein diet in regulating appetite and body composition of women with normal-weight obesity: A randomised controlled trial. Br. J. Nutr. 2020, 124, 1044–1051. [Google Scholar] [CrossRef]
- Mesa, M.S. Peer Reviewed: Health Care Disparities between Men and Women with Type 2 Diabetes. Prev. Chronic Dis. 2018, 15, E46. [Google Scholar] [CrossRef]
- Aryan, L.; Younessi, D.; Zargari, M.; Banerjee, S.; Agopian, J.; Rahman, S.; Borna, R.; Ruffenach, G.; Umar, S.; Eghbali, M. The role of estrogen receptors in cardiovascular disease. Int. J. Mol. Sci. 2020, 21, 4314. [Google Scholar] [CrossRef]
- Warner, S.O.; Yao, M.V.; Cason, R.L.; Winnick, J.J. Exercise-Induced Improvements to Whole Body Glucose Metabolism in Type 2 Diabetes: The Essential Role of the Liver. Front. Endocrinol. 2020, 11, 567. [Google Scholar]
- Zouhal, H.; Bagheri, R.; Ashtary-Larky, D.; Wong, A.; Triki, R.; Hackney, A.C.; Laher, I.; Abderrahman, A.B. Effects of Ramadan intermittent fasting on inflammatory and biochemical biomarkers in males with obesity. Physiol. Behav. 2020, 225, 113090. [Google Scholar] [CrossRef]
- Tremblay, A.; Clinchamps, M.; Pereira, B.; Courteix, D.; Lesourd, B.; Chapier, R.; Obert, P.; Vinet, A.; Walther, G.; Chaplais, E. Dietary fibres and the management of obesity and metabolic syndrome: The RESOLVE study. Nutrients 2020, 12, 2911. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; Tauveron, I.; Magdasy, S.; Benichou, T.; Bagheri, R.; Ugbolue, U.C.; Navel, V.; Dutheil, F. Effect of exercise training on heart rate variability in type 2 diabetes mellitus patients: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251863. [Google Scholar]
- Schwingshackl, L.; Missbach, B.; Dias, S.; König, J.; Hoffmann, G. Impact of different training modalities on glycaemic control and blood lipids in patients with type 2 diabetes: A systematic review and network meta-analysis. Diabetologia 2014, 57, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.; Beedie, C.; Balducci, S.; Zanuso, S.; Allgrove, J.; Bertiato, F.; Jimenez, A. Changes in insulin sensitivity in response to different modalities of exercise: A review of the evidence. Diabetes/Metab. Res. Rev. 2014, 30, 257–268. [Google Scholar] [CrossRef]
- Shultz, J.A.; Sprague, M.A.; Branen, L.J.; Lambeth, S. A comparison of views of individuals with type 2 diabetes mellitus and diabetes educators about barriers to diet and exercise. J. Health Commun. 2001, 6, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Guérin, E.; Fortier, M.S. Situational motivation and perceived intensity: Their interaction in predicting changes in positive affect from physical activity. J. Obes. 2012, 2012, 1–7. [Google Scholar]
- Smith, L.E.; Snow, J.; Fargo, J.S.; Buchanan, C.A.; Dalleck, L.C. The acute and chronic health benefits of TRX Suspension Training® in healthy adults. Int. J. Res. Ex Phys. 2016, 11, 1–15. [Google Scholar]
- Aminaei, M.; Shamsi, E.H.; Nikoei, R. The impact of eight weeks of calcium intake and vitamin D along with TRX exercise on body composition and lipid profiles of overweight women. Obes. Med. 2020, 19, 100249. [Google Scholar] [CrossRef]
- Sadeghi, A.; Pourrazi, H.; Yazdi, H.-R. The Effect of Eight-Week Total Body Resistance Exercise on Liver Functional Parameters in Patients with Non-Alcoholic Fatty Liver Disease. Hormozgan Med. J. 2019, 23, e97644. [Google Scholar] [CrossRef]
- Gaedtke, A.; Morat, T. TRX suspension training: A new functional training approach for older adults–development, training control and feasibility. Int. J. Exerc. Sci. 2015, 8, 224. [Google Scholar] [PubMed]
- Wibowo, S.; Fathir, L.W. Effect of total body weight resistance exercise (TRX) on arms muscle power. In Proceedings of the 4th International Conference on Physical Education, Sport and Health (Ismina) and Workshop: Enhancing Sport, Physical Activity, and Health Promotion for a Better Quality of Life, Semarang, Indonesia, 12–13 April 2017; pp. 735–743. [Google Scholar]
- Janot, J.; Heltne, T.; Welles, C.; Riedl, J.; Anderson, H.; Howard, A.; Myhre, S.L. Effects of TRX versus traditional resistance training programs on measures of muscular performance in adults. J. Fit. Res. 2013, 2, 23–38. [Google Scholar]
- Khoshkam, F.; Taghian, F.; Jalali Dehkordi, K. Effect of eight weeks of supplementation of omega-3 supplementation and TRX training on visfatin and insulin resistance in women with polycystic ovary syndrome. Iran. J. Obstet. Gynecol. Infertil. 2018, 21, 58–70. [Google Scholar]
- Huxtable, R. Physiological actions of taurine. Physiol. Rev. 1992, 72, 101–163. [Google Scholar]
- Chang, K.J.; Kwon, W. Immunohistochemical Localization of Insulin in Pancreatic β-Cells of Taurine-Supplemented or Taurine-Depleted Diabetic Rats. In Taurine 4; Springer: Cham, Switzerland, 2002; pp. 579–587. [Google Scholar]
- Franconi, F.; Loizzo, A.; Ghirlanda, G.; Seghieri, G. Taurine supplementation and diabetes mellitus. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 32–36. [Google Scholar] [CrossRef]
- Cherif, H.; Reusens, B.; Ahn, M.; Hoet, J.; Remacle, C. Effects of taurine on the insulin secretion of rat fetal islets from dams fed a low-protein diet. J. Endocrinol. 1998, 159, 341–348. [Google Scholar] [CrossRef]
- Kim, K.S.; Kim, J.Y.; Lee, B.G.; You, J.S.; Chang, K.J.; Chung, H.; Yoo, M.C.; Yang, H.-I.; Kang, J.-H.; Hwang, Y.C. Taurine ameliorates hyperglycemia and dyslipidemia by reducing insulin resistance and leptin level in Otsuka Long-Evans Tokushima fatty (OLETF) rats with long-term diabetes. Exp. Mol. Med. 2012, 44, 665–673. [Google Scholar] [CrossRef]
- Gomez, R.; Caletti, G.; Arbo, B.D.; Hoefel, A.L.; Schneider Jr, R.; Hansen, A.W.; Pulcinelli, R.R.; Freese, L.; Bandiera, S.; Kucharski, L.C. Acute intraperitoneal administration of taurine decreases the glycemia and reduces food intake in type 1 diabetic rats. Biomed. Pharmacother. 2018, 103, 1028–1034. [Google Scholar] [CrossRef]
- Foda, D.S.; Farrag, E.K.; Metwally, N.S.; Maghraby, A.S.; Farrag, A.R.H.; Rawi, S. Protective and therapeutic impact of taurine on some biochemical, immunological and histological parameters in diabetic rats. J. Appl. Pharm. Sci. 2016, 6, 45–54. [Google Scholar]
- Nakaya, Y.; Minami, A.; Harada, N.; Sakamoto, S.; Niwa, Y.; Ohnaka, M. Taurine improves insulin sensitivity in the Otsuka Long-Evans Tokushima Fatty rat, a model of spontaneous type 2 diabetes. Am. J. Clin. Nutr. 2000, 71, 54–58. [Google Scholar]
- Das, J.; Vasan, V.; Sil, P.C. Taurine exerts hypoglycemic effect in alloxan-induced diabetic rats, improves insulin-mediated glucose transport signaling pathway in heart and ameliorates cardiac oxidative stress and apoptosis. Toxicol. Appl. Pharmacol. 2012, 258, 296–308. [Google Scholar] [PubMed]
- Maleki, V.; Alizadeh, M.; Esmaeili, F.; Mahdavi, R.J.A.A. The effects of taurine supplementation on glycemic control and serum lipid profile in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Amino Acids 2020, 52, 905–914. [Google Scholar] [CrossRef]
- Motevalli, M.S.; Dalbo, V.J.; Attarzadeh, R.S.; Rashidlamir, A.; Tucker, P.S.; Scanlan, A.T. The effect of rate of weight reduction on serum myostatin and follistatin concentrations in competitive wrestlers. Int. J. Sports Physiol. Perform. 2015, 10, 139–146. [Google Scholar] [PubMed]
- Jackson, A.S.; Pollock, M.L.; Ward, A. Generalized equations for predicting body density of women. Med. Sci. Sports Exerc. 1980, 12, 175–181. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.; Rudenski, A.; Naylor, B.; Treacher, D.; Turner, R. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Cacho, J.; Sevillano, J.; de Castro, J.; Herrera, E.; Ramos, M. Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am. J. Physiol.-Endocrinol. Metab. 2008, 295, E1269–E1276. [Google Scholar] [CrossRef]
- Majid, H.; Masood, Q.; Khan, A.H. Homeostatic model assessment for insulin resistance (HOMA-IR): A better marker for evaluating insulin resistance than fasting insulin in women with polycystic ovarian syndrome. J. Coll. Physicians Surg. Pak. 2017, 27, 123–126. [Google Scholar] [PubMed]
- Bagheri, R.; Rashidlamir, A.; Motevalli, M.S.; Elliott, B.T.; Mehrabani, J.; Wong, A. Effects of upper-body, lower-body, or combined resistance training on the ratio of follistatin and myostatin in middle-aged men. Eur. J. Appl. Physiol. 2019, 119, 1–11. [Google Scholar]
- Bagheri, R.; Moghadam, B.H.; Ashtary-Larky, D.; Forbes, S.C.; Candow, D.G.; Galpin, A.J.; Eskandari, M.; Kreider, R.B.; Wong, A. Whole egg vs. egg white ingestion during 12 weeks of resistance training in trained young males: A randomized controlled trial. J. Strength Cond. Res. 2021, 35, 411–419. [Google Scholar]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Shari, F.H.; Dawood, H.; Hassan, J.K.; ALJazeari, Q.A.; Najm, M.A.; Salahuddin, A.; Al-Salman, H. To study the effect of taurine on the effects of vital bones and regulate the level of glucose in type II diabetes. Int. J. Res. Pharm. Sci. 2019, 10, 2545–2551. [Google Scholar] [CrossRef]
- Saleh, A.A.S. Effects of taurine and/or ginseng and their mixture on lipid profile and some parameters indicative of myocardial status in streptozotocin-diabetic rats. J. Basic Appl. Zool. 2012, 65, 267–273. [Google Scholar]
- Nishimura, N.; Umeda, C.; Oda, H.; Yokogoshi, H. The effect of taurine on plasma cholesterol concentration in genetic type 2 diabetic GK rats. J. Nutr. Sci. Vitaminol. 2002, 48, 483–490. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, S.; Yang, J.; Wu, G.; Liu, M.; Luan, X.; Lv, Q.; Zhao, H.; Hu, J. Preventive effect of taurine on experimental type II diabetic nephropathy. J. Biomed. Sci. 2010, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Buonani, C.; Rossi, F.E.; Diniz, T.A.; Fortaleza, A.C.; Viezel, J.; Picolo, M.R.; Fernandes, R.A.; Freitas Júnior, I.F. Concurrent training and taurine improve lipid profile in postmenopausal women. Rev. Bras. Med. Esporte 2019, 25, 121–126. [Google Scholar] [CrossRef]
- Carneiro, E.M.; Latorraca, M.Q.; Araujo, E.; Beltrá, M.; Oliveras, M.J.; Navarro, M.; Berná, G.; Bedoya, F.J.; Velloso, L.A.; Soria, B.; et al. Taurine supplementation modulates glucose homeostasis and islet function. J. Nutr. Biochem. 2009, 20, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.A.; Santos-Silva, J.C.; Vettorazzi, J.F.; Cotrim, B.B.; Mobiolli, D.D.; Boschero, A.C.; Carneiro, E.M. Taurine supplementation prevents morpho-physiological alterations in high-fat diet mice pancreatic β-cells. Amino Acids 2012, 43, 1791–1801. [Google Scholar] [PubMed]
- Batista, T.M.; Ribeiro, R.A.; da Silva, P.M.; Camargo, R.L.; Lollo, P.C.; Boschero, A.C.; Carneiro, E.M. Taurine supplementation improves liver glucose control in normal protein and malnourished mice fed a high-fat diet. Mol. Nutr. Food Res. 2013, 57, 423–434. [Google Scholar]
- Camargo, R.L.; Branco, R.C.S.; de Rezende, L.F.; Vettorazzi, J.F.; Borck, P.C.; Boschero, A.C.; Carneiro, E.M. The Effect of Taurine Supplementation on Glucose Homeostasis: The Role of Insulin-Degrading Enzyme. In Taurine 9; Springer: Cham, Switzerland, 2015; pp. 715–724. [Google Scholar]
- Borck, P.C.; Vettorazzi, J.F.; Branco, R.C.S.; Batista, T.M.; Santos-Silva, J.C.; Nakanishi, V.Y.; Boschero, A.C.; Ribeiro, R.A.; Carneiro, E.M. Taurine supplementation induces long-term beneficial effects on glucose homeostasis in ob/ob mice. Amino Acids 2018, 50, 765–774. [Google Scholar] [CrossRef]
- Contreras, C.; González-García, I.; Seoane-Collazo, P.; Martínez-Sánchez, N.; Liñares-Pose, L.; Rial-Pensado, E.; Fernø, J.; Tena-Sempere, M.; Casals, N.; Diéguez, C.J.D. Reduction of hypothalamic endoplasmic reticulum stress activates browning of white fat and ameliorates obesity. Diabetes 2017, 66, 87–99. [Google Scholar] [CrossRef]
- Cao, P.-J.; Jin, Y.-J.; Li, M.-E.; Zhou, R.; Yang, M. PGC-1α may associated with the anti-obesity effect of taurine on rats induced by arcuate nucleus lesion. Nutr. Neurosci. 2016, 19, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Santos-Silva, J.C.; Ribeiro, R.A.; Vettorazzi, J.F.; Irles, E.; Rickli, S.; Borck, P.C.; Porciuncula, P.M.; Quesada, I.; Nadal, A.; Boschero, A.C. Taurine supplementation ameliorates glucose homeostasis, prevents insulin and glucagon hypersecretion, and controls β, α, and δ-cell masses in genetic obese mice. Amino Acids 2015, 47, 1533–1548. [Google Scholar] [CrossRef]
- Vettorazzi, J.F.; Ribeiro, R.A.; Santos-Silva, J.C.; Borck, P.C.; Batista, T.M.; Nardelli, T.R.; Boschero, A.C.; Carneiro, E.M. Taurine supplementation increases K ATP channel protein content, improving Ca2+ handling and insulin secretion in islets from malnourished mice fed on a high-fat diet. Amino Acids 2014, 46, 2123–2136. [Google Scholar]
- Chen, W.; Guo, J.-X.; Chang, P. The effect of taurine on cholesterol metabolism. Mol. Nutr. Food Res. 2012, 56, 681–690. [Google Scholar] [PubMed]
- Murakami, S. Role of taurine in the pathogenesis of obesity. Mol. Nutr. Food. Res. 2015, 59, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Sak, D.; Erdenen, F.; Müderrisoglu, C.; Altunoglu, E.; Sozer, V.; Gungel, H.; Guler, P.A.; Sak, T.; Uzun, H. The Relationship between Plasma Taurine Levels and Diabetic Complications in Patients with Type 2 Diabetes Mellitus. Biomolecules 2019, 9, 96. [Google Scholar] [CrossRef]
- Zheng, Y.; Ceglarek, U.; Huang, T.; Wang, T.; Heianza, Y.; Ma, W.; Bray, G.A.; Thiery, J.; Sacks, F.M.; Qi, L. Plasma Taurine, Diabetes Genetic Predisposition, and Changes of Insulin Sensitivity in Response to Weight-Loss Diets. J. Clin. Endocrinol. Metab. 2016, 101, 3820–3826. [Google Scholar] [CrossRef]
- Franconi, F.; Bennardini, F.; Mattana, A.; Miceli, M.; Ciuti, M.; Mian, M.; Gironi, A.; Anichini, R.; Seghieri, G. Plasma and platelet taurine are reduced in subjects with insulin-dependent diabetes mellitus: Effects of taurine supplementation. Am. J. Clin. Nutr. 1995, 61, 1115–1119. [Google Scholar] [CrossRef]
- Haidari, F.; Asadi, M.; Mohammadi-Asl, J.; Angali, K.A. Effect of weight-loss diet combined with taurine supplementation on body composition and some biochemical markers in obese women: A randomized clinical trial. Amino Acids 2020, 52, 1–10. [Google Scholar] [CrossRef]
- De Carvalho, M.B.; Brandao, C.F.C.; Fassini, P.G.; Bianco, T.M.; Batitucci, G.; Galan, B.S.M.; De Carvalho, F.G.; Vieira, T.S.; Ferriolli, E.; Marchini, J.S.; et al. Taurine Supplementation Increases Post-Exercise Lipid Oxidation at Moderate Intensity in Fasted Healthy Males. Nutrients 2020, 12, 1540. [Google Scholar] [CrossRef]
- De Carvalho, F.G.; Brandao, C.F.C.; Batitucci, G.; Souza, A.D.O.; Ferrari, G.D.; Alberici, L.C.; Muñoz, V.R.; Pauli, J.R.; De Moura, L.P.; Ropelle, E.R.; et al. Taurine supplementation associated with exercise increases mitochondrial activity and fatty acid oxidation gene expression in the subcutaneous white adipose tissue of obese women. Clin. Nutr. 2020, 40, 2180–2187. [Google Scholar] [CrossRef]
- de Almeida Martiniano, A.C.; De Carvalho, F.G.; Marchini, J.S.; Garcia, S.B.; Júnior, J.E.; Mauad, F.M.; da Silva, A.S.R.; de Moraes, C.; de Freitas, E.C. Effects of taurine supplementation on adipose tissue of obese trained rats. In Taurine 9; Springer: Cham, Switzerland, 2015; pp. 707–714. [Google Scholar]
- Tomljanović, M.; Spasić, M.; Gabrilo, G.; Uljević, O.; Foretić, N. Effects of five weeks of functional vs. traditional resistance training on anthropometric and motor performance variables. Kinesiology 2011, 43, 145–154. [Google Scholar]
- Wideman, L.; Weltman, J.Y.; Hartman, M.L.; Veldhuis, J.D.; Weltman, A. Growth Hormone Release During Acute and Chronic Aerobic and Resistance Exercise. Sports Med. 2002, 32, 987–1004. [Google Scholar] [CrossRef]
- Yarizadeh, H.; Eftekhar, R.; Anjom-Shoae, J.; Speakman, J.R.; Djafarian, K. The Effect of Aerobic and Resistance Training and Combined Exercise Modalities on Subcutaneous Abdominal Fat: A Systematic Review and Meta-analysis of Randomized Clinical Trials. Adv. Nutr. 2020, 12, 179–196. [Google Scholar] [CrossRef]
- Shakiba, E.; Sheikholeslami-Vatani, D.; Rostamzadeh, N.; Karim, H. The type of training program affects appetite-regulating hormones and body weight in overweight sedentary men. Appl. Physiol. Nutr. Metab. 2019, 44, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Kerr, L.; Wilkerson, S.; Bandy, W.D.; Ishee, J. Reliability and Validity of Skinfold Measurements of Trained Versus Untrained Testers. Isokinet. Exerc. Sci. 1994, 4, 137–140. [Google Scholar] [CrossRef]
- Aandstad, A.; Holtberget, K.; Hageberg, R.; Holme, I.; Anderssen, S.A. Validity and Reliability of Bioelectrical Impedance Analysis and Skinfold Thickness in Predicting Body Fat in Military Personnel. Mil. Med. 2014, 179, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Kasper, A.; Langan-Evans, C.; Hudson, J.; Brownlee, T.; Harper, L.; Naughton, R.; Morton, J.; Close, G. Come Back Skinfolds, All Is Forgiven: A Narrative Review of the Efficacy of Common Body Composition Methods in Applied Sports Practice. Nutrients 2021, 13, 1075. [Google Scholar] [CrossRef] [PubMed]
| T | TP | TT | C | |
|---|---|---|---|---|
| Age (y) | 53.3 ± 3.8 | 55 ± 5.7 | 49.4 ± 6.3 | 54.4 ± 3.7 |
| Body mass (kg) | 86.1 ± 5 | 84.5 ± 4.6 | 83 ± 5.3 | 83.6 ± 5.7 |
| BMI (kg·m−2) | 27.9 ± 2.1 | 28.9 ± 2.5 | 27.6 ± 1.8 | 27 ± 1.6 |
| BFP (%) | 29.1 ± 2.4 | 30.5 ± 2.5 | 29.3 ± 3.3 | 30.3 ± 2.9 |
| FBS (mg/dL) | 157.2 ± 12.3 | 146.7 ± 7.4 | 156.2 ± 6.2 | 159.4 ± 7.3 |
| HbA1C (%) | 8 ± 0.6 | 7.4 ± 1.2 | 7.7 ± 0.9 | 7.3 ± 0.5 |
| HDL (mg/dL) | 43.8 ± 3.9 | 47.7 ± 5.5 | 42 ± 3.8 | 44.5 ± 5.6 |
| LDL mg/dL) | 99.4 ± 7.7 | 105.6 ± 12.6 | 92.2 ± 11.4 | 105.5 ± 21.8 |
| TG (mg/dL) | 141.6 ± 27.7 | 152.2 ± 21.4 | 134.2 ± 17.8 | 151.8 ± 15.5 |
| TC (mg/dL) | 167.4 ± 6.3 | 177.4 ± 8.5 | 162.2 ± 6.6 | 175.2 ± 12.1 |
| Insulin (mIU/L) | 11.4 ± 0.6 | 11.9 ± 1.4 | 11.7 ± 0.9 | 10.9 ± 0.8 |
| HOMA-IR | 4.4 ± 0.4 | 4.3 ± 0.4 | 4.5 ± 0.4 | 4.3 ± 0.4 |
| Week | RPE | Repetition |
|---|---|---|
| 1–2 | 14 | 12 |
| 3–4 | 14–15 | 12 |
| 5–6 | 15–16 | 10 |
| 7–8 | 16–17 | 8 |
| Variable | Contrast | β (Std. Error) | 95% CI | p-Value |
|---|---|---|---|---|
| body mass-post | T vs. C | −3.10 (0.67) | −4.98, −1.22 | <0.001 |
| TP vs. C | −3.40 (0.66) | −5.26, −1.54 | <0.001 | |
| TT vs. C | −3.80 (0.66) | −5.66, −1.95 | <0.001 | |
| T vs. TP | 0.30 (0.67) | −1.57, 2.17 | 1.000 | |
| T vs. TT | 0.70 (0.68) | −1.20, 2.60 | 1.000 | |
| TP vs. TT | 0.40 (0.67) | −1.46, 2.27 | 1.000 | |
| BMI-post | T vs. C | −1.04 (0.22) | −1.64, −0.44 | <0.001 |
| TP vs. C | −1.22 (0.23) | −1.85, −0.59 | <0.001 | |
| TT vs. C | −1.28 (0.21) | −1.88, −0.68 | <0.001 | |
| T vs. TP | 0.18 (0.22) | −0.43, 0.78 | 1.000 | |
| T vs. TT | 0.24 (0.21) | −0.36, 0.84 | 1.000 | |
| TP vs. TT | 0.06 (0.22) | −0.55, 0.67 | 1.000 | |
| BFP-post | T vs. C | −0.70 (0.25) | −1.39, −0.02 | 0.043 |
| TP vs. C | −1.26 (0.24) | −1.95, −0.58 | <0.001 | |
| TT vs. C | −2.15 (0.25) | −2.84, −1.46 | <0.001 | |
| T vs. TP | 0.56 (0.25) | −0.13, 1.25 | 0.181 | |
| T vs. TT | 1.44 (0.24) | 0.76, 2.13 | <0.001 | |
| TP vs. TT | 0.88 (0.25) | 0.20, 1.57 | 0.006 | |
| FBS-post | T vs. C | −6.97 (3.28) | −16.13, 2.20 | 0.244 |
| TP vs. C | −15.63 (3.72) | −26.03, −5.23 | 0.001 | |
| TT vs. C | −22.66 (3.29) | −31.86, −13.45 | <0.001 | |
| T vs. TP | 8.67 (3.58) | −1.34, 18.68 | 0.125 | |
| T vs. TT | 15.69 (3.27) | 6.56, 24.82 | <0.001 | |
| TP vs. TT | 7.02 (3.53) | −2.84, 16.88 | 0.325 | |
| HbA1C-post | T vs. C | −0.05 (0.19) | −0.57, 0.48 | 1.000 |
| TP vs. C | −0.21 (0.18) | −0.71, 0.29 | 1.000 | |
| TT vs. C | −0.897 (0.18) | −1.41, −0.39 | <0.001 | |
| T vs. TP | 0.17 (0.19) | −0.35, 0.68 | 1.000 | |
| T vs. TT | 0.85 (0.18) | 0.35, 1.36 | <0.001 | |
| TP vs. TT | 0.69 (0.18) | 0.18, 1.19 | 0.003 | |
| HOMA-IR | T vs. C | −0.58 (0.13) | −0.94, −0.22 | <0.001 |
| TP vs. C | −0.68 (0.13) | −1, −0.32 | <0.001 | |
| TT vs. C | −1 (0.13) | −1.46, −0.72 | <0.001 | |
| T vs. TP | 0.098 (0.13) | −0.26, 0.46 | 1.000 | |
| T vs. TT | 0.5 (0.13) | 0.14, 0.87 | 0.002 | |
| TP vs. TT | 0.41 (0.13) | 0.04, 0.77 | 0.021 | |
| HDL-post | T vs. C | 3.11 (1.67) | −1.56, 7.78 | 0.428 |
| TP vs. C | 3.15 (1.71) | −1.65, 7.95 | 0.450 | |
| TT vs. C | 8.50 (1.70) | 3.74, 13.26 | <0.001 | |
| T vs. TP | −0.04 (1.74) | −4.91, 4.83 | 1.000 | |
| T vs. TT | −5.39 (1.68) | −10.11, −0.68 | 0.017 | |
| TP vs. TT | −5.35 (1.82) | −10.45, −0.24 | 0.035 | |
| TG-post | T vs. C | −4.99 (1.80) | −10.03, 0.05 | 0.054 |
| TP vs. C | −3.72 (1.77) | −8.68, 1.24 | 0.260 | |
| TT vs. C | −16.71 (1.86) | −21.90, −11.52 | <0.001 | |
| T vs. TP | −1.27 (1.80) | −6.32, 3.78 | 1.000 | |
| T vs. TT | 11.72 (1.79) | 6.72, 16.72 | <0.001 | |
| TP vs. TT | 12.99 (1.86) | 7.79, 18.19 | <0.001 | |
| TC-post | T vs. C | −2.14 (2.51) | −9.16, 4.88 | 1.000 |
| TP vs. C | −2.34 (2.39) | −9.03, 4.35 | 1.000 | |
| TT vs. C | −14.94 (2.72) | −22.55, −7.32 | <0.001 | |
| T vs. TP | 0.20 (2.59) | −7.04, 7.44 | 1.000 | |
| T vs. TT | 12.80 (2.44) | 5.98, 19.62 | <0.001 | |
| TP vs. TT | 12.60 (2.83) | 4.67, 20.52 | 0.001 | |
| LDL-post | T vs. C | −5.84 (2.10) | −11.74, 0.061 | 0.054 |
| TP vs. C | −9.64 (2.08) | −15.47, −3.82 | <0.001 | |
| TT vs. C | −12.38 (2.20) | −18.54, −6.21 | <0.001 | |
| T vs. TP | 3.80 (2.11) | −2.09, 9.72 | 0.479 | |
| T vs. TT | 6.54 (2.12) | 0.61, 12.47 | 0.024 | |
| TP vs. TT | 2.73 (2.20) | −3.43, 8.90 | 1.000 | |
| Insulin-post | T vs. C | −1.14 (0.27) | −1.89, −0.38 | 0.001 |
| TP vs. C | −1.07 (0.28) | −1.86, −0.28 | 0.003 | |
| TT vs. C | −1.46 (0.27) | −2.27, −0.72 | <0.001 | |
| T vs. TP | −0.06 (0.27) | −0.82, 0.69 | 1.000 | |
| T vs. TT | 0.35 (0.26) | −0.39, 1.10 | 1.000 | |
| TP vs. TT | 0.42 (0.26) | −0.32, 1.17 | 0.751 |
| Variables | Group | Pre-Test | Post-Test | p-Value |
|---|---|---|---|---|
| Energy intake (kcal/day) | T | 2038 ± 285.2 | 1940.9 ± 263.8 | 0.434 |
| TP | 1954.9 ± 193 | 1904.3 ± 216.7 | 0.617 | |
| TT | 1945.3 ± 264.1 | 1858.6 ± 287.4 | 0.522 | |
| C | 1962.4 ± 238.8 | 2004.1 ± 338.1 | 0.433 | |
| Carbohydrate (g/day) | T | 235.8 ± 41.1 | 224.9 ± 38.7 | 0.392 |
| TP | 221.9 ± 31.6 | 219.4 ± 41.8 | 0.878 | |
| TT | 219.7 ± 35.1 | 212.5 ± 43.9 | 0.697 | |
| C | 228 ± 23.3 | 233.8 ± 38.5 | 0.560 | |
| Protein (g/day) | T | 67.4 ± 14 | 66 ± 13.1 | 0.689 |
| TP | 66.9 ± 12.4 | 63.4 ± 13.7 | 0.310 | |
| TT | 63.6 ± 13.3 | 59.8 ± 10.7 | 0.532 | |
| C | 64.4 ± 13.4 | 65 ± 14.3 | 0.853 | |
| Fat (g/day) | T | 91.6 ± 20.1 | 86.2 ± 20.1 | 0.580 |
| TP | 88.8 ± 13.9 | 85.8 ± 13.4 | 0.671 | |
| TT | 90.2 ± 13.1 | 85.4 ± 18.1 | 0.486 | |
| C | 88 ± 19.7 | 89.8 ± 21.1 | 0.624 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samadpour Masouleh, S.; Bagheri, R.; Ashtary-Larky, D.; Cheraghloo, N.; Wong, A.; Yousefi Bilesvar, O.; Suzuki, K.; Siahkouhian, M. The Effects of TRX Suspension Training Combined with Taurine Supplementation on Body Composition, Glycemic and Lipid Markers in Women with Type 2 Diabetes. Nutrients 2021, 13, 3958. https://doi.org/10.3390/nu13113958
Samadpour Masouleh S, Bagheri R, Ashtary-Larky D, Cheraghloo N, Wong A, Yousefi Bilesvar O, Suzuki K, Siahkouhian M. The Effects of TRX Suspension Training Combined with Taurine Supplementation on Body Composition, Glycemic and Lipid Markers in Women with Type 2 Diabetes. Nutrients. 2021; 13(11):3958. https://doi.org/10.3390/nu13113958
Chicago/Turabian StyleSamadpour Masouleh, Shohreh, Reza Bagheri, Damoon Ashtary-Larky, Neda Cheraghloo, Alexei Wong, Omid Yousefi Bilesvar, Katsuhiko Suzuki, and Marefat Siahkouhian. 2021. "The Effects of TRX Suspension Training Combined with Taurine Supplementation on Body Composition, Glycemic and Lipid Markers in Women with Type 2 Diabetes" Nutrients 13, no. 11: 3958. https://doi.org/10.3390/nu13113958
APA StyleSamadpour Masouleh, S., Bagheri, R., Ashtary-Larky, D., Cheraghloo, N., Wong, A., Yousefi Bilesvar, O., Suzuki, K., & Siahkouhian, M. (2021). The Effects of TRX Suspension Training Combined with Taurine Supplementation on Body Composition, Glycemic and Lipid Markers in Women with Type 2 Diabetes. Nutrients, 13(11), 3958. https://doi.org/10.3390/nu13113958









