How to Tackle the Relationship between Autoimmune Diseases and Diet: Well Begun Is Half-Done
Abstract
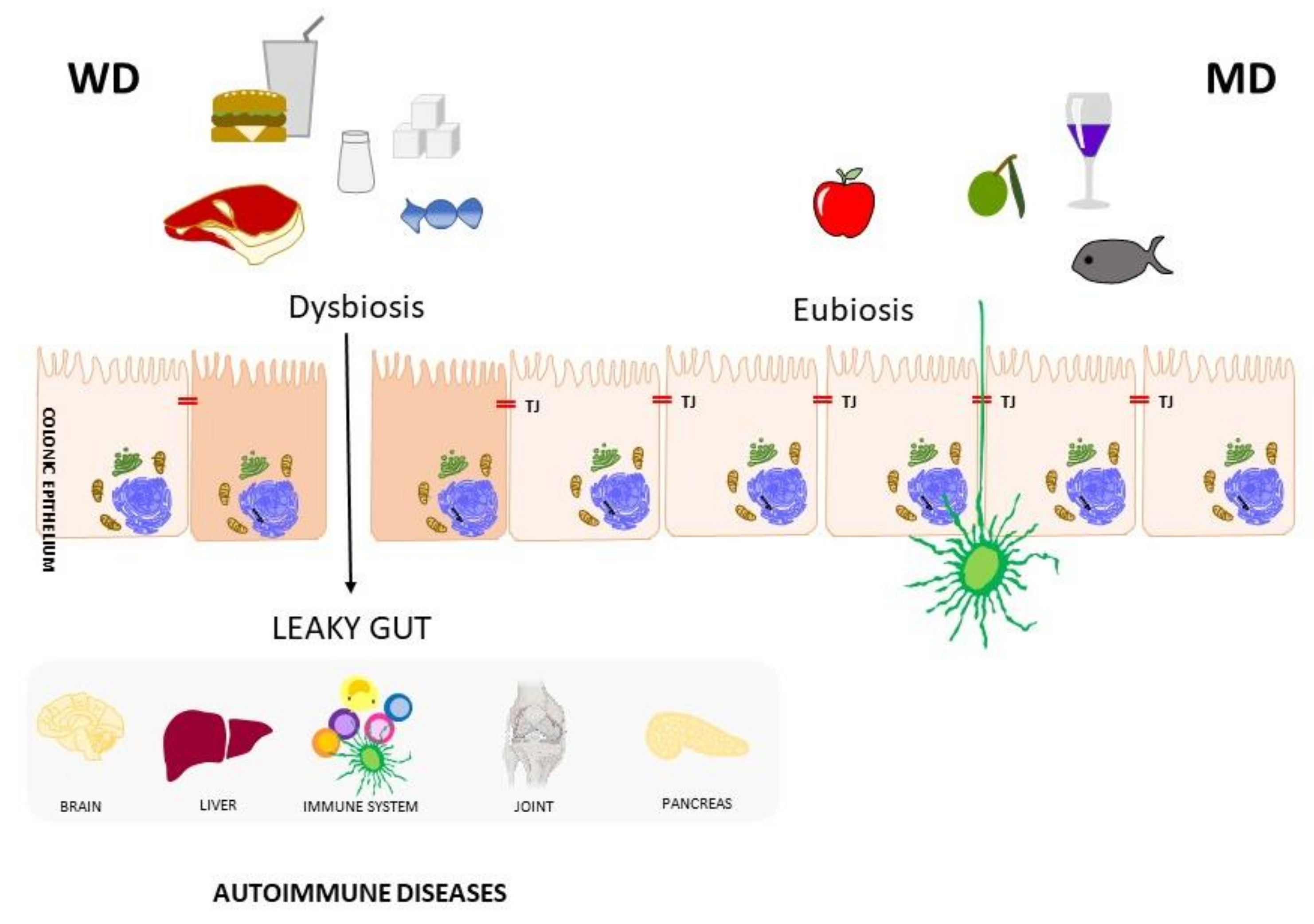
1. Diet and Autoimmune diseases
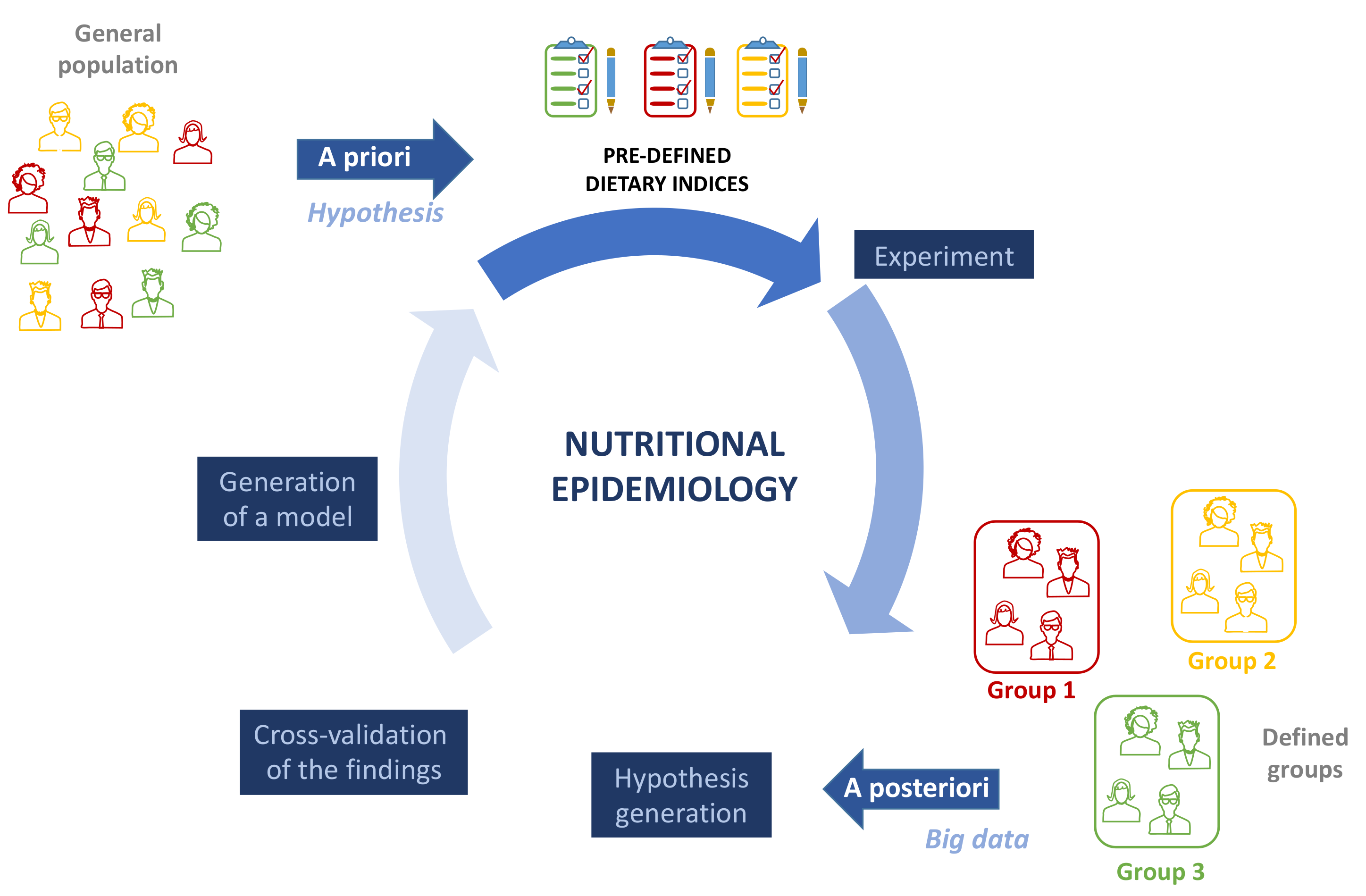
2. Starting from the Right Tools
3. Dietary Patterns and Evaluation Methods
4. Many MD Scores, Few WD Scores
5. Need for a Double Score
6. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Çehreli, R. Moleculer nutritional immunology and cancer. J. Oncol. Sci. 2018, 4, 40–46. [Google Scholar] [CrossRef]
- Platt, A.M. Immunity in the Gut. Viral Gastroenteritis 2017, 351, 1329–1333. [Google Scholar]
- Lerner, A.; Matthias, T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun. Rev. 2015, 14, 479–489. [Google Scholar] [CrossRef]
- Venter, C.; Eyerich, S.; Sarin, T.; Klatt, K.C. Nutrition and the immune system: A complicated tango. Nutrients 2020, 12, 818. [Google Scholar] [CrossRef]
- Rose, N.R. Prediction and prevention of autoimmune disease in the 21st century: A review and preview. Am. J. Epidemiol. 2016, 183, 403–406. [Google Scholar] [CrossRef]
- De Luca, F.; Shoenfeld, Y. The microbiome in autoimmune diseases. Clin. Exp. Immunol. 2019, 195, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Kuhn, C.; Feillet, H.; Bach, J.-F. The “hygiene hypothesis” for autoimmune and allergic diseases: An update. Clin. Exp. Immunol. 2010, 160, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Selmi, C. The worldwide gradient of autoimmune conditions. Autoimmun. Rev. 2010, 9, A247–A250. [Google Scholar] [CrossRef] [PubMed]
- Brady, B.D.M. Autoimmune disease: A modern epidemic? Molecular mimicry, the hygiene hypothesis, stealth infections, and other examples of disconnect between medical research and the practice of clinical medicine. N. Engl. J. Med. 2014, 347, 911–920. [Google Scholar] [CrossRef]
- Fatoye, F.; Gebrye, T.; Svenson, L.W. Real-world incidence and prevalence of systemic lupus erythematosus in Alberta, Canada. Rheumatol. Int. 2018, 38, 1721–1726. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Shea-Donohue, T. Mechanisms of disease: The role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005, 2, 416–422. [Google Scholar] [CrossRef]
- Buckland, G.; Agudo, A.; Travier, N.; Huerta, J.M.; Cirera, L.; Tormo, M.-J.; Navarro, C.; Chirlaque, M.D.; Moreno-Iribas, C.; Ardanaz, E.; et al. Adherence to the Mediterranean diet reduces mortality in the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain). Br. J. Nutr. 2011, 106, 1581–1591. [Google Scholar] [CrossRef]
- Naska, A.; Trichopoulou, A. Back to the future: The Mediterranean diet paradigm. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 216–219. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.C.; Louzada, M.L.C.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Varlamov, O. Western-style diet, sex steroids and metabolism. Biochim. Biophys. Acta -Mol. Basis Dis. 2017, 1863, 1147–1155. [Google Scholar] [CrossRef]
- Gioia, C.; Lucchino, B.; Tarsitano, M.G.; Iannuccelli, C.; Di Franco, M. Dietary habits and nutrition in rheumatoid arthritis: Can diet influence disease development and clinical manifestations? Nutrients 2020, 12, 1456. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 2016, 167, 1339–1353. [Google Scholar] [CrossRef] [PubMed]
- Maione, F.; Cappellano, G.; Bellan, M.; Raineri, D.; Chiocchetti, A. Chicken-or-egg question: Which came first, extracellular vesicles or autoimmune diseases? J. Leukoc. Biol. 2020, 108, 601–616. [Google Scholar] [CrossRef] [PubMed]
- Munger, K.L.; Levin, L.I.; Hollis, B.W.; Howard, N.S.; Ascherio, A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006, 296, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- Christ, A.; Lauterbach, M.; Latz, E. Western diet and the immune system: An inflammatory connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef]
- Huang, S.; Rutkowsky, J.M.; Snodgrass, R.G.; Ono-Moore, K.D.; Schneider, D.A.; Newman, J.W.; Adams, S.H.; Hwang, D.H. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J. Lipid Res. 2012, 53, 2002–2013. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, S.; Bogie, J.F.J.; Vanmierlo, T.; Lütjohann, D.; Stinissen, P.; Hellings, N.; Hendriks, J.J.A. High fat diet exacerbates neuroinflammation in an animal model of multiple sclerosis by activation of the renin angiotensin system. J. Neuroimmune Pharmacol. 2014, 9, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Katz Sand, I. The role of diet in multiple sclerosis: Mechanistic connections and current evidence. Curr. Nutr. Rep. 2018, 7, 150–160. [Google Scholar] [CrossRef]
- Hagen, K.B.; Byfuglien, M.G.; Falzon, L.; Olsen, S.U.; Smedslund, G. Dietary interventions for rheumatoid arthritis. Cochrane Database Syst. Rev. 2009. [Google Scholar] [CrossRef]
- Cena, H.; Calder, P.C. Defining a healthy diet: Evidence for the role of contemporary dietary patterns in health and disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Sánchez-Villegas, A. The emerging role of Mediterranean diets in cardiovascular epidemiology: Monounsaturated fats, olive oil, red wine or the whole pattern? Eur. J. Epidemiol. 2004, 19, 9–13. [Google Scholar] [CrossRef]
- Forsyth, C.; Kouvari, M.; D’Cunha, N.M.; Georgousopoulou, E.N.; Panagiotakos, D.B.; Mellor, D.D.; Kellett, J.; Naumovski, N. The effects of the Mediterranean diet on rheumatoid arthritis prevention and treatment: A systematic review of human prospective studies. Rheumatol. Int. 2018, 38, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the mediterranean diet: A literature review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.; Sjöblom, H.; Gjertsson, I.; Ulven, S.M.; Lindqvist, H.M.; Bärebring, L. Do interventions with diet or dietary supplements reduce the disease activity score in rheumatoid arthritis? A systematic review of randomized controlled trials. Nutrients 2020, 12, 2991. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.M.; Ma, D.W.L. Are all n-3 polyunsaturated fatty acids created equal? Lipids Health Dis. 2009, 8, 1–20. [Google Scholar] [CrossRef]
- Statovci, D.; Aguilera, M.; MacSharry, J.; Melgar, S. The impact of western diet and nutrients on the microbiota and immune response at mucosal interfaces. Front. Immunol. 2017, 8, 838. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; de Roos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Strate, L.L.; Keeley, B.R.; Cao, Y.; Wu, K.; Giovannucci, E.L.; Chan, A.T. Western dietary pattern increases, and prudent dietary pattern decreases, risk of incident diverticulitis in a prospective cohort study. Gastroenterology 2017, 152, 1023–1030. [Google Scholar] [CrossRef]
- Bennett, E.; Peters, S.A.E.; Woodward, M. Sex differences in macronutrient intake and adherence to dietary recommendations: Findings from the UK Biobank. BMJ Open 2018, 8, e020017. [Google Scholar] [CrossRef]
- Zaccardelli, A.; Friedlander, H.M.; Ford, J.A.; Sparks, J.A. Potential of lifestyle changes for reducing the risk of developing rheumatoid arthritis: Is an ounce of prevention worth a pound of cure? Clin. Ther. 2019, 41, 1323–1345. [Google Scholar] [CrossRef]
- Sharma, M.; Rao, M.; Jacob, S.; Jacob, C.K. Validation of 24-hour dietary recall: A study in hemodialysis patients. J. Ren. Nutr. 1998, 8, 199–202. [Google Scholar] [CrossRef]
- Steinemann, N.; Grize, L.; Ziesemer, K.; Kauf, P.; Probst-Hensch, N.; Brombach, C. Relative validation of a food frequency questionnaire to estimate food intake in an adult population. Food Nutr. Res. 2017, 61, 1305193. [Google Scholar] [CrossRef]
- Fatihah, F.; Ng, B.K.; Hazwanie, H.; Karim Norimah, A.; Shanita, S.N.; Ruzita, A.T.; Poh, B.K. Development and validation of a food frequency questionnaire for dietary intake assessment among multi-ethnic primary school-aged children. Singap. Med. J. 2015, 56, 687–694. [Google Scholar] [CrossRef]
- Affret, A.; El Fatouhi, D.; Dow, C.; Correia, E.; Boutron-Ruault, M.C.; Fagherazzi, G. Relative validity and reproducibility of a new 44-item diet and food frequency questionnaire among adults: Online assessment. J. Med. Internet Res. 2018, 20, e9113. [Google Scholar]
- García-Conesa, M.T.; Philippou, E.; Pafilas, C.; Massaro, M.; Quarta, S.; Andrade, V.; Jorge, R.; Chervenkov, M.; Ivanova, T.; Dimitrova, D.; et al. Exploring the validity of the 14-item mediterranean diet adherence screener (Medas): A cross-national study in seven european countries around the mediterranean region. Nutrients 2020, 12, 2960. [Google Scholar] [CrossRef]
- Turconi, G.; Celsa, M.; Rezzani, C.; Biino, G.; Sartirana, M.A.; Roggi, C. Reliability of a dietary questionnaire on food habits, eating behaviour and nutritional knowledge of adolescents. Eur. J. Clin. Nutr. 2003, 57, 753–763. [Google Scholar] [CrossRef]
- Goldbohm, R.A.; Van’t Veer, P.; Van den Brandt, P.A.; Van’t Hof, M.A.; Brants, H.A.M.; Sturmans, F.; Hermus, R.J.J. Reproducibility of a food frequency questionnaire and stability of dietary habits determined from five annually repeated measurements. Eur. J. Clin. Nutr. 1995, 49, 420–429. [Google Scholar] [PubMed]
- Riboli, E.; Kaaks, R. The EPIC project: Rationale and study design. European Prospective Investigation into Cancer and Nutrition. Int. J. Epidemiol. 1997, 26, S6–S14. [Google Scholar] [CrossRef] [PubMed]
- Slimani, N.; Deharveng, G.; Unwin, I.; Southgate, D.A.T.; Vignat, J.; Skeie, G.; Salvini, S.; Parpinel, M.; Møller, A.; Ireland, J.; et al. The EPIC nutrient database project (ENDB): A first attempt to standardize nutrient databases across the 10 European countries participating in the EPIC study. Eur. J. Clin. Nutr. 2007, 61, 1037–1056. [Google Scholar] [CrossRef] [PubMed]
- de Pablo, P.; Romaguera, D.; Fisk, H.L.; Calder, P.C.; Quirke, A.-M.; Cartwright, A.J.; Panico, S.; Mattiello, A.; Gavrila, D.; Navarro, C.; et al. High erythrocyte levels of the n-6 polyunsaturated fatty acid linoleic acid are associated with lower risk of subsequent rheumatoid arthritis in a southern European nested case—control study. Ann. Rheum. Dis. 2018, 77, 981–987. [Google Scholar] [CrossRef]
- Vagnani, S.; Tani, C.; Carli, L.; Querci, F.; Della Rossa, A.; D’Ascanio, A.; Ermini, I.; Ceroti, M.; Caini, S.; Palli, D.; et al. Nutritional assessment in patients with systemic lupus erythematosus and systemic sclerosis. Arthritis Rheumatol. 2014, 66, S1061–S1062. [Google Scholar] [CrossRef]
- Keeble, M.; Adams, J.; Sacks, G.; Vanderlee, L.; White, C.M.; Hammond, D.; Burgoine, T. Use of online food delivery services to order food prepared away-from-home and associated sociodemographic characteristics: A cross-sectional, multi-country analysis. Int. J. Environ. Res. Public Health 2020, 17, 5190. [Google Scholar] [CrossRef] [PubMed]
- Salgado, E.; Bes-Rastrollo, M.; de Irala, J.; Carmona, L.; Gómez-Reino, J.J. High sodium intake is associated with self-reported rheumatoid arthritis: A cross sectional and case control analysis within the SUN cohort. Medicine 2015, 94, e924. [Google Scholar] [CrossRef] [PubMed]
- McGuire, S.; Todd, J.E.; Mancino, L.; Lin, B.; Jessica, E. The impact of food away from home on adult diet quality. USDA-ERS Econ. Res. Rep. Pap. Adv. Nutr. 2011, 2, 442–443. [Google Scholar] [CrossRef] [PubMed]
- Demmler, K.M.; Klasen, S.; Nzuma, J.M.; Qaim, M. Supermarket purchase contributes to nutrition-related non-communicable diseases in urban Kenya. PLoS ONE 2017, 12, e0185148. [Google Scholar] [CrossRef]
- Heidemann, C.; Schulze, M.B.; Franco, O.H.; van Dam, R.M.; Mantzoros, C.S.; Hu, F.B. Dietary patterns and risk of mortality from cardiovascular disease, cancer, and all causes in a prospective cohort of women. Circulation 2008, 118, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.V.; Diez Roux, A.V.; Nettleton, J.A.; Jacobs, D.R.; Franco, M. Fast-food consumption, diet quality, and neighborhood exposure to fast food: The multi-ethnic study of atherosclerosis. Am. J. Epidemiol. 2009, 170, 29–36. [Google Scholar] [CrossRef]
- Ramezankhani, A.; Hosseini-Esfahani, F.; Mirmiran, P.; Azizi, F.; Hadaegh, F. The association of priori and posteriori dietary patterns with the risk of incident hypertension: Tehran Lipid and Glucose Study. J. Transl. Med. 2021, 19, 1–11. [Google Scholar] [CrossRef]
- Newby, P.K.; Tucker, K.L. Empirically derived eating patterns using factor or cluster analysis: A review. Nutr. Rev. 2004, 62, 177–203. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Rimm, E.; Smith-Warner, S.A.; Feskanich, D.; Stampfer, M.J.; Ascherio, A.; Sampson, L.; Willett, W.C. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am. J. Clin. Nutr. 1999, 69, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C. α-priori and α-posterior dietary pattern analyses have similar estimating and discriminating ability in predicting 5-y incidence of cardiovascular disease: Methodological issues in nutrition assessment. J. Food Sci. 2009, 74, H218–H224. [Google Scholar] [CrossRef] [PubMed]
- Leermakers, E.T.M.; van den Hooven, E.H.; Franco, O.H.; Jaddoe, V.W.V.; Moll, H.A.; Kiefte-de Jong, J.C.; Voortman, T. A priori and a posteriori derived dietary patterns in infancy and cardiometabolic health in childhood: The role of body composition. Clin. Nutr. 2018, 37, 1589–1595. [Google Scholar] [CrossRef]
- Khaled, K.; Hundley, V.; Almilaji, O.; Koeppen, M.; Tsofliou, F. A priori and a posteriori dietary patterns in women of childbearing age in the UK. Nutrients 2020, 12, 2921. [Google Scholar] [CrossRef]
- Serra-Majem, L.; Ribas, L.; Ngo, J.; Ortega, R.M.; García, A.; Pérez-Rodrigo, C.; Aranceta, J. Food, youth and the Mediterranean diet in Spain. Development of KIDMED, Mediterranean Diet Quality Index in children and adolescents. Public Health Nutr. 2004, 7, 931–935. [Google Scholar] [CrossRef]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health status: An updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2013, 17, 2769–2782. [Google Scholar] [CrossRef]
- Monteagudo, C.; Mariscal-Arcas, M.; Rivas, A.; Lorenzo-Tovar, M.L.; Tur, J.A.; Olea-Serrano, F. Proposal of a Mediterranean diet serving score. PLoS ONE 2015, 10, e0128594. [Google Scholar] [CrossRef]
- Hodge, A.; Bassett, J. What can we learn from dietary pattern analysis? Public Health Nutr. 2016, 19, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Solans, M.; Benavente, Y.; Saez, M.; Agudo, A.; Naudin, S.; Hosnijeh, F.S.; Noh, H.; Freisling, H.; Ferrari, P.; Besson, C.; et al. Adherence to the Mediterranean diet and lymphoma risk in the European prospective investigation into cancer and nutrition. Int. J. Cancer 2019, 145, 122–131. [Google Scholar] [CrossRef]
- Shannon, O.M.; Stephan, B.C.M.; Granic, A.; Lentjes, M.; Hayat, S.; Mulligan, A.; Brayne, C.; Khaw, K.-T.; Bundy, R.; Aldred, S.; et al. Mediterranean diet adherence and cognitive function in older UK adults: The European Prospective Investigation into Cancer and Nutrition-Norfolk (EPIC-Norfolk). Study. Am. J. Clin. Nutr. 2019, 110, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Orfanos, P.; Norat, T.; Bueno-de-Mesquita, B.; Ocké, M.C.; Peeters, P.H.M.; van der Schouw, Y.T.; Boeing, H.; Hoffmann, K.; Boffetta, P.; et al. Modified Mediterranean diet and survival: EPIC-elderly prospective cohort study. BMJ 2005, 330, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Dinu, M.; Pagliai, G.; Marcucci, R.; Casini, A. Validation of a literature-based adherence score to Mediterranean diet: The MEDI-LITE score. Int. J. Food Sci. Nutr. 2017, 68, 757–762. [Google Scholar] [CrossRef]
- Shakersain, B.; Santoni, G.; Larsson, S.C.; Faxén-Irving, G.; Fastbom, J.; Fratiglioni, L.; Xu, W. Prudent diet may attenuate the adverse effects of Western diet on cognitive decline. Alzheimer’s Dement. 2016, 12, 100–109. [Google Scholar] [CrossRef]
- Cunnane, S.C. Origins and evolution of the Western diet: Implications of iodine and seafood intakes for the human brain. Am. J. Clin. Nutr. 2005, 82, 483. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzucca, C.B.; Raineri, D.; Cappellano, G.; Chiocchetti, A. How to Tackle the Relationship between Autoimmune Diseases and Diet: Well Begun Is Half-Done. Nutrients 2021, 13, 3956. https://doi.org/10.3390/nu13113956
Mazzucca CB, Raineri D, Cappellano G, Chiocchetti A. How to Tackle the Relationship between Autoimmune Diseases and Diet: Well Begun Is Half-Done. Nutrients. 2021; 13(11):3956. https://doi.org/10.3390/nu13113956
Chicago/Turabian StyleMazzucca, Camilla Barbero, Davide Raineri, Giuseppe Cappellano, and Annalisa Chiocchetti. 2021. "How to Tackle the Relationship between Autoimmune Diseases and Diet: Well Begun Is Half-Done" Nutrients 13, no. 11: 3956. https://doi.org/10.3390/nu13113956
APA StyleMazzucca, C. B., Raineri, D., Cappellano, G., & Chiocchetti, A. (2021). How to Tackle the Relationship between Autoimmune Diseases and Diet: Well Begun Is Half-Done. Nutrients, 13(11), 3956. https://doi.org/10.3390/nu13113956








