Relationship between Nutritional Screening Tools and GLIM in Complicated IBD Requiring Surgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Design
- (1)
- Anthropometric parameters (consistent with GLIM phenotypic criteria)
- -
- BMI: values under <18.5 kg/m2 are associated with poor outcome and higher mortality rates [13,14]. GLIM BMI cut-off for malnutrition risk is <20 kg/m2. In older adults, the cut-off for the definition of underweight is higher (<22 kg/m2) as carrying some extra weight seems to be protective in this population [6,15];
- -
- (2)
- Disease-related parameters (consistent with GLIM etiologic criteria)
- -
- -
2.2. Statistical Analysis
3. Results
3.1. Demographics and Clinical Characteristics of IBD Patients
3.2. Nutritional Characteristics of IBD Patients
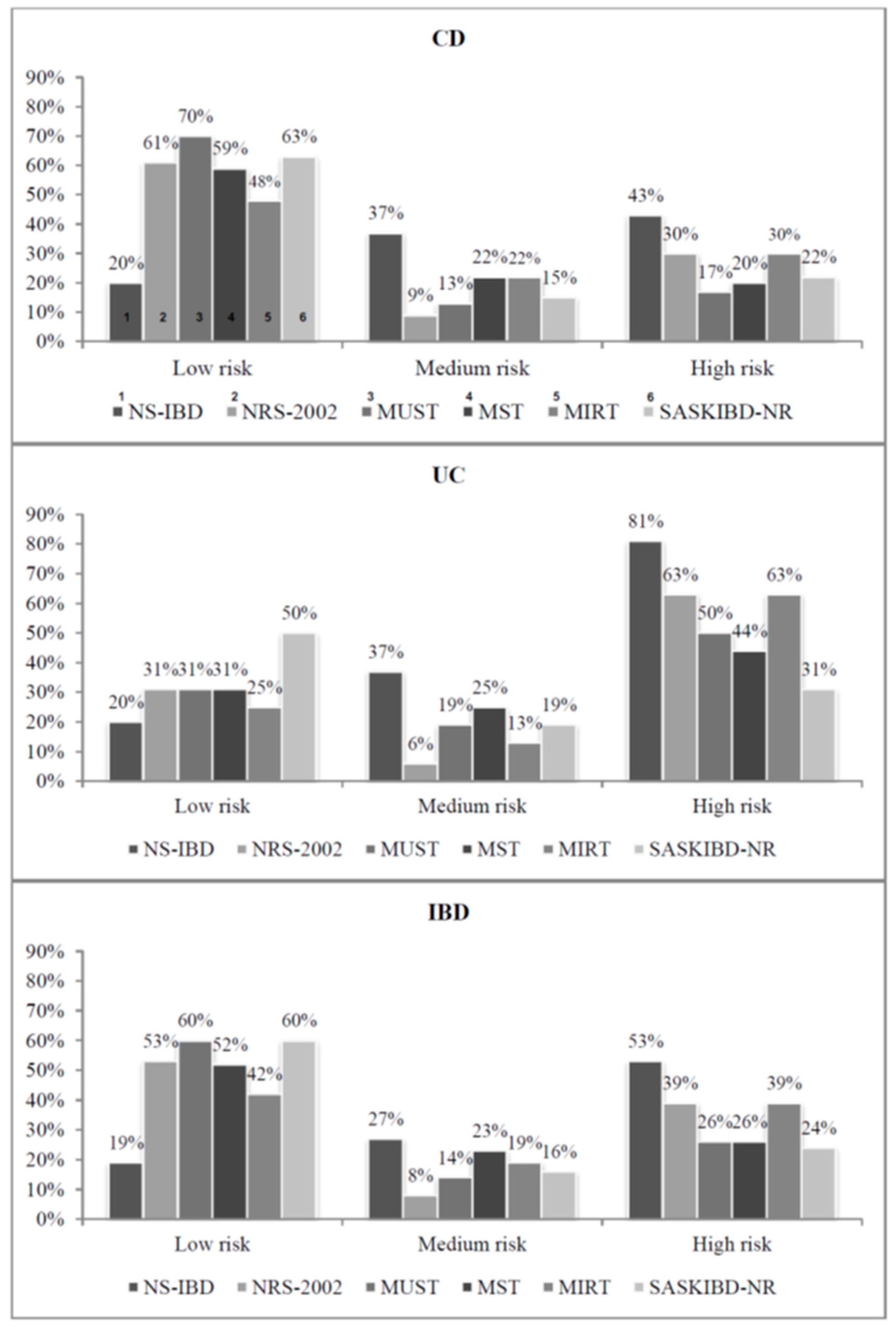
3.3. Prevalence of Nutritional Risk
3.4. Screening Tests Agreement
3.5. Reliability of the NS-IBD and Other Screening Tests with GLIM Malnutrition Diagnosis
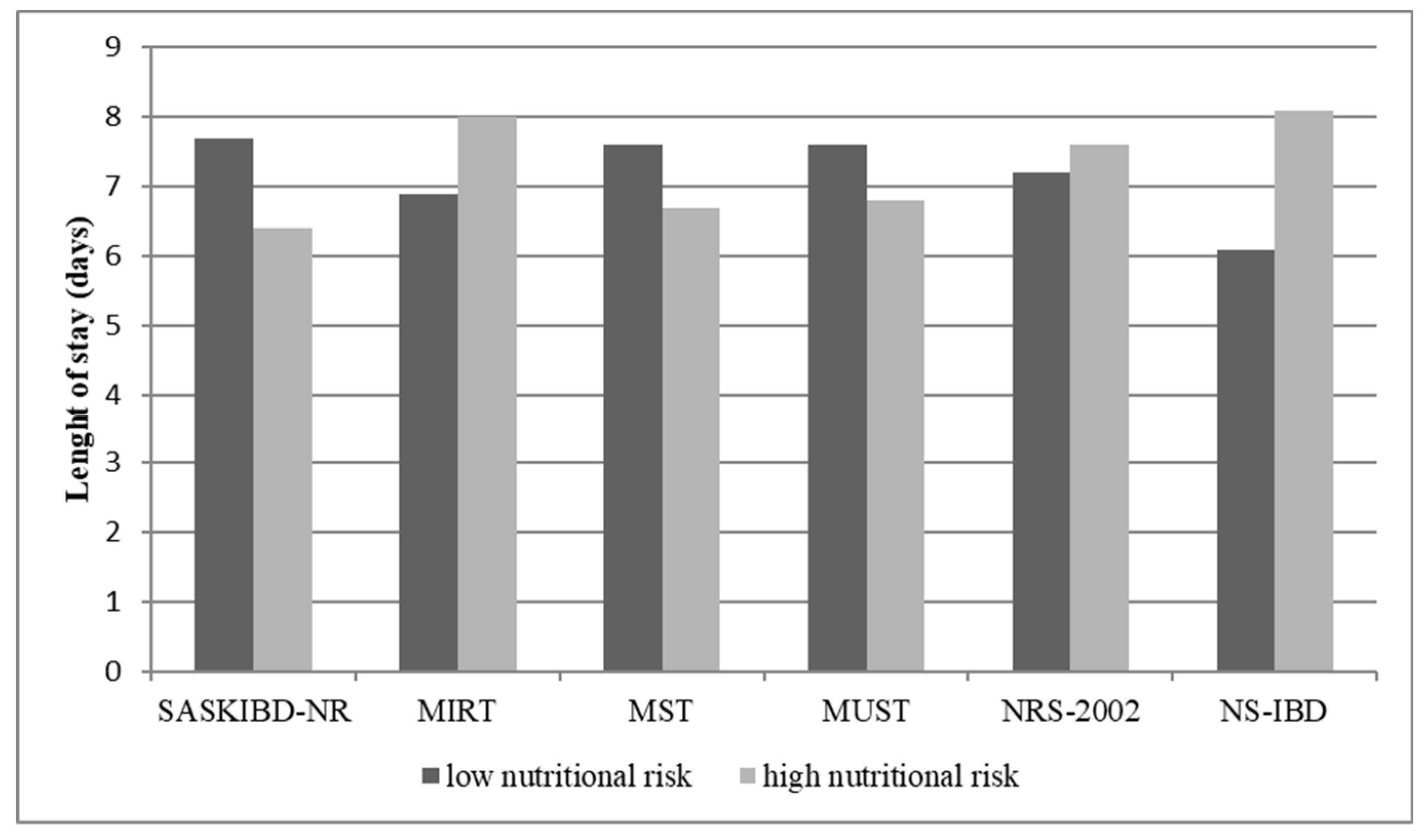
3.6. Postoperative Length of Stay and Nutritional Risk
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Forbes, A.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN guideline: Clinical nutrition in inflammatory bowel disease. Clin. Nutr. 2016, 36, 321–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Power, L.; Mullally, D.; Gibney, E.R.; Clarke, M.; Visser, M.; Volkert, D.; Bardon, L.; De Van Der Schueren, M.A.; Corish, C.A. A review of the validity of malnutrition screening tools used in older adults in community and healthcare settings—A MaNuEL study. Clin. Nutr. ESPEN 2018, 24, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Suqing, L. Systematic review of nutrition screening and assessment in inflammatory bowel disease. World J. Gastroenterol. 2019, 25, 3823–3837. [Google Scholar] [CrossRef]
- Goh, J.; O’Morain, C.A. Review article: Nutrition and adult inflammatory bowel disease. Aliment. Pharmacol. Ther. 2003, 17, 307–320. [Google Scholar] [CrossRef]
- Wedrychowicz, A.; Zając, A.; Tomasik, P. Advances in nutritional therapy in inflammatory bowel diseases: Review. World J. Gastroenterol. 2016, 22, 1045–1066. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2018, 38, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorindi, C.; Luceri, C.; Dragoni, G.; Piemonte, G.; Scaringi, S.; Staderini, F.; Nannoni, A.; Ficari, F.; Giudici, F. GLIM Criteria for Malnutrition in Surgical IBD Patients: A Pilot Study. Nutrients 2020, 12, 2222. [Google Scholar] [CrossRef]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M. Educational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN). ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef]
- Weekes, C.E.; Elia, M.; Emery, P.W. The development, validation and reliability of a nutrition screening tool based on the recommendations of the British Association for Parenteral and Enteral Nutrition (BAPEN). Clin. Nutr. 2004, 23, 1104–1112. [Google Scholar] [PubMed]
- Ferguson, M.; Capra, S.; Bauer, J.; Banks, M. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition 1999, 15, 458–464. [Google Scholar] [CrossRef]
- Jansen, I.; Prager, M.; Valentini, L.; Büning, C. Inflammation-driven malnutrition: A new screening tool predicts outcome in Crohn’s disease. Br. J. Nutr. 2016, 116, 1061–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haskey, N.; Peña-Sánchez, J.N.; Jones, J.L.; Fowler, S.A. Development of a screening tool to detect nutrition risk in patients with inflammatory bowel disease. Asia Pac. J. Clin. Nutr. 2018, 27, 756–762. [Google Scholar] [CrossRef]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritolig, M.; Nyulasih, I.; Ockenga, J.; Schneider, S.M.; et al. Diagnostic criteria for malnutrition e an ESPEN consensus statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Sen, A.; Prasad, M.; Norat, T.; Janszky, I.; Tonstad, S.; Romundstad, P.; Vatten, L.J. BMI and all cause mortality: Systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ 2016, 353, i2156. [Google Scholar] [CrossRef] [Green Version]
- Winter, J.E.; MacInnis, R.J.; Nowson, C.A. The influence of age on the BMI and all-cause mortality association: A meta-analysis. J. Nutr. Health Aging 2016, 21, 1254–1258. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.D.; Newman, A.B.; Hirsch, C.; Gottdiener, J.S.; Seeman, T.E.; Tracy, R.P.; Kop, W.J.; Burke, G.L.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Nguyen, G.C.; Munsell, M.; Harris, M.L. Nationwide prevalence and prognostic significance of clinically diagnosable protein-calorie malnutrition in hospitalized inflammatory bowel disease patients. Inflamm. Bowel Dis. 2008, 14, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Prince, A.; Whelan, K.; Moosa, A.; Lomer, M.C.; Reidlinger, D.P. Nutritional problems in inflammatory bowel disease: The patient perspective. J. Crohn’s Colitis 2011, 5, 443–450. [Google Scholar] [CrossRef] [Green Version]
- Casanova, M.J.; Chaparro, M.; Molina, B.; Merino, O.; Batanero, R.; Dueñas-Sadornil, C.; Robledo, P.; Garcia-Albert, A.M.; Gómez-Sánchez, M.B.; Calvet, X.; et al. Prevalence of Malnutrition and Nutritional Characteristics of Patients With Inflammatory Bowel Disease. J. Crohn’s Colitis 2017, 11, 1430–1439. [Google Scholar] [CrossRef]
- Pironi, L.; Arends, J.; Baxter, J.; Bozzetti, F.; Peláez, R.B.; Cuerda, C.; Forbes, A.; Gabe, S.; Gillanders, L.; Holst, M.; et al. ESPEN endorsed recommendations. Definition and classification of intestinal failure in adults. Clin. Nutr. 2015, 34, 171–180. [Google Scholar] [CrossRef]
- Hart, J.W.; Wootton, S.A.; Bremner, A.R.; Beattie, R.M. P0779 measured versus predicted energy expenditure in children with inactive crohn’s disease. J. Pediatr. Gastroenterol. Nutr. 2004, 39, S352–S353. [Google Scholar] [CrossRef]
- Steenhagen, E.; de Roos, N.M.; Bouwman, C.A.; van Laarhoven, C.J.; van Staveren, W.A. Sources and Severity of Self-Reported Food Intolerance after Ileal Pouch–Anal Anastomosis. J. Am. Diet. Assoc. 2006, 106, 1459–1462. [Google Scholar] [CrossRef] [PubMed]
- Hebuterne, X.; Filippi, J.; Al-Jaouni, R.; Schneider, S. Nutritional consequences and nutrition therapy in Crohn’s disease. Gastroenterol. Clin. Biol. 2009, 33 (Suppl. 3), S235–S244. [Google Scholar] [CrossRef]
- Lucendo, A.J.; De Rezende, L.C. Importance of nutrition in inflammatory bowel disease. World J. Gastroenterol. 2009, 15, 2081–2088. [Google Scholar] [CrossRef] [PubMed]
- Skipper, A.; Ferguson, M.; Thompson, K.; Castellanos, V.H.; Porcari, J. Nutrition screening tools: An analysis of the evidence. J. Parenter Enter. Nutr. 2012, 36, 292–298. [Google Scholar] [CrossRef]
- Schueren, M.A.V.B.-D.V.D.; Guaitoli, P.R.; Jansma, E.P.; De Vet, H.C. Nutrition screening tools: Does one size fit all? A systematic review of screening tools for the hospital setting. Clin. Nutr. 2014, 33, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Ljungqvist, O.; Scott, M.; Fearon, K.C. Enhanced Recovery After Surgery: A Review. JAMA Surg. 2017, 152, 292–298. [Google Scholar] [CrossRef]
- Tiberi, A.; Pesi, B.; Giudici, F.; Zambonin, D.; Nelli, T.; Cupellini, C.; Ficari, F.; Cianchi, F.; Scaringi, S. Laparoscopic ileo-colic resection and right hemicolectomy for Crohn’s disease and colon cancer: A preliminary comparative study on post-operative outcome. Updat. Surg. 2020, 72, 821–826. [Google Scholar] [CrossRef]
- Tonelli, F.; Giudici, F.; Di Martino, C.; Scaringi, S.; Ficari, F.; Addasi, R. Outcome after ileal pouch-anal anastomosis in ulcerative colitis patients: Experience during a 27-year period. ANZ J. Surg. 2016, 86, 768–772. [Google Scholar] [CrossRef]
- Ladd, M.R.; Garcia, A.V.; Leeds, I.; Haney, C.; Oliva-Hemker, M.M.; Alaish, S.; Boss, E.; Rhee, D.S. Malnutrition increases the risk of 30-day complications after surgery in pediatric patients with Crohn disease. J. Pediatr. Surg. 2018, 53, 2336–2345. [Google Scholar] [CrossRef]
- Vaisman, N.; Dotan, I.; Halack, A.; Niv, E. Malabsorption is a major contributor to underweight in Crohn’s disease patients in remission. Nutrition 2006, 22, 855–859. [Google Scholar] [CrossRef]
- Yadav, D.P.; Kedia, S.; Madhusudhan, K.S.; Bopanna, S.; Goyal, S.; Jain, S.; Vikram, N.K.; Sharma, R.; Makharia, G.K.; Ahuja, V. Body Composition in Crohn’s Disease and Ulcerative Colitis: Correlation with Disease Severity and Duration. Can. J. Gastroenterol. Hepatol. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skeie, E.; Tangvik, R.J.; Nymo, L.S.; Harthug, S.; Lassen, K.; Viste, A. Weight loss and BMI criteria in GLIM’s definition of malnutrition is associated with postoperative complications following abdominal resections—Results from a National Quality Registry. Clin Nutr. 2020, 39, 1593–1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leuenberger, M.S.; Joray, M.L.; Kurmann, S.; Stanga, Z. How to assess the nutritional status of my patient. Praxis 2012, 101, 307–315. [Google Scholar] [CrossRef] [PubMed]

| BMI | Weight Loss | Reduced Food Intake | Severity of Disease | CRP | GI Symptoms ** | AGE | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NRS-2002 | Score 1 | x | Score 1 | >5% in 3 months | Score 1 | Intake of 50–75% of normal requirement in preceding week | Score 1 | Hip fracture, chronic patients, in particular with acute complications: cirrhosis, COPD. chronic hemodialysis, diabetes, oncology | ≥70: + 1 point | ||||
| Score 2 | 18.5–20.5 | Score 2 | >5% in 2 months | Score 2 | Intake of 25–60% of normal requirement in preceding week | Score 2 | Major abdominal surgery, stroke, severe pneumonia, hematologic malignancy | ||||||
| Score 3 | <18.5 | Score 3 | >5% in 1 month or >15% in 3 months | Score 3 | Intake of 0–25 of normal requirement in preceding week | Score 3 | Head injury, bone marrow transplantation, intensive care patients. | ||||||
| MUST | Score 1 | 18.5–20 | Score 1 | 5–10% in 3–6 months | Score 2 | There has been or is likely to be no nutritional intake for >5 days OR the patients is acutely ill * | |||||||
| Score 2 | <18.5 | Score 2 | >10% in 3–6 months | ||||||||||
| MST | Score 1 | 1–5 kg | Score 1 | Patient been eating poorly because of a decreased appetite | |||||||||
| Score 2 | 6–10 kg | ||||||||||||
| Score 3 | 5–11 kg | ||||||||||||
| Score 4 | >15 kg | ||||||||||||
| Score 2 | Unsure | ||||||||||||
| SASKIBD-NR | Score 1 | 2.3–4.5 kg in 1 month | Score 2 | Eating poorly because of a decreased appetite | Score 1 | N° 1–2 | |||||||
| Score 2 | 4.5–7 kg in 1 month | Score 2 | Restricting any foods or food groups | Score 2 | N° ≥ 3 | ||||||||
| Score 3 | >7 kg in 1 month | ||||||||||||
| MIRT | Score 1 | 18.5–20 | Score 2 | 5–10% in 3 months | Score 2 | 5–50 mg/L | |||||||
| Score 2 | <18.5 | Score 3 | ≥10% in 3 months | Score 3 | ≥50 mg/L | ||||||||
| Score 0 | Score 1 | Score 2 | |
|---|---|---|---|
| BMI, kg/m2 | >20.5 or 22 if >70 years | 18.5–20.5 or 20–22 if >70 years | <18.5 or <20 if >70 years |
| UWL, % | <5% within past 6 mths | 5–10% within past 3–6 mths or >10% beyond 6 mths | 5–10% within last mth or ≥10% in 3–6 mths |
| Chronic diarrhea or ileostomy | no | yes | / |
| Other GI symptoms, n° | 0–2 | ≥3 | / |
| Previuos surgery for IBD | no | yes | / |
| Total score: 0 = low risk; 1 = medium risk; ≥2 high risk | |||
| IBD | CD | UC | p * | |
|---|---|---|---|---|
| Patients, n (%) | 62 | 46 (74%) | 16 (26%) | |
| Age, years, median (IQR) | 54.5 (42.3–62.8) | 53.5 (43–62.2) | 57 (35.7–62.2) | 0.51952 |
| Male, n (%) | 36 (58%) | 25 (54%) | 11 (69%) | 0.31461 |
| Duration of disease, years, median (IQR) | 10.5 (2.2–18) | 10.5 (2.2–10.5) | 11 (2.5–18.2) | 0.92241 |
| First operation, n (%) | 33 (53%) | 28 (61%) | 5 (31%) | 0.04083 |
| Presence of stoma, n (%) | 11 (18%) | 1 (2%) | 10 (63%) | <0.001 |
| Chronic diarrhoea, n (%) | 18 (29%) | 16 (35%) | 2 (13%) | 0.11746 |
| N° of GI symptoms, mean, SD | 0.98 ± 1.13 | 1.21 ± 1.17 | 0.31 ± 0.70 | <0.001 |
| <3, n (%) | 55 (89%) | 39 (85%) | 16 (100%) | 0.17498 |
| ≥3, n (%) | 7 (11%) | 7 (15%) | 0 | |
| Crohn’s Disease behaviour | ||||
| -stricturing, n (%) | 31 (67%) | |||
| -fistulizing, n (%) | 11 (24%) | |||
| -inflammatory | 4 (9%) | |||
| Crohn’s Disease’s localization | ||||
| -Ileal, n (%) | 36 (78%) | |||
| -Ileocolonic, n (%) | 5 (11%) | |||
| -Colonic, n (%) | 5 (11%) | |||
| UC, n (%) | ||||
| -Proctitis | 8 (50%) | |||
| -Left side colitis | 1 (6%) | |||
| -Extensive colitis | 7 (44%) | |||
| Postoperative complications | 0.501 | |||
| -Anastomotic Leak | 3 (5%) | 2 (4%) | 1 (6%) | |
| -Wound infection | 1 (2%) | 0 | 1 (6%) | |
| -PONV | 5 (8%) | 5 (11%) | 0 | |
| Nutritional Status | ||||
| Weight, kg, mean, SD | 64.4 ± 13.75 | 64.1 ± 13.04 | 65.4 ± 16.05 | 0.74574 |
| BMI, kg/m2, mean, SD | 22.9 ± 4.03 | 22.9 ± 3.74 | 22.7 ± 4.90 | 0.6582 |
| <18.5, n (%) | 8 (13%) | 4 (9%) | 4 (25%) | 0.18724 |
| 18.5–25, n (%) | 40 (65%) | 30 (65%) | 10 (62%) | 0.84486 |
| 25–30, n (%) | 11 (18%) | 10 (22%) | 1 (6%) | 0.26074 |
| >30, n (%) | 3 (5%) | 2 (4%) | 1 (6%) | 1 |
| UWL | ||||
| n (%) | 30 (48%) | 19 (41%) | 11 (69%) | 0.08269 |
| <5%, n (%) | 7 (11%) | 6 (13%) | 1 (6%) | 0.66553 |
| 5–10%, n (%) | 13 (21%) | 7 (15%) | 6 (38%) | 0.0791 |
| ≥10%, n (%) | 10 (16%) | 6 (13%) | 4 (25%) | 0.26605 |
| FFM %, mean, SD | 78.7 ± 8.22 | 79.1 ± 8.13 | 77.4 ± 8.62 | 0.46595 |
| FFM % (M), mean, SD | 80.9 ± 7.84 | 82.1 ± 7.56 | 78.3 ± 8.17 | 0.18269 |
| FFM % (F), mean, SD | 75.3 ± 7.76 | 75.3 ± 7.36 | 75.2 ± 10.18 | 0.9771 |
| FFMI, kg/m2, mean, SD | 17.5 ± 2.22 | 17.7 ± 2.21 | 17.2 ± 2.29 | 0.41296 |
| FFMI (M), kg/m2, mean, SD | 18.5 ± 2.20 | 18.7 ± 2.27 | 18.1 ± 2.07 | 0.47845 |
| FFMI (F), kg/m2, mean, SD | 16.1 ± 1.34 | 16.4 ± 1.13 | 15.0 ± 0.85 | 0.0355 |
| FFMI < 17 (M) or < 15 (F), n (%) | 15 (25%) | 9 (20%) | 6 (37.5%) | 0.16266 |
| Reduced food intake, n (%) | 12 (19%) | 9 (19%) | 3 (19%) | 1 |
| Intake > 75% of TEE, n (%) | 59 (95%) | 43 (94%) | 16 (100%) | |
| Intake < 75% of TEE, n (%) | 3 (5%) | 3 (6%) | 0 | |
| IBD | CD | UC | p * | ||||
|---|---|---|---|---|---|---|---|
| Nutritional screening tools | n° | % | n° | % | n° | % | |
| NS-IBD | 33 | 53 | 20 | 43 | 13 | 81 | 0.01051 |
| NRS-2002 | 24 | 39 | 14 | 30 | 10 | 63 | 0.02332 |
| MUST | 16 | 26 | 8 | 17 | 8 | 50 | 0.01024 |
| MST | 16 | 26 | 9 | 20 | 7 | 44 | 0.05687 |
| MIRT | 24 | 39 | 14 | 30 | 10 | 63 | 0.02332 |
| SASKIBD-NR | 15 | 24 | 10 | 22 | 5 | 31 | 0.44417 |
| Malnutrition diagnosis | n° | % | n° | % | n° | % | |
| GLIM | 25 | 40 | 15 | 33 | 10 | 63 | 0.03578 |
| - GLIM stage 1 | 10 | 16 | 7 | 15 | 3 | 19 | 0.70878 |
| - GLIM stage 2 | 15 | 24 | 8 | 17 | 7 | 44 | 0.03395 |
| IBD | |||||||||
| Sensitivity | 95% CI | Specificity | 95% CI | PPV | 95% CI | NPV | 95% CI | Youden index | |
| NS-IBD | 0.92 | 0.72–0.98 | 0.73 | 0.56–0.86 | 0.7 | 0.51–0.84 | 0.93 | 0.76–0.99 | 0.65 |
| NRS-2002 | 0.84 | 0.63–0.95 | 0.92 | 0.77–0.98 | 0.87 | 0.66–0.97 | 0.89 | 0.74–0.96 | 0.76 |
| MUST | 0.6 | 0.39–0.78 | 0.97 | 0.84–0.99 | 0.94 | 0.68–0.99 | 0.78 | 0.63–0.88 | 0.57 |
| MST | 0.6 | 0.39–0.78 | 0.97 | 0.84–0.99 | 0.94 | 0.68–0.99 | 0.78 | 0.63–0.88 | 0.57 |
| MIRT SASKIBD-NR | 0.84 0.52 | 0.63–0.95 0.31–0.72 | 0.92 0.95 | 0.77–0.98 0.80–0.99 | 0.87 0.87 | 0.66–0.97 0.58–0.98 | 0.89 0.74 | 0.74–0.96 0.59–0.85 | 0.76 0.47 |
| CD | |||||||||
| Sensitivity | 95% CI | Specificity | 95% CI | PPV | 95% CI | NPV | 95% CI | Youden index | |
| NS-IBD | 0.86 | 0.58–0.97 | 0.77 | 0.58–0.89 | 0.65 | 0.40–0.83 | 0.92 | 0.73–0.98 | 0.63 |
| NRS-2002 | 0.8 | 0.51–0.94 | 0.93 | 0.77–0.98 | 0.85 | 0.56–0.97 | 0.90 | 0.73–0.97 | 0.73 |
| MUST | 0.53 | 0.27–0.77 | 1 | 0.86–1 | 1 | 0.59–1 | 0.81 | 0.65–0.91 | 0.53 |
| MST | 0.6 | 0.32–0.82 | 1 | 0.86–1 | 1 | 0.62–1 | 0.83 | 0.67–0.93 | 0.53 |
| MIRT SASKIBD-NR | 0.8 0.6 | 0.51–0.94 0.32–0.82 | 0.93 0.96 | 0.77–0.98 0.81–0.99 | 0.85 0.9 | 0.56–0.97 0.54–0.99 | 0.90 0.83 | 0.73–0.97 0.66–0.93 | 0.73 0.56 |
| UC | |||||||||
| Sensitivity | 95% CI | Specificity | 95% CI | PPV | 95% CI | NPV | 95% CI | Youden index | |
| NS-IBD | 1 | 0.65–1 | 0.5 | 0.13–0.86 | 0.76 | 0.45–0.93 | 1 | 0.30–1 | 0.50 |
| NRS-2002 | 0.9 | 0.54–0.99 | 0.83 | 0.36–0.99 | 0.9 | 0.54–0.99 | 0.83 | 0.34–0.99 | 0.73 |
| MUST | 0.7 | 0.35–0.91 | 0.83 | 0.36–0.99 | 0.87 | 0.46–0.99 | 0.62 | 0.25–0.89 | 0.53 |
| MST | 0.6 | 0.27–0.86 | 0.83 | 0.36–0.99 | 0.85 | 0.42–0.99 | 0.55 | 0.22–0.84 | 0.43 |
| MIRT SASKIBD-NR | 0.9 0.4 | 0.54–0.99 0.13–0.72 | 0.83 0.83 | 0.36–0.99 0.36–0.99 | 0.9 0.8 | 0.54–0.99 0.29–0.98 | 0.83 0.45 | 0.36–0.99 0.18–0.75 | 0.73 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorindi, C.; Dragoni, G.; Scaringi, S.; Staderini, F.; Nannoni, A.; Ficari, F.; Giudici, F. Relationship between Nutritional Screening Tools and GLIM in Complicated IBD Requiring Surgery. Nutrients 2021, 13, 3899. https://doi.org/10.3390/nu13113899
Fiorindi C, Dragoni G, Scaringi S, Staderini F, Nannoni A, Ficari F, Giudici F. Relationship between Nutritional Screening Tools and GLIM in Complicated IBD Requiring Surgery. Nutrients. 2021; 13(11):3899. https://doi.org/10.3390/nu13113899
Chicago/Turabian StyleFiorindi, Camilla, Gabriele Dragoni, Stefano Scaringi, Fabio Staderini, Anita Nannoni, Ferdinando Ficari, and Francesco Giudici. 2021. "Relationship between Nutritional Screening Tools and GLIM in Complicated IBD Requiring Surgery" Nutrients 13, no. 11: 3899. https://doi.org/10.3390/nu13113899
APA StyleFiorindi, C., Dragoni, G., Scaringi, S., Staderini, F., Nannoni, A., Ficari, F., & Giudici, F. (2021). Relationship between Nutritional Screening Tools and GLIM in Complicated IBD Requiring Surgery. Nutrients, 13(11), 3899. https://doi.org/10.3390/nu13113899







