A Pilot Study of the Effect of Lactobacillus casei Obtained from Long-Lived Elderly on Blood Biochemical, Oxidative, and Inflammatory Markers, and on Gut Microbiota in Young Volunteers
Abstract
:1. Introduction
2. Materials and Methods
2.1. LTL1879 Powder Preparation
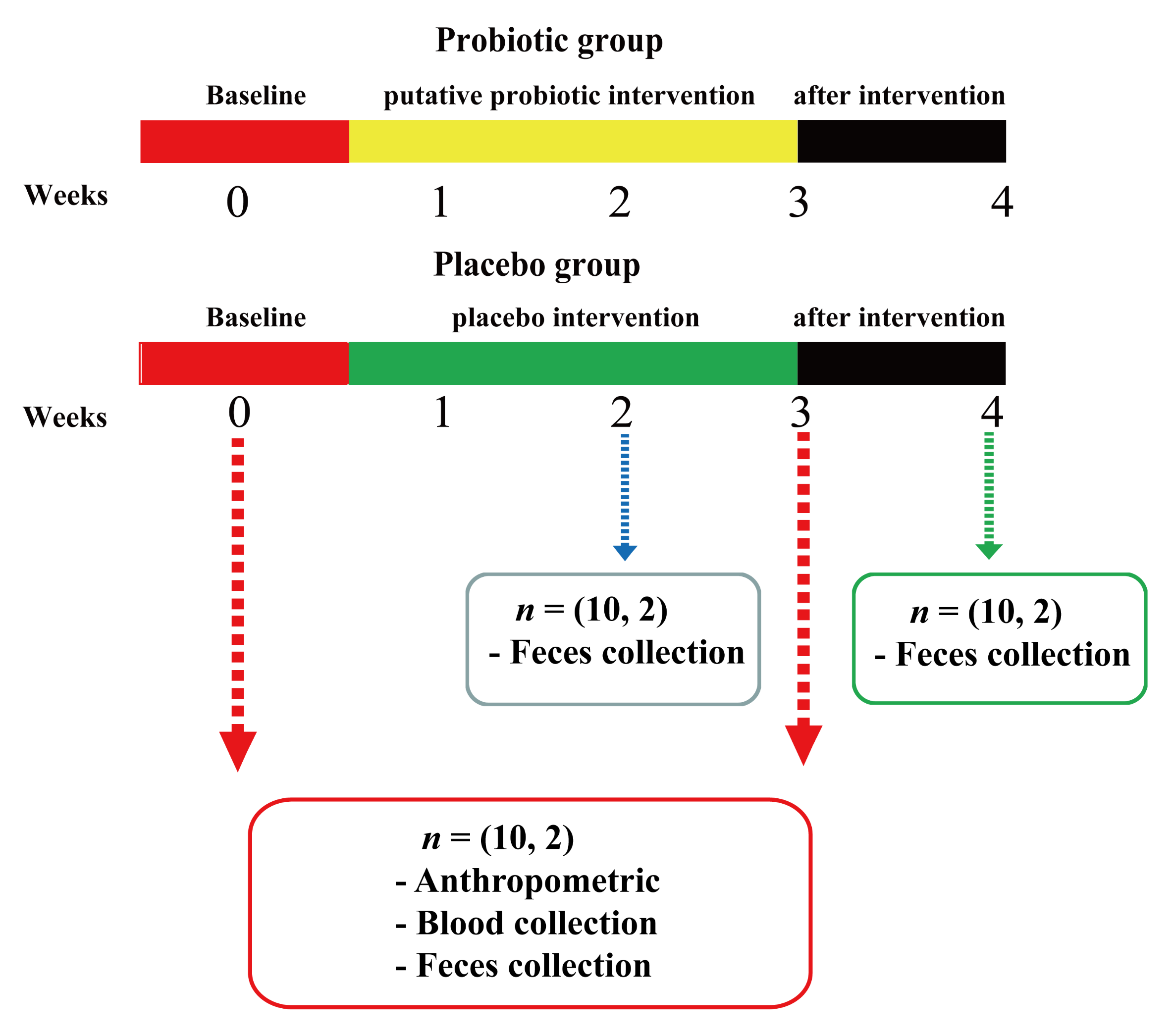
2.2. Participants and Study Design
2.3. Anthropometric Assessment
2.4. Blood Sample Collection and Biochemical Parameter Measurement
2.5. Oxidative Marker Measurements
2.6. Inflammatory Marker Measurements
2.7. Investigation of Gut Microbiota
2.7.1. Fecal Sample Collection
2.7.2. Extraction of Fecal DNA
2.7.3. Real-Time PCR
2.8. Statistical Analysis of Comprehensive Health Indicators and Quantitative Evaluation of Health Indicators
2.9. Statistical Analysis
3. Results
3.1. Effects of LTL1879 Intervention on Blood Parameters and Anthropometric Measurements
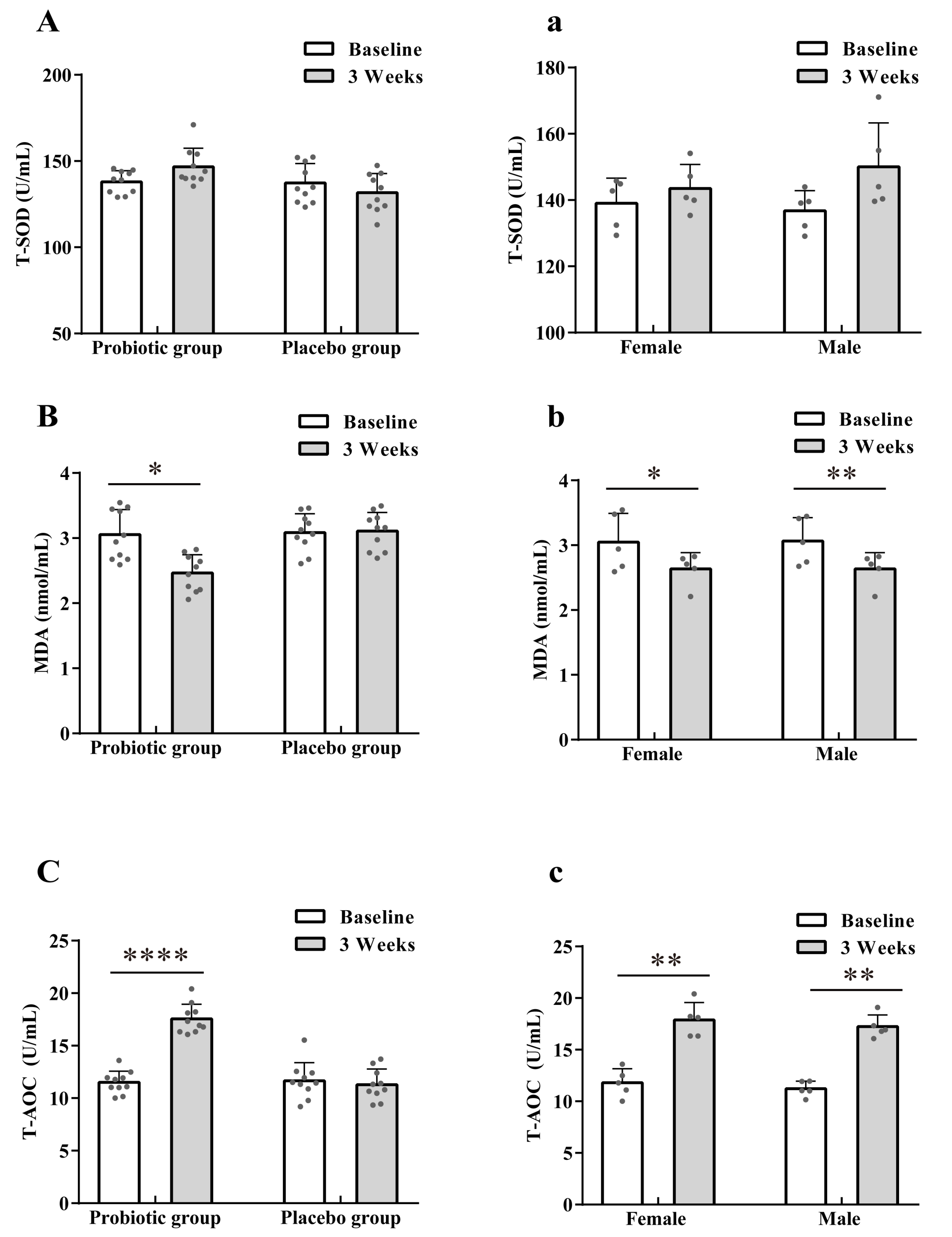
3.2. Effect of LTL1879 Intervention on Oxidation Indexes
3.3. Effect of LTL1879 Intervention on Immune Parameters
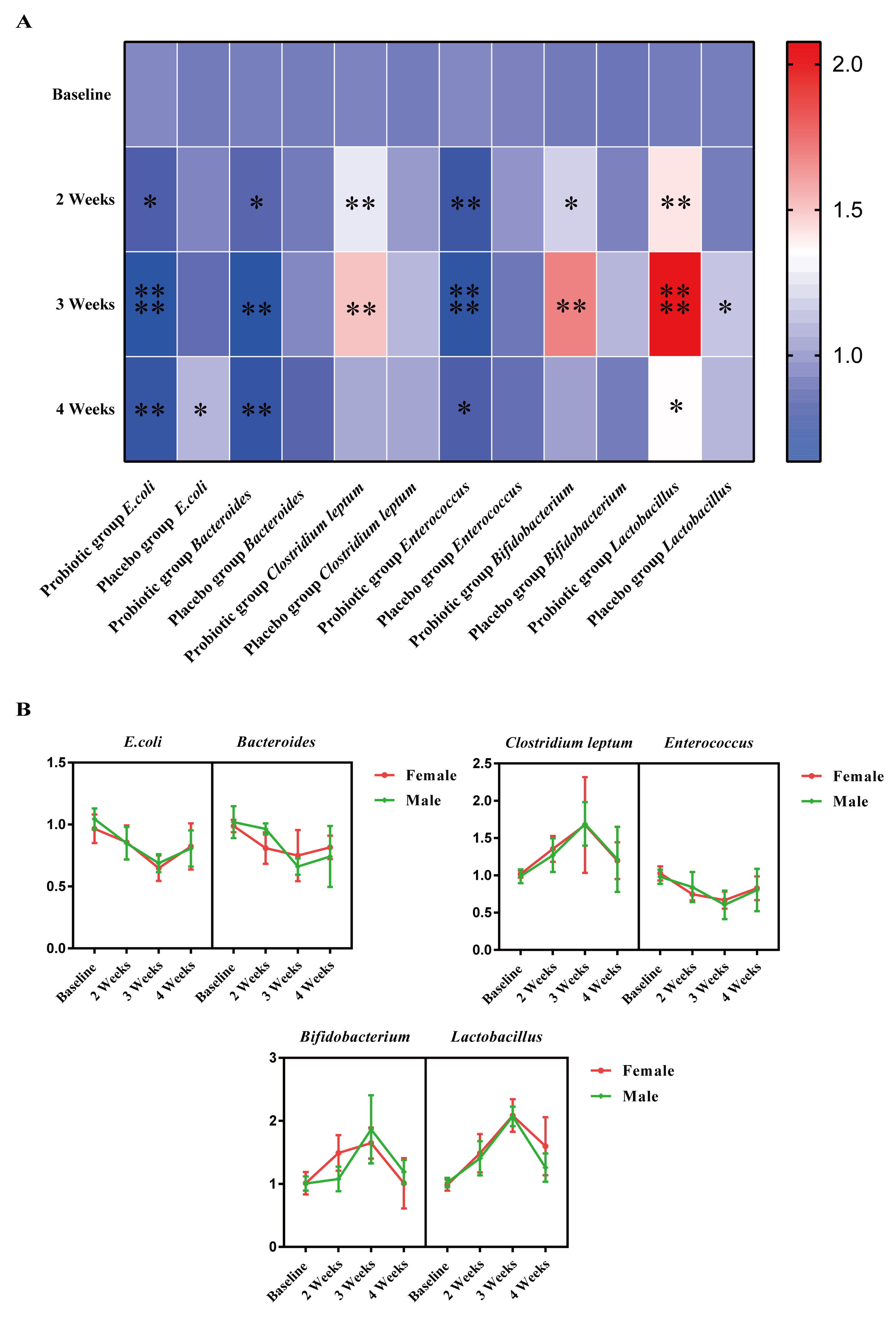
3.4. Effect of LTL1879 Intervention on Typical Fecal Microorganisms
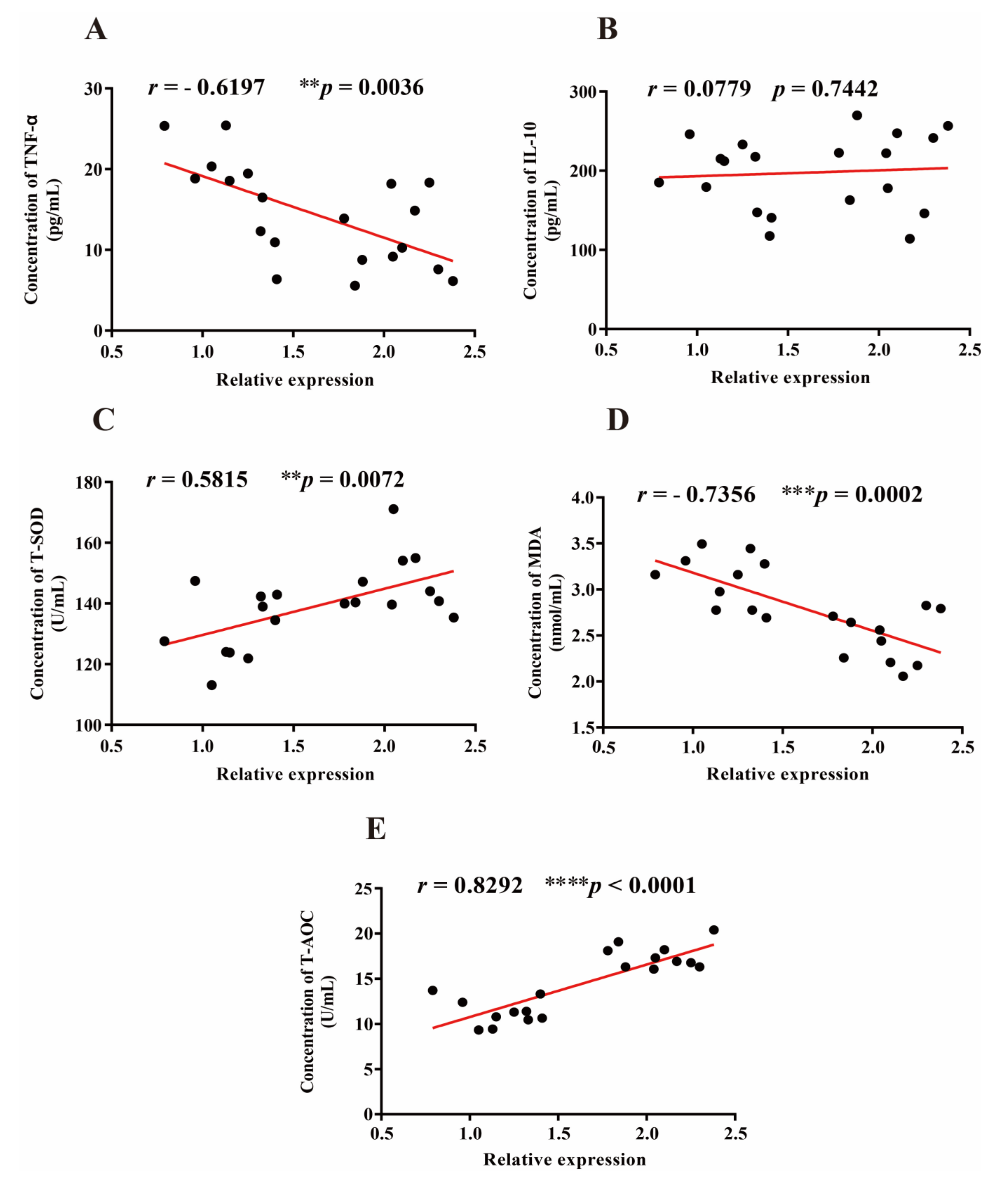
3.5. Correlation between Lactobacillus, Oxidative, and Inflammatory Markers
3.6. Quantitative Evaluation of Volunteer Health Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lederberg, J. Infectious History. Science 2000, 288, 287–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, F.; Hua, Y.; Zeng, B.; Ning, R.; Li, Y.; Zhao, J. Gut microbiota signatures of longevity. Curr. Biol. 2016, 26, R832–R833. [Google Scholar] [CrossRef] [Green Version]
- DeJong, E.N.; Surette, M.G.; Bowdish, D.M.E. The Gut Microbiota and Unhealthy Aging: Disentangling Cause from Consequence. Cell Host Microbe 2020, 28, 180–189. [Google Scholar] [CrossRef]
- Kundu, P.; Lee, H.U.; Garcia-Perez, I.; Tay, E.; Pettersson, S. Neurogenesis and prolongevity signaling in young germ-free mice transplanted with the gut microbiota of old mice. Sci. Transl. Med. 2019, 11, eaau4760. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Wilms, E.; Masclee, A.A.M.; Smidt, H.; Zoetendal, E.G.; Jonkers, D. Age-dependent changes in GI physiology and microbiota: Time to reconsider? Gut 2018, 67, 2213–2222. [Google Scholar] [CrossRef]
- Wu, L.; Zeng, T.; Zinellu, A.; Rubino, S.; Carru, C. A Cross-Sectional Study of Compositional and Functional Profiles of Gut Microbiota in Sardinian Centenarians. mSystems 2019, 4, e00325-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.S.; Shin, E.; Hong, H.; Shin, H.J.; Lee, Y. Characterization of Lactobacillus fermentum PL9988 Isolated from Healthy Elderly Korean in a Longevity Village. J. Microbiol. Biotechnol. 2015, 25, 1510–1518. [Google Scholar] [CrossRef]
- Yu, X.; Li, S.; Dong, Y.; Liang, Q.; Wu, Y.; Wang, D.; Shah, N.P.; Feng, X.; Hua, W. A novel strain of Lactobacillus mucosae isolated from a Gaotian villager improves in vitro and in vivo antioxidant as well as biological properties in d-galactose-induced aging mice. J. Dairy Sci. 2016, 99, 903–914. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Feng, N.; Zhang, C.; Liu, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Lactobacillus reuteri A9 and Lactobacillus mucosae A13 isolated from Chinese superlongevity people modulate lipid metabolism in a hypercholesterolemia rat model. FEMS Microbiol. Lett. 2019, 366, 24. [Google Scholar] [CrossRef]
- Yang, H.Y.; Liu, S.L.; Ibrahim, S.A.; Zhao, L.; Jiang, J.L.; Sun, W.F.; Ren, F.Z. Oral administration of live Bifidobacterium substrains isolated from healthy centenarians enhanced immune function in BALB/c mice. Nutr. Res. 2009, 29, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhu, C.; Ge, S.; Zhang, M.; Jiang, L.; Cui, J.; Ren, F. Lactobacillus salivarius Ren prevent the early colorectal carcinogenesis in 1, 2-dimethylhydrazine-induced rat model. J. Appl. Microbiol. 2014, 117, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.; Campbell, J.I.; King, T.P.; Grant, G.; Jansson, E.A.; Coutts, A.G.; Pettersson, S.; Conway, S. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-γ and RelA. Nat. Immunol. 2004, 5, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Long, H.N.; Song, M.; Wang, D.D.; Chan, A.T. Dietary fiber intake, the gut microbiome, and chronic systemic inflammation in a cohort of adult men. Genome Med. 2021, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Pietzner, M.; Budde, K.; Rühlemann, M.; Vlzke, H.; Frost, F. Exocrine Pancreatic Function Modulates Plasma Metabolites through Changes in Gut Microbiota Composition. J. Clin. Endocrinol. Metab. 2021, 106, e2290–e2298. [Google Scholar] [CrossRef]
- Roy, T.L.; Lécuyer, E.; Chassaing, B.; Rhimi, M.; Lesnik, P. The intestinal microbiota regulates host cholesterol homeostasis. BMC Biol. 2019, 17, 94. [Google Scholar]
- Ohnmacht, C.; Park, J.H.; Cording, S.; Wing, J.B.; Atarashi, K.; Obata, Y.; Gaboriau-Routhiau, V.; Marques, R.; Dulauroy, S.; Fedoseeva, M. The microbiota regulates type 2 immunity through ROR t+ T cells. Science 2015, 349, aac4263. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lem, A.; Ll, B.; Mjl, B. Short-term probiotic supplementation enhances cellular immune function in healthy elderly: Systematic review and meta-analysis of controlled studies. Nutr. Res. 2019, 64, 1–8. [Google Scholar]
- Sabico, S.; Al-Mashharawi, A.; Al-Daghri, N.M.; Yakout, S.; Alnaami, A.M.; Alokail, M.S.; Mcternan, P.G. Effects of a multi-strain probiotic supplement for 12weeks in circulating endotoxin levels and cardiometabolic profiles of medication nave T2DM patients: A randomized clinical trial. J. Transl. Med. 2017, 15, 249. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; Lu, W.; Zhao, J.; Zhang, H.; Chen, W. Probiotics modulate the gut microbiota composition and immune responses in patients with atopic dermatitis: A pilot study. Eur. J. Nutr. 2019, 58, 2119–2130. [Google Scholar] [CrossRef]
- Infusino, F.; Marazzato, M.; Mancone, M.; Fedele, F.; D’Ettorre, G. Diet Supplementation, Probiotics, and Nutraceuticals in SARS-CoV-2 Infection: A Scoping Review. Nutrients 2020, 12, 1718. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, N.B.; Bryrup, T.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Pedersen, O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: A systematic review of randomized controlled trials. Genome Med. 2016, 8, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Q.; Zhao, F.; Liu, W.; Lv, R.; Khine, W.W.T.; Han, J.; Sun, Z.; Lee, Y.K.; Zhang, H. Probiotic-directed modulation of gut microbiota is basal microbiome dependent. Gut Microbes 2020, 12, 1736974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Yu, X.; Yi, L.; Yun, G.; Lin, G.; Pu, F.; Ma, X.; Cui, W.; Fran Ce Sco, M.; Fang, H. Bifidobacterium bifidum TMC3115 Can Characteristically Influence Glucose and Lipid Profile and Intestinal Microbiota in the Middle-Aged and Elderly. Probiotics Antimicrob. Proteins 2018, 11, 1182–1194. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, H.; Xu, M.; Wang, X.; Wang, C.; Lian, Y.; Mehmood, A.; Dai, H. Stevia residue extract ameliorates oxidative stress in d. J. Funct. Foods 2019, 52, 587–595. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Yu, J.P.; Liu, Y.F.; Teng, X.J.; Ming, M.; Lv, P.; An, P.; Liu, S.Q.; Yu, H.G. Effects of Ginkgo biloba Extract on Inflammatory Mediators (SOD, MDA, TNF-α, NF-κBp65, IL-6) in TNBS-Induced Colitis in Rats. Mediat. Inflamm. 2006, 2016, 092642. [Google Scholar]
- Cheng, Y.; Zuo, H.J.; Liao, H.Y.; Chen, J.Y.; Zhang, T.L.; Pei, X.F.; Xu, X. Establishment of real-time PCR method for detection of intestinal bacteria. Mod. Prev. Med. 2014, 41, 4338–4341. [Google Scholar]
- Nelson, E.A.; Palombo, E.A.; Knowles, S.R. Comparison of methods for the extraction of bacterial DNA from human faecal samples for analysis by real-time PCR. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 2010, 2, 1479–1485. [Google Scholar]
- Liu, R. R-T PCR Preliminary Analysis of Gut Flora in Patients with Elderly Hypertension; Jilin University: Changchun, China, 2014. [Google Scholar]
- Walter, J.; Hertel, C.; Tannock, G.W.; Lis, C.M.; Munro, K.; Hammes, W.P. Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella Species in Human Feces by Using Group-Specific PCR Primers and Denaturing Gradient Gel Electrophoresis. Appl. Environ. Microbiol. 2001, 67, 2578–2585. [Google Scholar] [CrossRef] [Green Version]
- Rinttilä, T.; Kassinen, A.; Malinen, E.; Krogius, L.; Palva, A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 2004, 97, 1166–1177. [Google Scholar] [CrossRef]
- Pang, X.; Ding, D.; Wei, G.; Zhang, M.; Wang, L.; Zhao, L. Molecular profiling of Bacteroides spp. in human feces by PCR-temperature gradient gel electrophoresis. J. Microbiol. Methods 2007, 61, 413–417. [Google Scholar] [CrossRef]
- Wu, X.; Xiao, P.P.; Xie, X.R.; Sun, X.Q.; Fu, B.B.; Sun, X.P. Analysis of the Changes of Intestinal Bifidobacterium and Clostridium Tenella in Patients with Recurrent SLE by qRT-PCR. Med. Inf. 2018, 31, 73–77. [Google Scholar]
- Lee, S.H.; You, H.S.; Kang, H.G.; Kang, S.S.; Hyun, S.H. Association between Altered Blood Parameters and Gut Microbiota after Synbiotic Intake in Healthy, Elderly Korean Women. Nutrients 2020, 12, 3112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, Y.; Fei, X. Effect of probiotics on body weight and body-mass index: A systematic review and meta-analysis of randomized, controlled trials. Int. J. Food Sci. Nutr. 2016, 67, 571–580. [Google Scholar] [CrossRef]
- Bohlouli, J.; Namjoo, I.; Borzoo-Isfahani, M.; Zehi, Z.; Moravejolahkami, A.R.; Kermani, M. Effect of probiotics on oxidative stress and inflammatory status in diabetic nephropathy: A systematic review and meta-analysis of clinical trials. Heliyon 2021, 7, e05925. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Nie, X.L.; Zhang, J.J. The effect of probiotics supplementation on blood pressure: A systemic review and meta-analysis. Lipids Health Dis. 2020, 19, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivey, K.L.; Hodgson, J.M.; Kerr, D.A.; Thompson, P.L.; Stojceski, B.; Prince, R.L. The effect of yoghurt and its probiotics on blood pressure and serum lipid profile; a randomised controlled trial. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 46–51. [Google Scholar] [CrossRef] [Green Version]
- Khalesi, S.; Sun, J.; Buys, N.; Jayasinghe, R. Effect of Probiotics on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized, Controlled Trials. Hypertension 2014, 64, 897–903. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.P. Redefining Oxidative Stress. Antioxidiants Redox Signal. 2006, 8, 1865–1879. [Google Scholar] [CrossRef]
- Asemi, Z.; Zare, Z.; Shakeri, H.; Sabihi, S.S.; Esmaillzadeh, A. Effect of Multispecies Probiotic Supplements on Metabolic Profiles, hs-CRP, and Oxidative Stress in Patients with Type 2 Diabetes. Ann. Nutr. Metab. 2013, 63, 1–9. [Google Scholar] [CrossRef]
- Badehnoosh, B.; Karamali, M.; Zarrati, M.; Jamilian, M.; Asemi, Z. The effects of probiotic supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in gestational diabetes. J. Matern.-Fetal Neonatal Med. 2017, 31, 1–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Y.M.; Lee, B.J.; Kim, J.I.; Nam, B.H.; Cha, J.Y.; Kim, Y.M.; Ahn, C.B.; Choi, J.S.; Choi, I.S.; Je, J.Y. Antioxidant effects of fermented sea tangle (Laminaria japonica) by Lactobacillus brevis BJ20 in individuals with high level of gamma-GT: A randomized, double-blind, and placebo-controlled clinical study. Food Chem. Toxicol. 2012, 50, 1166–1169. [Google Scholar] [CrossRef] [PubMed]
- Valentini, L.; Pinto, A.; Bourdel-Marchasson, I.; Ostan, R.; Brigidi, P. Impact of personalized diet and probiotic supplementation on inflammation, nutritional parameters and intestinal microbiota—The “RISTOMED project”: Randomized controlled trial in healthy older people. Clin. Nutr. 2015, 34, 593–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozkan, O.V.; Yuzbasioglu, M.F.; Ciralik, H.; Kurutas, E.B.; Yonden, Z.; Aydin, M.; Bulbuloglu, E.; Semerci, E.; Goksu, M.; Atli, Y.; et al. Resveratrol, a natural antioxidant, attenuates intestinal ischemia/reperfusion injury in rats. Tohoku J. Exp. Med. 2009, 218, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Zamani, B.; Farshbaf, S.; Golkar, H.R.; Bahmani, F.; Asemi, Z. Synbiotic supplementation and the effects on clinical and metabolic responses in patients with rheumatoid arthritis: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2017, 117, 1095–1102. [Google Scholar] [CrossRef]
- Sanaie, S.; Mameghani, M.E.; Mahmoodpoor, A.; Hamishehkar, H. Effect of a probiotic preparation (VSL#3) in critically ill patients: A randomized, double-blind, placebo-controlled trial (Pilot Study). Pak. J. Med. Sci. 2013, 29, 490–494. [Google Scholar]
- Soleimani, A.; Mojarrad, M.Z.; Bahmani, F.; Taghizadeh, M.; Ramezani, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Esmaillzadeh, A.; Asemi, Z. Probiotic supplementation in diabetic hemodialysis patients has beneficial metabolic effects. Kidney Int. 2017, 91, 435–442. [Google Scholar] [CrossRef]
- Jamilian, M.; Bahmani, F.; Vahedpoor, Z.; Salmani, A.; Tajabadi-Ebrahimi, M.; Jafari, P.; Hashemi Dizaji, S.; Asemi, Z. Effects of Probiotic Supplementation on Metabolic Status in Pregnant Women: A Randomized, Double-blind, Placebo-Controlled Trial. Arch. Iran. Med. 2016, 19, 687–692. [Google Scholar]
- Shakeri, H.; Hadaegh, H.; Abedi, F.; Tajabadi-Ebrahimi, M.; Mazroii, N.; Ghandi, Y.; Asemi, Z. Consumption of synbiotic bread decreases triacylglycerol and VLDL levels while increasing HDL levels in serum from patients with type-2 diabetes. Lipids 2014, 49, 695–701. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [Green Version]
- Webster, N.R.; Galley, H.F. Inflammation and immunity. BJA CEPD Rev. 2003, 3, 54–58. [Google Scholar] [CrossRef]
- Sichetti, M.; Marco, S.D.; Pagiotti, R.; Traina, G.; Pietrella, D. Anti-inflammatory effect of multistrain probiotic formulation (L. rhamnosus, B. lactis, and B. longum). Nutrition 2018, 53, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Levast, B.; Li, Z.; Madrenas, J. The role of IL-10 in microbiome-associated immune modulation and disease tolerance. Cytokine 2015, 75, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.N.; Saboori, S.; Asbaghi, O. Effect of daily probiotic yogurt consumption on inflammation: A Systematic Review and Meta-analysis of Randomized Controlled Clinical Trials. Obes. Med. 2020, 18. [Google Scholar] [CrossRef]
- Mehta, N.N.; Mcgillicuddy, F.C.; Anderson, P.D.; Hinkle, C.C.; Shah, R.; Pruscino, L.; Tabita-Martinez, J.; Sellers, K.F.; Rickels, M.R.; Reilly, M.P. Experimental Endotoxemia Induces Adipose Inflammation and Insulin Resistance in Humans. Diabetes 2010, 59, 172–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Kelleher, S.L.; Casas, I.; Carbajal, N.; Lönnerdal, B. Supplementation of infant formula with the probiotic lactobacillus reuteri and zinc: Impact on enteric infection and nutrition in infant rhesus monkeys. J. Pediatric Gastroenterol. Nutr. Metab. 2002, 35, 162–168. [Google Scholar] [CrossRef]
- Cruchet, S.; Obregon, M.C.; Salazar, G.; Diaz, E.; Gotteland, M. Effect of the ingestion of a dietary product containing Lactobacillus johnsonii La1 on Helicobacter pylori colonization in children. Nutrition 2003, 19, 716–721. [Google Scholar] [CrossRef]
- Chen, L.; Wang, M.-F.; Chang, C.-C.; Huang, S.-Y.; Pan, C.-H.; Yeh, Y.-T.; Huang, C.-H.; Chan, C.-H.; Huang, H.-Y. Lacticaseibacillus paracasei PS23 Effectively Modulates Gut Microbiota Composition and Improves Gastrointestinal Function in Aged SAMP8 Mice. Nutrients 2021, 13, 1116. [Google Scholar] [CrossRef]
- Spanhaak, S.; Havenaar, R.; Schaafsma, G. The effect of consumption of milk fermented by Lactobacillus casei strain Shirota on the intestinal microflora and immune parameters in humans. Eur. J. Clin. Nutr. 1998, 52, 899–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsuoka, T. Bifidobacteria and their role in human health. J. Ind. Microbiol. 1990, 6, 263–267. [Google Scholar] [CrossRef]
- Derrien, M.; Van, H.V.; Johan, E.T. Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends Microbiol. 2015, 23, 354–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuohy, K.M.; Pinart-Gilberga, M.; Jones, M.; Hoyles, L.; Mccartney, A.L.; Gibson, G.R. Survivability of a probiotic Lactobacillus casei in the gastrointestinal tract of healthy human volunteers and its impact on the faecal microflora. J. Appl. Microbiol. 2010, 102, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Tao, D.; Schloss, P.D. Dynamics and associations of microbial community types across the human body. Nature 2014, 509, 357–360. [Google Scholar]
- Falony, G.; Joossens, M.; Vieira-Silva, S.; Wang, J.; Darzi, Y.; Faust, K.; Kurilshikov, A.; Bonder, M.J.; Valles-Colomer, M.; Vandeputte, D.; et al. Population-level analysis of gut microbiome variation. Science 2016, 352, 560–564. [Google Scholar] [CrossRef]
- Steegenga, W.T.; Mischke, M.; Lute, C.; Boekschoten, M.V.; Pruis, M.G.M.; Lendvai, A.; Verkade, H.J.; Boekhorst, J.; Timmerman, H.M.; Plösch, T.; et al. Sexually dimorphic characteristics of the small intestine and colon of prepubescent C57BL/6 mice. Biol. Sex Differ. 2014, 5, 11. [Google Scholar] [CrossRef]
- Chen, K.L.; Madak-Erdogan, Z. Estrogen and Microbiota Crosstalk: Should We Pay Attention? Trends Endocrinol. Metab. Tem 2016, 27, 752–755. [Google Scholar] [CrossRef]
- Vaughan, E.; Mollet, B.; Devos, W.M. Functionality of probiotics and intestinal lactobacilli: Light in the intestinal tract tunnel. Curr. Opin. Biotechnol. 1999, 10, 505–510. [Google Scholar] [CrossRef]

| Probiotic Group | Placebo Group | p-Value | |
|---|---|---|---|
| Age | 24.9 ± 1.66 | 23.9 ± 0.99 | 0.120 |
| Height (m) | 1.67 ± 0.07 | 1.66 ± 0.09 | 0.828 |
| Weight (kg) | 59.36 ± 7.4 | 60.77 ± 19.53 | 0.835 |
| Male/Female | 5/5 | 4/6 | - |
| Bacteria | Primer Sequence (5′–3′) | Annealing Temperature | References |
|---|---|---|---|
| Total intestinal flora | F: ACTCCTACGGGAGGCAGCAG R: ATTACCGCGGCTGCTGG-3′ | 64 °C | Cheng, Y. et al. [27] |
| Escherichia coli | F: GTTAATACCTTTGCTCATTGA R: ACCAGGGTATVTTAATCCTGTT | 60 °C | Nelson, E. A. et al. [28] |
| Bifidobacterium | F: GGGTGGTAATGCCGGATG R: CCACCGTTACACCGGGAA | 65 °C | Liu, R. [29] |
| Lactobacillus | F: AGCAGTAGGGAATCTTCCA R: ATTTCACCGCTACACATG | 62 °C | Walter, J et al. [30] |
| Enterococcus | F: CCCTTATTGTTAGTTGCCATCATT R: ACTCGTTGTTGTACTTCCCATTGTT | 60 °C | Rinttilä, T. et al. [31] Nelson, E. A. et al. [28] |
| Bacteroides | F: CTGAACCAGCCAAGTAGCG R: CCGCAAACTTTCACAACTGACTTA | 68 °C | Pang, X. et al. [32] |
| Clostridium leptum | F: CCCTTCAGTGCCGCAGT R: GTCGCAGGATGTCAAGAC | 58 °C | Wu, X. et al. [33] |
| Parameter | Probiotic Group | Placebo Group | ||||
|---|---|---|---|---|---|---|
| Baseline | 3 Weeks | p-Value | Baseline | 3 Weeks | p-Value | |
| Weight (kg) | 59.36 ± 7.4 | 59.72 ± 7.41 | 0.300 | 60.77 ± 19.53 | 61.19 ± 19.19 | 0.629 |
| BMI (kg/m2) | 21.25 ± 2.12 | 21.36 ± 1.97 | 0.360 | 21.64 ± 5.13 | 21.80 ± 5.00 | 0.569 |
| Systolic blood pressure (mmHg) | 107.00 ± 11.54 | 109.90 ± 8.67 | 0.483 | 110.40 ± 17.79 | 112.80 ± 12.95 | 0.227 |
| Diastolic blood pressure (mmHg) | 68.40 ± 6.57 | 70.90 ± 7.80 | 0.426 | 73.50 ± 11.70 | 75.30 ± 7.27 | 0.466 |
| FBG (mmol/L) | 4.86 ± 0.4 | 4.73 ± 0.43 | 0.340 | 4.87 ± 0.33 | 4.9 ± 0.52 | 0.855 |
| Triglyceride (mmol/L) | 1.04 ± 0.17 | 1.05 ± 0.28 | 0.862 | 1.5 ± 1.61 | 1.99 ± 2.26 | 0.058 |
| HDL (mmol/L) | 1.42 ± 0.36 | 1.7 ± 0.38 | 0.078 | 1.47 ± 0.36 | 1.63 ± 0.46 | 0.455 |
| LDL (mmol/L) | 2.43 ± 0.33 | 2.42 ± 0.53 | 0.952 | 2.98 ± 0.94 | 2.75 ± 0.88 | 0.115 |
| UA (μmol/L) | 382.4 ± 56.87 | 342.6 ± 111.03 | 0.313 | 426.9 ± 135.51 | 428.6 ± 74.69 | 0.939 |
| Creatinine (μmol/L) | 79.8 ± 11.34 | 78.1 ± 22.06 | 0.746 | 82.1 ± 17.89 | 78.4 ± 18.49 | 0.387 |
| ALP (U/L) | 73.2 ± 17.33 | 70.4 ± 19.65 | 0.612 | 72.8 ± 18.12 | 72.9 ± 13.27 | 0.984 |
| AST (U/L) | 21.1 ± 7.5 | 17.8 ± 6.25 | 0.133 | 24.4 ± 9.03 | 22.6 ± 5.52 | 0.420 |
| GGT (U/L) | 18.4 ± 8.58 | 11.2 ± 3.43 | 0.052 | 23.7 ± 16.68 | 27.6 ± 13.72 | 0.200 |
| Principal Component | Eigenvalues | Variance Contribution/% | Accumulative Variance Contribution/% |
|---|---|---|---|
| 1 | 5.596 | 50.870 | 50.870 |
| 2 | 1.226 | 11.145 | 62.015 |
| 3 | 1.040 | 9.453 | 71.468 |
| Health Index | Principal Component | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| T-SOD | 0.558 | 0.093 | 0.512 |
| MDA | 0.834 | 0.041 | −0.191 |
| T-AOC | 0.940 | 0.085 | −0.043 |
| IL-10 | 0.267 | 0.790 | −0.428 |
| TNF-α | 0.373 | 0.362 | 0.724 |
| E. coli | 0.752 | 0.337 | −0.083 |
| Bacteroides | 0.670 | −0.128 | −0.041 |
| Clostridium leptum | 0.684 | −0.197 | 0.046 |
| Enterococcus | 0.802 | −0.328 | −0.085 |
| Bifidobacterium | 0.773 | −0.418 | −0.109 |
| Lactobacillus | 0.879 | 0.047 | −0.036 |
| Number | F1 | F2 | F3 | Fsum | Rank |
|---|---|---|---|---|---|
| 1 | 2.396 | 0.178 | 0.166 | 1.255 | 1 |
| 2 | 1.949 | 0.579 | 0.568 | 1.110 | 7 |
| 3 | 0.575 | 0.177 | 0.146 | 0.326 | 19 |
| 4 | 0.926 | 0.058 | 0.172 | 0.494 | 13 |
| 5 | 2.448 | −0.035 | −0.205 | 1.223 | 5 |
| 6 | 1.110 | 0.622 | 0.292 | 0.662 | 11 |
| 7 | 1.807 | 0.176 | −0.385 | 0.903 | 10 |
| 8 | 2.173 | 0.533 | 0.274 | 1.191 | 6 |
| 9 | 2.286 | 0.875 | −0.149 | 1.247 | 2 |
| 10 | 0.800 | 0.395 | 0.074 | 0.458 | 17 |
| 11 | 1.040 | 0.548 | 0.181 | 0.607 | 12 |
| 12 | 2.267 | 0.227 | 0.649 | 1.241 | 3 |
| 13 | 0.756 | 0.683 | 0.263 | 0.485 | 14 |
| 14 | 0.765 | 0.455 | 0.260 | 0.465 | 16 |
| 15 | 2.252 | 0.257 | 0.660 | 1.237 | 4 |
| 16 | 0.758 | −0.041 | 0.304 | 0.410 | 18 |
| 17 | 1.921 | 0.517 | 0.044 | 1.039 | 8 |
| 18 | 0.524 | 0.316 | 0.152 | 0.316 | 20 |
| 19 | 1.791 | 0.780 | 0.428 | 1.039 | 9 |
| 20 | 0.783 | 0.510 | 0.281 | 0.482 | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mei, L.-H.; Zheng, W.-X.; Zhao, Z.-T.; Meng, N.; Zhang, Q.-R.; Zhu, W.-J.; Li, R.-D.; Liang, X.-L.; Li, Q.-Y. A Pilot Study of the Effect of Lactobacillus casei Obtained from Long-Lived Elderly on Blood Biochemical, Oxidative, and Inflammatory Markers, and on Gut Microbiota in Young Volunteers. Nutrients 2021, 13, 3891. https://doi.org/10.3390/nu13113891
Mei L-H, Zheng W-X, Zhao Z-T, Meng N, Zhang Q-R, Zhu W-J, Li R-D, Liang X-L, Li Q-Y. A Pilot Study of the Effect of Lactobacillus casei Obtained from Long-Lived Elderly on Blood Biochemical, Oxidative, and Inflammatory Markers, and on Gut Microbiota in Young Volunteers. Nutrients. 2021; 13(11):3891. https://doi.org/10.3390/nu13113891
Chicago/Turabian StyleMei, Li-Hua, Wen-Xuan Zheng, Zheng-Tao Zhao, Ning Meng, Qin-Ren Zhang, Wen-Jun Zhu, Rui-Ding Li, Xiao-Lin Liang, and Quan-Yang Li. 2021. "A Pilot Study of the Effect of Lactobacillus casei Obtained from Long-Lived Elderly on Blood Biochemical, Oxidative, and Inflammatory Markers, and on Gut Microbiota in Young Volunteers" Nutrients 13, no. 11: 3891. https://doi.org/10.3390/nu13113891
APA StyleMei, L.-H., Zheng, W.-X., Zhao, Z.-T., Meng, N., Zhang, Q.-R., Zhu, W.-J., Li, R.-D., Liang, X.-L., & Li, Q.-Y. (2021). A Pilot Study of the Effect of Lactobacillus casei Obtained from Long-Lived Elderly on Blood Biochemical, Oxidative, and Inflammatory Markers, and on Gut Microbiota in Young Volunteers. Nutrients, 13(11), 3891. https://doi.org/10.3390/nu13113891





