Abstract
Marine n-3 fatty acids are well known to have health benefits. Recently, krill oil, which contains phospholipids, has been in the spotlight as an n-3 PUFA-containing oil. Euphausia pacifica (E. pacifica), also called North Pacific krill, is a small, red crustacean similar to shrimp that flourishes in the North Pacific Ocean. E. pacifica oil contains 8-hydroxyeicosapentaenoic acid (8-HEPE) at a level more than 10 times higher than Euphausia superba oil. 8-HEPE can activate the transcription of peroxisome proliferator-activated receptor alpha (PPARα), PPARγ, and PPARδ to levels 10, 5, and 3 times greater than eicosapentaenoic acid, respectively. 8-HEPE has beneficial effects against metabolic syndrome (reduction in body weight gain, visceral fat area, amount of gonadal white adipose tissue, and gonadal adipocyte cell size), dyslipidemia (reduction in serum triacylglycerol and low-density lipoprotein cholesterol and induction of serum high-density lipoprotein cholesterol), atherosclerosis, and nonalcoholic fatty liver disease (reduction in triglyceride accumulation and hepatic steatosis in the liver) in mice. Further studies should focus on the beneficial effects of North Pacific krill oil products and 8-HEPE on human health.
1. Introduction
It is well known that the liver has detoxification, metabolic, and storage functions. Consequently, when the liver is injured by disease, these functions are impaired. Obesity-related nonalcoholic fatty liver disease (NAFLD), which is a hepatic steatosis arising in the absence of alcohol intake, impairs the liver’s functions. NAFLD is known to participate in the development of other liver diseases, such as liver cirrhosis and hepatocellular carcinoma [1,2,3]. NAFLD is also associated with the development of diseases other than the liver, such as cardiovascular disease [4,5] and cognitive impairment [6,7,8]. These results indicate that the treatment of NAFLD is important to prevent several diseases and maintain normal liver function. Moreover, because metabolic syndrome (central adiposity, hyperglycemia, dyslipidemia, and arterial hypertension), weight gain, and insulin resistance/diabetes are risk factors for NAFLD [9,10], the elimination of these factors is important for suppressing the appearance of NAFLD, which would prevent the NAFLD-related diseases described above.
Marine n-3 polyunsaturated fatty acids (PUFA) are well known to have health benefits [11]. A diet containing n-3 PUFA, including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), reduces the incidence and mortality of cardiovascular disease through multiple mechanisms, such as the reduction in serum triacylglycerol (TAG) levels and anti-inflammatory effects [12]. Moreover, n-3 PUFAs play a positive role in the prevention and treatment of metabolic syndrome, including dyslipidemia [13,14] and NAFLD [15]. TAG-type fish oil is most commonly used as a source of n-3 PUFA [16]. In addition, krill oil, which contains phospholipids, has been in the spotlight as an n-3 PUFA-containing oil [17]. The intake of phospholipids has been reported to reduce serum total cholesterol and low-density lipoprotein (LDL) cholesterol levels [18]. It also inhibits intestinal cholesterol absorption [19], atherosclerosis, and liver damage [20] and improves brain function [21,22]. Therefore, as a health-promoting food, krill oil with phospholipids is considered to be better than TAG-type fish oil. In fact, a previous study reported that krill oil intake increases serum high-density lipoprotein (HDL) cholesterol levels more than TAG-type fish oil in humans [23].
Euphausia pacifica (E. pacifica) (Figure 1A), also called North Pacific krill, is a small, red crustacean similar to shrimp that flourishes in the North Pacific Ocean and is eaten in Japan. E. pacifica has large amounts of 8-hydroxyeicosapentaenoic acid (8-HEPE), which has physiological effects. This review discusses the chemical features of E. pacifica and the potential benefits of 8-hydroxyeicosapentaenoic acid (8-HEPE) extracted from E. pacifica against metabolic syndrome, dyslipidemia, NAFLD, and atherosclerosis in mice under the condition of a high-fat diet (HFD) or Western diet (WD).
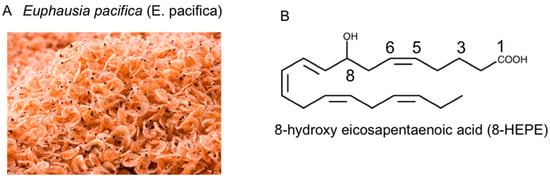
Figure 1.
(A) Photograph of Euphausia pacifica (E. pacifica), also called North Pacific krill. (B) The structural formula of 8-hydroxyeicosapentaenoic acid (8-HEPE).
2. Chemical Features of E. pacifica
2.1. Lipids of E. pacifica
“Krill” refers to Euphausiids, which are widespread in oceans worldwide. E. pacifica is a good source of marine n-3 PUFAs, which include EPA and DHA [24]. Both fish oil and krill oil are a source of EPA and DHA; however, the compositions of these oils are different. Fish oil is mostly composed of TAG, and krill oil is mostly composed of TAG and phospholipids. Krill oil also contains the phospholipid form of n-3 PUFA. Because the phospholipid form of n-3 PUFA is incorporated into plasma faster than the TAG form, krill oil can increase the n-3 index at a lower dose in humans [23,25]. Krill oil also contains the antioxidant astaxanthin.
At present, krill oil is generally E. superba (Antarctic krill) oil. There are several differences between E. pacifica oil and E. superba oil. The proportion of phospholipids in E. pacifica oil is higher than E. superba oil, and the content of oil is higher in E. superba than in E. pacifica. However, the most conspicuous difference between E. pacifica oil and E. superba oil is the content of 8-HEPE (Figure 1B), which is higher in E. pacifica oil [24]. In contrast, 8-HEPE in fish oil has not been detected. We analyzed the 8-HEPE content in several species, including Trachurus japonicus, Scomber japonicus, Haliotis, Patinopecten yessoensis, Heliocidaris crassispina, Pandalus eous, Metapenaeopsis barbaraz, Marsupenaeus japonicus and Balanus rostratus Hoek, but detected it only in Pandalus eous, Metapenaeopsis barbaraz, and Balanus rostratus Hoek [24]. Furthermore, the content of 8-HEPE in these crustaceans was less than one-twentieth of that in E. pacifica. From our analysis, E. pacifica is the best source of 8-HEPE. Furthermore, the 8-HEPE in E. pacifica is 8R-HEPE, which is metabolized from EPA by 8R-lipoxygenase [26,27].
2.2. 8-HEPE Extracted from E. pacifica and PPAR Activation
Methanol extract from E. pacifica activates the transcription of peroxisome proliferator-activated receptor alpha (PPARα), PPARγ, and PPARδ [28]. In addition, 5-HEPE, 8-HEPE, 9-HEPE, 12-HEPE, and 18-HEPE (hydroxylation products of EPA) obtained from methanol extracts of E. pacifica act as PPAR ligands. Two of these products, 8-HEPE and 9-HEPE, enhance the transcription levels of PPARs to a significantly greater extent than 5-HEPE, 12-HEPE, 18-HEPE, EPA, or EPA ethyl ester in NIH-3T3 cells [28]. In fact, 8-HEPE activates the transcription of PPARα, PPARγ, and PPARδ to levels 10, 5, and 3 times greater than EPA, respectively. 8-HEPE also increases the expression levels of genes regulated by PPARs, such as liver fatty-acid-binding protein, enoyl-CoA hydratase/3-hydroxyacyl CoA dehydrogenase, and carnitine palmitoyltransferase, in FaO cells. In contrast to 8-HEPE, EPA at the same concentration has weak or little effect on these gene expression levels and functions, indicating that 8-HEPE is the more potent inducer of physiological effects. As another good source of marine n-3 PUFAs, Antarctic krill oil has been reported to change PPARγ expression in bone diseases, such as osteoarthritis [29] and dexamethasone-induced osteoporosis [30]. Fish oil increases the hepatic mRNA levels of PPARα, liver fatty acid-binding protein, acyl CoA oxidase, cytochrome P450 4a14, and uncoupling protein 2, indicating PPARα activation [31]. It also increases PPARγ protein levels in pancreatic islets [32].
3. Potential Benefits of 8-HEPE Extracted from E. pacifica against NAFLD and Its Associated Diseases
3.1. Effects of 8-HEPE Extracted from E. pacifica on Metabolic Syndrome
Metabolic syndrome is the medical term for a combination of diabetes, hypertension, and obesity and causes dyslipidemia and fatty liver. Moreover, it is associated with greater risk of developing blood vessel diseases, such as coronary heart disease and stroke. The activation of hepatic PPARα could ameliorate body weight gain and improve insulin sensitivity in HFD-fed obese mice [33]. Moreover, adipocyte hypertrophy and the functional disorder of adipose tissue, such as reduced adiponectin secretion, have been reported to be associated with obesity [34]. 8-HEPE (9.5 mg/kg) extracted from E. pacifica reduced the amount of visceral fat (Figure 2A) [35], gonadal white adipose tissue, and the size of gonadal adipocyte cells in HFD-fed mice [36]. PPARα activators can increase hepatic fatty acid oxidation and decrease serum TAG levels, which are responsible for adipose cell hypertrophy and hyperplasia, leading to the regulation of obesity. Compared to EPA, 8-HEPE, which is a potent activator of PPARα, might improve metabolic syndrome.
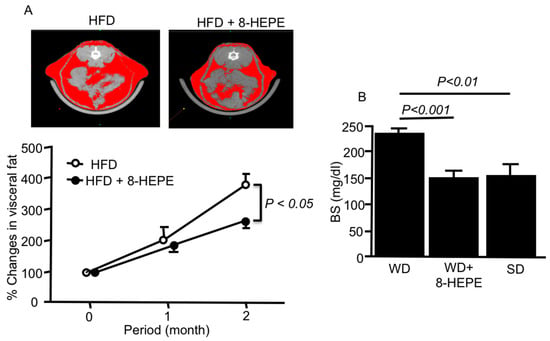
Figure 2.
(A) Representative computed tomography images of the abdomen (upper) and percentage changes in visceral fat area (lower) in mice fed an HFD only or an HFD with 8-HEPE. Visceral and subcutaneous fat areas are shown in red. Data are the mean ± SE obtained from eight mice for each group. (B) Blood sugar (BS) in apoE knock-out (apoE-KO) mice fed a WD only or WD with 8-HEPE. Data are the mean ± SE obtained from 10 mice for each group. An analysis of variance (ANOVA) with Dunnett’s test was used for the statistical analysis. HFD, high fat diet; 8-HEPE, 8-hydroxyeicosapentaenoic acid; WD, western diet.
It is well known that adipose tissue regulates energy homeostasis and insulin sensitivity through the secretion of leptin and adiponectin [37]. 8-HEPE increased angiopoietin-like protein 4 expression through PPARδ activation [28,38] more than EPA, leading to the enhancement of glucose uptake [28,39] in mouse myoblasts (C2C12). 8-HEPE (47 mg/kg) also decreased blood glucose levels in WD-fed apoE knock-out (apoE-KO) mice (Figure 2B) [40].
3.2. Effects of 8-HEPE Extracted from E. pacifica on Dyslipidemia
Dyslipidemia is defined by abnormal levels of plasma lipoproteins and TAG, and several types are known. Furthermore, dyslipidemia causes several complications, such as NAFLD and atherosclerosis, and its improvement is important for preventing these complications. Fibrates are an important group of drugs used to treat dyslipidemia in clinical practice. They are agonists of PPARα, which plays important roles in the normalization of plasma lipoproteins and TAG levels. GW590735, a PPARα agonist, increased HDL cholesterol and decreased LDL cholesterol, very low-density lipoprotein (VLDL) cholesterol, and TAG in hApoB100/hCETP mice [41]. Plasma TAG levels were significantly decreased in mice fed an HFD with 10 mg/kg 8-HEPE compared with HFD with EPA or HFD alone [36]. In contrast, plasma total cholesterol levels were similar between mice fed an HFD with 10 mg/kg 8-HEPE and HFD only [36]. Interestingly, 8-HEPE (83 mg/kg) suppressed the WD-induced increases in plasma LDL cholesterol in LDL cholesterol receptor knock-out (LDLR-KO) mice [42]. Moreover, it also increased plasma HDL cholesterol levels in WD-fed LDLR-KO mice. These results suggest that a low dose of 8-HEPE is enough to suppress plasma TAG, but a high dose of 8-HEPE might be needed to decrease plasma LDL cholesterol. Although n-3 PUFAs, especially EPA and DHA, play a positive role in the treatment of dyslipidemia [13], these beneficial effects are considered to be mainly due to the ability of n-3 PUFAs to reduce plasma TAG levels [43]. ATP-binding cassette transporter A1 (ABCA1) transports phospholipids and free cholesterol from macrophages to lipid-free apoA-I [44,45], leading to the generation of HDL particles [46,47]. 8-HEPE, but not EPA, increased the gene expression of ABCA1 in murine OxLDL-treated J774.1 macrophages [42]. Rayner et al. [48] showed that the elevated ABCA1 expression increased plasma HDL cholesterol in LDLR-KO mice. Therefore, 8-HEPE may be more effective at increasing circulating HDL cholesterol than EPA.
3.3. Effects of 8-HEPE Extracted from E. pacifica on NAFLD
Excessive calorie intake and lack of exercise have contributed to increased obesity and the prevalence of NAFLD in recent years. NAFLD is now the most important cause of chronic liver disease in the absence of excess alcohol consumption. NAFLD includes hepatic steatosis, which may progress to nonalcoholic steatohepatitis (NASH), fibrosis, and cirrhosis. Hepatic steatosis is characterized by the accumulation of TAG lipid droplets in the hepatocyte cytoplasm. Nonesterified fatty acids derived from the plasma are transported into the liver via transport proteins, such as CD36, which causes hepatic TAG accumulation. CD36 is expressed in a variety of cells, such as macrophages, and tissues [49]. Hajri et al. [50] showed that the deletion of nonhepatic CD36 gene expression causes hepatic steatosis and reduces muscle TAG contents in mice. Oil Red O histological staining, a marker of fat accumulation in the liver, was reduced in the liver of LDL-KO mice fed a WD with 83 mg/kg 8-HEPE compared with WD alone [42]. The content of TAG in the liver was also decreased in the WD with 8-HEPE than WD alone in these mice, suggesting that 8-HEPE can improve hepatic steatosis. We showed that 8-HEPE (50 μM) significantly increased CD36 gene expression in OxLDL-treated murine J774.1 macrophages [42]. This effect may improve hepatic steatosis through the relative increase in fatty acid uptake from plasma into the macrophages compared to the liver.
Fatty acids synthesized from glucose in the liver also play a role in the development of hepatic steatosis [51]. Muscle and liver insulin resistance promotes the accumulation of specific lipid metabolites [52]. Insulin resistance also promotes lipogenesis within the liver, leading to the development of hepatic steatosis. IL-6 signaling leads to a STAT3-dependent upregulation of SOCS3, which in turn induces insulin resistance in the liver [53]. Awazawa et al. [54] reported that IL-6 derived from macrophages contributes to the enhancement of hepatic insulin sensitivity through adiponectin. 8-HEPE increased IL-6 gene expression in macrophages. Moreover, 8-HEPE made adipocytes smaller in HFD-induced obese mice, suggesting increased adiponectin release from the adipocytes [37]. Therefore, 8-HEPE may improve hepatic steatosis by improving insulin sensitivity in the liver via higher IL-6 gene expression in macrophages and inhibited adipocyte hypertrophy. 8-HEPE decreased serum alanine aminotransferase (ALT) and hepatic TAG in HFD-fed mice [37]. ALT levels in the plasma are used as a marker to indicate hepatic disorders. In fact, Marinho et al. [55] showed that capybara oil decreased plasma ALT levels and improved hepatic steatosis. Moreover, an n-3 PUFA-enriched diet decreased serum ALT levels and damaged areas of the liver, including hepatocyte necrosis, in a Con-A-induced hepatitis mouse model [56]. Our previous studies indicate that 8-HEPE activates PPARα and increases fatty acid oxidation in the liver. In contrast to 8-HEPE, EPA failed to decrease liver TAG levels or plasma ALT or increase the levels of enoyl-CoA hydratase/3-hydroxyacyl CoA dehydrogenase, carnitine palmitoyltransferase, and expression of cytochrome P450 4a14 in the liver of HFD-fed mice [37]. Tanaka et al. [57] showed that PPARα eliminates fatty acids from the liver by increasing the expressions of several genes involved in hepatic fatty acid/triglyceride metabolism. Therefore, 8-HEPE may improve hepatic steatosis by increasing fatty acid oxidation via hepatic PPARα activation.
3.4. Effects of 8-HEPE Extracted from E. Pacifica on Atherosclerosis
Several studies have highlighted the association between NAFLD and increased carotid and coronary atherosclerosis [4,58,59]. Palolini et al. [20] demonstrated that Antarctic krill oil inhibits aortic atherosclerosis in WD-fed apoE-KO mice. We elucidated the effects of 8-HEPE extracted from North Pacific krill oil on aortic atherosclerosis using apoE-KO mice. Sudan IV staining demonstrated that 8-HEPE (47 mg/kg) reduced the area of aortic atherosclerosis in WD-fed apoE-KO mice (Figure 3) [40], suggesting that 8-HEPE works as an inhibitor of atherosclerosis. CD36 macrophages participate in atherosclerotic arterial lesion formation by interacting with oxLDL, and CD36 deficiency reduces atherosclerotic lesion formation [60]. Moreover, plasma OxLDL levels were increased in apoE-KO mice [61]. Therefore, 8-HEPE seems to aggravate atherosclerosis by increasing CD36 gene expression in macrophages. However, Moore et al. [62] showed that the loss of CD36 in apoE-KO mice did not alleviate atherosclerotic lesions. Moreover, Zhu et al. [63] showed that the scavenger receptor activity of CD16, which is different from that of CD36, also contributed to the progression of atherosclerosis in apoE-KO mice. Therefore, increased CD36 gene expression in macrophages may not always aggravate atherosclerosis.
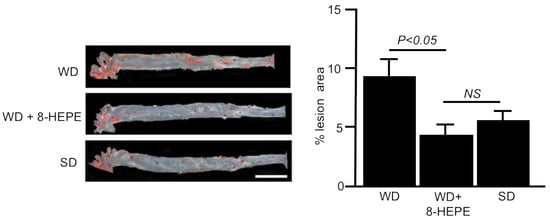
Figure 3.
Representative images of the aorta (left) stained with Sudan IV and percentage lesion area of atherosclerosis (right) in apoE-KO mice fed a Western diet (WD) only or a WD with 8-HEPE (WD + 8-HEPE). Data are the mean ± SE obtained from eight mice for each group. An analysis of variance (ANOVA) with Dunnett’s test was used for the statistical analysis. SD, standard diet; NS, not significant.
4. Conclusions
In this review, we explain the potentially beneficial effects of 8-HEPE against metabolic syndrome, dyslipidemia, NAFLD, and atherosclerosis (Figure 4). It is known that fish and krill oils containing n-3 PUFAs have health benefits against NAFLD, dyslipidemia, cardiovascular disease, diabetes, cancer, age-related cognitive decline, and rheumatoid arthritis. However, the beneficial effects of 8-HEPE against cognitive impairment are still unknown. This is an important question for aging societies. In addition, studies regarding 8-HEPE have demonstrated that (1) apoE carrier causes greater increase in EPA-derived 8-HEPE [64] and (2) EPA ethyl esters inhibit HFD-induced fat mass accumulation through EPA-derived 8-HEPE in female mice [65]. These results suggest 8-HEPE plays important roles in human health, even if it is derived from EPA. Further research is needed to investigate the potential benefits of 8-HEPE on human health. Moreover, there are controversial points regarding the effects of n-3 PUFAs on pathological/physiological processes, such as cancer, stroke, diabetes, and brain development, and proper clinical trials of n-3 PUFA-containing therapeutic drugs are lacking because of funding constraints. Therefore, further studies, including clinical investigation, are needed to investigate the beneficial effects of North Pacific krill oil products on human health.
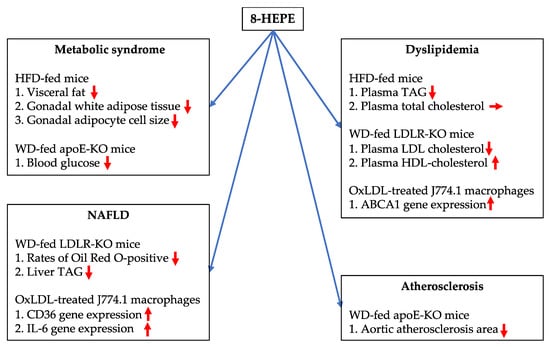
Figure 4.
Potential beneficial effects of 8-HEPE against metabolic syndrome, dyslipidemia, NAFLD, and atherosclerosis. HFD, high-fat diet; WD, Western diet; apoE-KO, apolipoprotein E knock out; LDLR-KO, low-density lipoprotein receptor knock out; TAG, triacylglycerol; IL-6, interleukin 6; OxLDL, oxidized LDL; ABCA1, ATP-binding cassette transporter 1.
Author Contributions
Conceptualization, M.H.; writing—original draft preparation, N.I. and H.Y.; writing—review and editing, N.I., H.Y. and M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funds from the Technology Agency and Project of the NARO Bio-oriented Technology Research Advancement Institution (special scheme project on vitalizing management entities of agriculture, forestry, and fisheries) # 16932713 (to H.Y. and M.H.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Starley, B.Q.; Calcagno, C.J.; Harrison, S.A. Nonalcoholic fatty liver disease and hepatocellular carcinoma: A weighty connection. Hepatology 2010, 51, 1820–1832. [Google Scholar] [CrossRef]
- Pennisi, G.; Celsa, C.; Giammanco, A.; Spatola, F.; Petta, S. The Burden of Hepatocellular Carcinoma in Non-Alcoholic Fatty Liver Disease: Screening Issue and Future Perspectives. Int. J. Mol. Sci. 2019, 20, 5613. [Google Scholar] [CrossRef] [Green Version]
- Kanda, T.; Goto, T.; Hirotsu, Y.; Masuzaki, R.; Moriyama, M.; Omata, M. Molecular Mechanisms: Connections between Nonalcoholic Fatty Liver Disease, Steatohepatitis and Hepatocellular Carcinoma. Int. J. Mol. Sci. 2020, 21, 1525. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, L.S.; Curzen, N.P.; Calder, P.C.; Byrne, C.D. Non-alcoholic fatty liver disease: A new and important cardiovascular risk factor? Eur. Heart J. 2012, 33, 1190–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Targher, G.; Bertolini, L.; Padovani, R.; Poli, F.; Scala, L.; Tessari, R.; Zenari, L.; Falezza, G. Increased prevalence of cardiovascular disease in Type 2 diabetic patients with non-alcoholic fatty liver disease. Diabet. Med. 2006, 23, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.W.; Gottesman, R.F.; Clark, J.M.; Hernaez, R.; Chang, Y.; Kim, C.; Ha, K.H.; Guallar, E.; Lazo, M. Nonalcoholic fatty liver disease is associated with cognitive function in adults. Neurology 2016, 86, 1136–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinstein, G.; Davis-Plourde, K.; Himali, J.J.; Zelber-Sagi, S.; Beiser, A.S.; Seshadri, S. Non-alcoholic fatty liver disease, liver fibrosis score and cognitive function in middle-aged adults: The Framingham Study. Liver Int. Off. J. Int. Assoc. Study Liver 2019, 39, 1713–1721. [Google Scholar] [CrossRef]
- Celikbilek, A.; Celikbilek, M.; Bozkurt, G. Cognitive assessment of patients with nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2018, 30, 944–950. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global Epidemiology of Nonalcoholic Fatty Liver Disease-Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Lindenmeyer, C.C.; McCullough, A.J. The Natural History of Nonalcoholic Fatty Liver Disease—An Evolving View. Clin. Liver. Dis. 2018, 22, 11–21. [Google Scholar] [CrossRef]
- Lorente-Cebrián, S.; Costa, A.G.; Navas-Carretero, S.; Zabala, M.; Martínez, J.A.; Moreno-Aliaga, M.J. Role of omega-3 fatty acids in obesity, metabolic syndrome, and cardiovascular diseases: A review of the evidence. J. Physiol. Biochem. 2013, 69, 633–651. [Google Scholar] [CrossRef]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.; AlAbdulghafoor, F.K.; Summerbell, C.D.; Worthington, H.V.; et al. N-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018, 11, CD003177. [Google Scholar] [PubMed]
- Wen, Y.; Dai, J.H.; Gao, Q. Effects of Omega-3 fatty acid on major cardiovascular events and mortality in patients with coronary heart disease: A meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Albracht-Schulte, K.; Kalupahana, N.S.; Ramalingam, L.; Wang, S.; Rahman, S.M.; Robert-McComb, J.; Moustaid-Moussa, N. Omega-3 fatty acids in obesity and metabolic syndrome: A mechanistic update. J. Nutr. Biochem. 2018, 58, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Spooner, M.H.; Jump, D.B. Omega-3 fatty acids and nonalcoholic fatty liver disease in adults and children: Where do we stand? Curr. Opin. Clin. Nutr. Metab. Care. 2019, 22, 103–110. [Google Scholar] [CrossRef]
- Takahashi, K.; Fukunaga, K.; Yoshioka, T. Purely domestic giant scallop oil as a more health beneficial oil than fish oil. Oleoscience 2018, 18, 341–348. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, N.; Suzuki, T.; Yamazaki, Y.; Sugiyama, K.; Koike, S.; Nishimukai, M. Supplemental feeding of phospholipid-enriched alkyl phospholipid from krill relieves spontaneous atopic dermatitis and strengthens skin intercellular lipid barriers in NC/Nga mice. Biosci. Biotechnol. Biochem. 2018, 83, 717–727. [Google Scholar] [CrossRef]
- Childs, M.T.; Bowlin, J.A.; Ogilvie, J.T.; Hazzard, W.R.; Albers, J.J. The contrasting effects of a dietary soya lecithin product and corn oil on lipoprotein lipids in normolipidemic and familial hypercholesterolemic subjects. Atherosclerosis 1981, 38, 217–228. [Google Scholar] [CrossRef]
- Rampone, A.J.; Machida, C.M. Mode of action of lecithin in suppressing cholesterol absorption. J. Lipid Res. 1981, 22, 744–752. [Google Scholar] [CrossRef]
- Parolini, C.; Bjorndal, B.; Busnelli, M.; Manzini, S.; Ganzetti, G.S.; Dellera, F.; Ramsvik, M.; Bruheim, I.; Berge, R.K.; Chiesa, G. Effect of Dietary Components from Antarctic Krill on Atherosclerosis in apoE-Deficient Mice. Mol. Nutr. Food Res. 2017, 61, 1700098. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.Y.; Moriyama, T.; Uezu, E.; Uezu, K.; Hirata, R.; Yohena, N.; Masuda, Y.; Kokubu, T.; Yamamoto, S. Administration of phosphatidylcholine increases brain acetylcholine concentration and improves memory in mice with dementia. J. Nutr. 1995, 125, 1484–1489. [Google Scholar]
- McDaniel, M.A.; Maier, S.F.; Einstein, G.O. “Brain-specific” nutrients: A memory cure? Nutrition 2003, 19, 957–975. [Google Scholar] [CrossRef]
- Ulven, S.M.; Kirkhus, B.; Lamglait, A.; Basu, S.; Elind, E.; Haider, T.; Berge, K.; Vik, H.; Pedersen, I.J. Metabolic effects of krill oil are essentially similar to those of fish oil but at lower dose of EPA and DHA, in healthy volunteers. Lipids 2011, 46, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Yamada, H.; Yamazaki, Y.; Koike, S.; Hakozaki, M.; Nagahora, N.; Yuki, S.; Yano, A.; Tsurumi, K.; Okumura, T. Lipids, fatty acids and hydroxy-fatty acids of Euphausia pacifica. Sci. Rep. 2017, 7, 9944. [Google Scholar] [CrossRef] [Green Version]
- Ramprasath, V.R.; Eyal, I.; Zchut, S.; Jones, P.J.H. Enhanced increase of omega-3 index in healthy individuals with response to 4-week n-3 fatty acid supplementation from krill oil versus fish oil. Lipids Health Dis. 2013, 12, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, H.; Kumagai, K.; Uemura, A.; Yuki, S. Euphausia pacifica as a source of 8(R)-hydroxy-eicosapentaenoic acid (8R-HEPE), 8(R)-hydroxy-eicosatetraenoic acid (8R-HETE) and 10(R)-hydroxy-docosahexaenoic acid (10R-HDHA). Biosci. Biotechnol. Biochem. 2020, 84, 455–462. [Google Scholar] [CrossRef]
- Yuki, S.; Uemura, A.; Hakozaki, M.; Yano, A.; Abe, M.; Misawa, Y.; Baba, N.; Yamada, H. Identification of a novel enzyme from E. pacifica that acts as an eicosapentaenoic 8R- LOX and docosahexaenoic 10R-LOX. Sci. Rep. 2020, 10, 20592. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Oshiro, E.; Kikuchi, S.; Hakozaki, M.; Takahashi, H.; Kimura, K.I. Hydroxyeicosapentaenoic acids from the Pacific krill show high ligand activities for PPARs. J. Lipid Res. 2014, 55, 895–904. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.; Zhang, L.; Yan, Z.; Li, Z.; Fu, M.; Xue, C.; Wang, J. A low proportion n-6/n-3 PUFA diet supplemented with Antarctic krill (Euphausia superba) oil protects against osteoarthritis by attenuating inflammation in ovariectomized mice. Food Funct. 2021, 12, 6766–6779. [Google Scholar] [CrossRef]
- Mao, L.; Wang, F.; Li, Y.; Dai, Y.; Liu, Y.; Wang, J.; Xue, C. Oil from Antarctic krill (Euphausia superba) facilitates bone formation in dexamethasone-treated mice. Food Sci. Biotechnol. 2018, 28, 539–545. [Google Scholar] [CrossRef]
- Larter, C.Z.; Yeh, M.M.; Cheng, J.; Williams, J.; Brown, S.; dela Pena, A.; Bell-Anderson, K.S.; Farrell, G.C. Activation of peroxisome proliferator-activated receptor alpha by dietary fish oil attenuates steatosis, but does not prevent experimental steatohepatitis because of hepatic lipoperoxide accumulation. J. Gastroenterol Hepatol. 2008, 23, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.R.; Chicco, A.; Lombardo, Y.B. Dietary fish oil normalized glucose-stimulated insulin secretion in isolated pancreatic islets of dyslipemic rats through mechanisms involving glucose phosphorylation, peroxisome proliferator-activated receptor γ and uncoupling protein 2. Prostaglandins Leukot. Essent. Fatty Acids 2013, 89, 31–38. [Google Scholar] [CrossRef]
- Lee, D.; Shin, Y.; Roh, J.S.; Ahn, J.; Jeoong, S.; Shin, S.S.; Yoon, M. Lemon Balm Extract ALS-L1023 regulates obesity and improves insulin sensitivity via activation of hepatic PPARα in high-fat diet-fed obese C57BL/6J mice. Int. J. Mol. Sci. 2020, 21, 4256. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Ginsberg, H.N. Adipocyte signaling and lipid homeostasis: Sequelae of insulin-resistant adipose tissue. Circ. Res. 2005, 96, 1042–1052. [Google Scholar] [CrossRef]
- Hirose, M.; Yamada, H.; Kamata, Y.; Yoshida, K.; Sasaki, H.; Tanji, M.; Ibi, M. Preventive Effect of New Functional Material Derived from E. pacifica on Visceral Fat. Society of Cerebral-Cardio-Vascular Anti-Aging 2015 Abstract. Available online: http://www.plus-s-ac.com/ccvaa/english.html (accessed on 28 November 2015).
- Yamada, H.; Kikuchi, S.; Hakozaki, M.; Motodate, K.; Nagahora, N.; Hirose, M. 8-Hydroxyeicosapentaenoic acid Ddcreases plasma and hepatic triglycerides via activation of peroxisome proliferator-activated receptor alpha in high-fat diet-induced obese mice. J. Lipids. 2016, 7498508. [Google Scholar] [CrossRef] [Green Version]
- Yadav, A.; Kataria, M.A.; Saini, V.; Yadav, A. Role of leptin and adiponectin in insulin resistance. Clin. Chim. Acta. 2013, 417, 80–84. [Google Scholar] [CrossRef]
- Staiger, H.; Haas, C.; Machann, J.; Werner, R.; Weisser, M.; Schick, F.; Machicao, F.; Stefan, N.; Fritsche, A.; Häring, H.-U. Musclederived angiopoietin-like protein 4 is induced by fatty acids via peroxisome proliferator-activated receptor (PPAR)-delta and is of metabolic relevance in humans. Diabetes 2009, 58, 579–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krämer, D.K.; Al-Khalili, L.; Perrini, S.; Skogsberg, J.; Wretenberg, P.; Kannisto, K.; Wallberg-Henriksson, H.; Ehrenborg, E.; Zierath, J.R.; Krook, A. Direct activation of glucose transport in primary human myotubes after activation of peroxisome proliferatoractivated receptor delta. Diabetes 2005, 54, 1157–1163. [Google Scholar] [CrossRef] [Green Version]
- Ibi, M.; Saito, M.; Ishida, N.; Yamada, Y.; Hirose, M. 8-HEPE concentrated materials from pacific krill suppresses atherosclerosis in apoE-deficient mice. Circulation 2019, 140 (Suppl. 1), A11153. [Google Scholar]
- Hansen, M.K.; McVey, M.J.; White, R.F.; Legos, J.J.; Brusq, J.M.; Grillot, D.A.; Issandou, M.; Barone, F.C. Selective CETP inhibition and PPARalpha agonism increase HDL cholesterol and reduce LDL cholesterol in human ApoB100/human CETP transgenic mice. J. Cardiovasc. Pharmacol. Ther. 2010, 15, 196–202. [Google Scholar] [CrossRef]
- Saito, M.; Ishida, N.; Yamada, H.; Ibi, M.; Hirose, M. 8-HEPE- concentrated materials from Pacific krill improve plasma cholesterol levels and hepatic steatosis in high cholesterol diet-fed low-density lipoprotein (LDL) receptor-deficient mice. Biol. Pharm. Bull. 2020, 43, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Huertas, E. The effect of EPA and DHA on metabolic syndrome patients: A systematic review of randomised controlled trials. Br. J. Nutr. 2012, 107, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Park, Y.; Koo, S. ATP-binding cassette transporter A1 and HDL metabolism: Effects of fatty acids. J. Nutr. Biochem. 2012, 23, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Landry, Y.; Denis, M.; Nandi, S.; Bell, S.; Vaughan, A.; Zha, X. ATP-binding cassette transporter A1 expression disrupts raft membrane microdomains through its ATPase-related functions. J. Biol. Chem. 2006, 281, 36091–36101. [Google Scholar] [CrossRef] [Green Version]
- Phillips, M. Molecular mechanisms of cellular cholesterol efflux. J. Biol. Chem. 2014, 289, 24020–24029. [Google Scholar] [CrossRef] [Green Version]
- Vedhachalam, C.; Duong, P.; Nickel, M.; Nguyen, D.; Dhanasekaran, P.; Saito, H.; Rothblat, G.H.; Lund-Katz, S.; Phillips, M.C. Mechanism of ATP-binding cassette transporter A1-mediated cellular lipid efflux to apolipoprotein A-I and formation of high-density lipoprotein particles. J. Biol. Chem. 2007, 282, 25123–25130. [Google Scholar] [CrossRef] [Green Version]
- Rayner, K.J.; Sheedy, F.J.; Esau, C.C.; Hussain, F.N.; Temel, R.E.; Parathath, S.; van Gils, J.M.; Rayner, A.J.; Chang, A.N.; Suarez, Y.; et al. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J. Clin. Invest. 2011, 121, 2921–2931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, C.G.; Tran, J.L.; Erion, D.M.; Vera, N.B.; Febbraio, M.; Weiss, E.J. Hepatocyte-specific disruption of CD36 attenuates fatty liver and improves insulin sensitivity in HFD-fed mice. Endocrinology 2016, 157, 570–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajri, T.; Han, X.X.; Bonen, A.; Abumrad, N.A. Defective fatty acid uptake modulates insulin responsiveness and metabolic responses to diet in CD36-null mice. J. Clin. Invest. 2002, 109, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [Green Version]
- Samuel, V.T.; Shulman, G.I. Mechanisms for insulin resistance: Common threads and missing links. Cell. 2012, 148, 852–871. [Google Scholar] [CrossRef] [Green Version]
- Schmidt-Arras, D.; Rose-John, S. IL-6 pathway in the liver: From physiopathology to therapy. J. Hepatol. 2016, 64, 1403–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awazawa, M.; Ueki, K.; Inabe, K.; Yamauchi, T.; Kubota, N.; Kaneko, K.; Kobayashi, M.; Iwane, A.; Sasako, T.; Okazaki, Y.; et al. Adiponectin enhances insulin sensitivity by increasing hepatic IRS-2 expression via a macrophage-derived IL-6-dependent pathway. Cell Metab. 2011, 13, 401–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marinho, P.C.; Vieira, A.B.; Pereira, P.G.; Rabelo, K.; Ciambarella, B.T.; Nascimento, A.L.; Cortez, E.; Moura, A.S.; Guimarães, F.V.; Martins, M.A.; et al. Capybara oil improves hepatic mitochondrial dysfunction, steatosis, and inflammation in a murine model of nonalcoholic fatty liver disease. Evid. Based Complement Alternat Med. 2018, 2018, 4956079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lian, M.; Luo, W.; Sui, Y.; Li, Z.; Hua, J. Dietary n-3 PUFA Protects Mice from Con A Induced Liver Injury by Modulating Regulatory T Cells and PPAR-γ Expression. PLoS ONE. 2015, 10, e0132741. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, N.; Aoyama, T.; Kimura, S.; Gonzalez, F.J. Targeting nuclear receptors for the treatment of fatty liver disease. Pharmacol. Ther. 2017, 179, 142–157. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Kozakova, M.; Hojlund, K.; Flyvbjerg, A.; Favuzzi, A.; Mitrakou, A.; Balkau, B. Fatty liver is associated with insulin resistance, risk of coronary heart disease, and early atherosclerosis in a large European population. Hepatology 2009, 49, 1537–1544. [Google Scholar] [CrossRef]
- Sung, K.C.; Wild, S.H.; Kwag, H.J.; Byrne, C.D. Fatty liver, insulin resistance, and features of metabolic syndrome: Relationships with coronary artery calcium in 10,153 people. Diabetes Care. 2012, 35, 2359–2364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.M. CD36, a scavenger receptor implicated in atherosclerosis. Exp. Mol. Med. 2014, 46, e99. [Google Scholar] [CrossRef] [Green Version]
- Kato, R.; Mori, C.; Kitazato, K.; Arata, S.; Obama, T.; Mori, M.; Takahashi, K.; Aiuchi, T.; Takano, T.; Itabe, H. Transient increase in plasma oxidized LDL during the progression of atherosclerosis in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 33–39. [Google Scholar] [CrossRef]
- Moore, K.J.; Kunjathoor, V.V.; Koehn, S.L.; Manning, J.J.; Tseng, A.A.; Silver, J.M.; McKee, M.; Freeman, M.W. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J. Clin. Invest. 2005, 115, 2192–2201. [Google Scholar] [CrossRef]
- Zhu, X.; Ng, H.P.; Lai, Y.C.; Craigo, J.K.; Nagilla, P.S.; Raghani, P.; Nagarajan, S. Scavenger receptor function of mouse Fcγ receptor III contributes to progression of atherosclerosis in apolipoprotein E hyperlipidemic mice. J. Immunol. 2014, 193, 2483–2495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleh, R.N.M.; West, A.L.; Ostermann, A.I.; Schebb, N.H.; Calder, P.C.; Minihane, A.M. APOE genotype modifies the plasma oxylipin response to omega-3 polyunsaturated fatty acid supplementation in healthy individuals. Front. Nutr. 2021, 8, 723813. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Sun, S.; Armstrong, M.; Manke, J.; Reisdorph, N.; Adams, V.R.; Kennedy, A.; Zu, Y.; Moustaid-Moussa, N.; Carroll, I.; et al. Beneficial effects of eicosapentaenoic acid on the metabolic profile of obese female mice entails upregulation of HEPEs and increased abundance of enteric Akkermansia Muciniphila. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids 2021, 1867, 159059. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).