Amino Acid Profile in Malnourished Patients with Liver Cirrhosis and Its Modification with Oral Nutritional Supplements: Implications on Minimal Hepatic Encephalopathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Outcome Measures
2.3. Statistical Analyses
3. Results
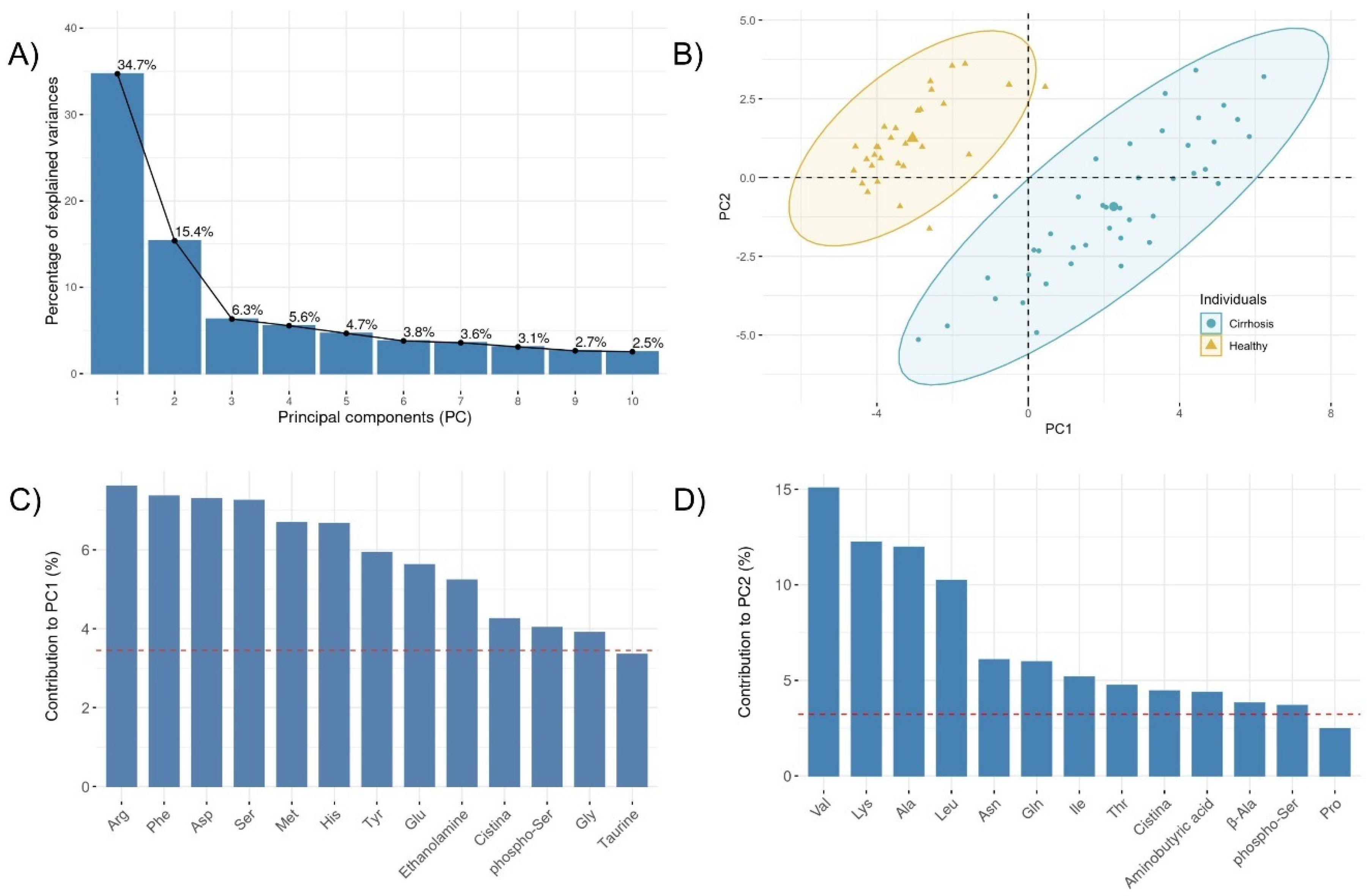
3.1. Specific Plasma Amino Acid Profile in Cirrhotic Patients
3.2. Baseline Characteristics of Cirrhotic Patients before Oral Nutritional Supplementation
3.3. Longitudinal Changes in Metabolic Parameters and Amino Acids during Oral Nutritional Supplementation
3.4. Amino Acid Signature of the Minimal Hepatic Encephalopathy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Periyalwar, P.; Dasarathy, S. Malnutrition in Cirrhosis: Contribution and Consequences of Sarcopenia on Metabolic and Clinical Responses. Clin. Liver Dis. 2012, 16, 95–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasarathy, S.; Merli, M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J. Hepatol. 2016, 65, 1232–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, J.E.; Rosen, H.M.; Ebeid, A.M.; James, J.H.; Keane, J.M.; Soeters, P.B. The effect of normalization of plasma amino acids on hepatic encephalopathy in man. Surgery 1976, 80, 77–91. [Google Scholar] [PubMed]
- Campollo, O.; Sprengers, D.; McIntyre, N. The BCAA/AAA ratio of plasma amino acids in three different groups of cirrhotics. Rev. Investig. Clin. 1992, 44, 513–518. [Google Scholar]
- Holeček, M. The Role of Skeletal Muscle in The Pathogenesis of Altered Concentrations of Branched-Chain Amino Acids (Valine, Leucine, and Isoleucine) in Liver Cirrhosis, Diabetes, and Other Diseases. Physiol. Res. 2021, 70, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, A.H.; McDonald, J.M.; E Reiman, R.; Gelbard, A.S.; Laughlin, J.S.; Duffy, T.E.; Plum, F. The dynamics of ammonia metabolism in man: Effects of liver disease and hyperammonemia. J. Clin. Investig. 1979, 63, 449–460. [Google Scholar] [CrossRef] [Green Version]
- Holecek, M. Branched-chain amino acids and ammonia metabolism in liver disease: Therapeutic implications. Nutrition 2013, 29, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Dasarathy, S. Cause and management of muscle wasting in chronic liver disease. Curr. Opin. Gastroenterol. 2016, 32, 159–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holecek, M. Evidence of a vicious cycle in glutamine synthesis and breakdown in pathogenesis of hepatic en-cephalopathy–therapeutic perspectives. Metab. Brain Dis. 2014, 29, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holecek, M. Ammonia and amino acid profiles in liver cirrhosis: Effects of variables leading to hepatic encephalopathy. Nutrition 2015, 31, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Eid, T.; Hassel, B.; Danbolt, N.C. Novel aspects of glutamine synthetase in ammonia homeostasis. Neurochem. Int. 2020, 140, 104809. [Google Scholar] [CrossRef] [PubMed]
- Merli, M.; Giusto, M.; Lucidi, C.; Giannelli, V.; Pentassuglio, I.; di Gregorio, V.; Lattanzi, B.; Riggio, O. Muscle depletion increases the risk of overt and minimal hepatic encephalopathy: Results of a prospective study. Metab. Brain Dis. 2012, 28, 281–284. [Google Scholar] [CrossRef]
- Patidar, K.R.; Bajaj, J.S. Covert and Overt Hepatic Encephalopathy: Diagnosis and Management. Clin. Gastroenterol. Hepatol. 2015, 13, 2048–2061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, C.F.; Amodio, P.; Bajaj, J.S.; Dhiman, R.K.; Montagnese, S.; Taylor-Robinson, S.D.; Vilstrup, H.; Jalan, R. Hepatic encephalopathy: Novel insights into classification, pathophysiology and therapy. J. Hepatol. 2020, 73, 1526–1547. [Google Scholar] [CrossRef] [PubMed]
- Weissenborn, K.; Ennen, J.C.; Schomerus, H.; Rückert, N.; Hecker, H. Neuropsychological characterization of hepatic encephalopathy. J. Hepatol. 2001, 34, 768–773. [Google Scholar] [CrossRef]
- Vasques, J.; Guerreiro, C.S.; Sousa, J.; Pinto, M.; Cortez-Pinto, H. Nutritional support in cirrhotic patients with sarcopenia. Clin. Nutr. ESPEN 2019, 33, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Taniguchi, E.; Sata, M. Effects of Oral Branched-Chain Amino Acids on Hepatic Encephalopathy and Outcome in Patients with Liver Cirrhosis. Nutr. Clin. Pr. 2013, 28, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Ooi, P.; Gilmour, S.; Yap, J.; Mager, D. Effects of branched chain amino acid supplementation on patient care outcomes in adults and children with liver cirrhosis: A systematic review. Clin. Nutr. ESPEN 2018, 28, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Kurpad, A.V.; Regan, M.M.; Raj, T.; Gnanou, J.V. Branched-Chain Amino Acid Requirements in Healthy Adult Human Subjects. J. Nutr. 2006, 136, 256S–263S. [Google Scholar] [CrossRef] [Green Version]
- Holeček, M. Branched-chain amino acid supplementation in treatment of liver cirrhosis: Updated views on how to attenuate their harmful effects on cataplerosis and ammonia formation. Nutrition 2017, 41, 80–85. [Google Scholar] [CrossRef]
- Holeček, M.; Šiman, P.; Vodenicarovova, M.; Kandar, R. Alterations in protein and amino acid metabolism in rats fed a branched-chain amino acid- or leucine-enriched diet during postprandial and postabsorptive states. Nutr. Metab. 2016, 13, 12. [Google Scholar] [CrossRef] [Green Version]
- Nissen, S.; Sharp, R.; Ray, M.; A Rathmacher, J.; Rice, D.; Fuller, J.C.; Connelly, A.S.; Abumrad, N. Effect of leucine metabolite beta-hydroxy-beta-methylbutyrate on muscle metabolism during resistance-exercise training. J. Appl. Physiol. 1996, 81, 2095–2104. [Google Scholar] [CrossRef]
- Holeček, M. Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J. Cachexia Sarcopenia Muscle 2017, 8, 529–541. [Google Scholar] [CrossRef]
- Girón, M.D.; Vílchez, J.D.; Salto, R.; Manzano, M.; Sevillano, N.; Campos, N.; Argilés, J.M.; Rueda, R.; López-Pedrosa, J.M. Conversion of leucine to β-hydroxy-β-methylbutyrate by α-keto isocaproate dioxygenase is required for a potent stimulation of protein synthesis in L6 rat myotubes. J. Cachexia Sarcopenia Muscle 2015, 7, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, B.; Bruni, A.; di Cola, S.; Molfino, A.; de Santis, A.; Muscaritoli, M.; Merli, M. The Effects of 12-Week Beta-Hydroxy-Beta-Methylbutyrate Supplementation in Patients with Liver Cirrhosis: Results from a Randomized Controlled Single-Blind Pilot Study. Nutrients 2021, 13, 2296. [Google Scholar] [CrossRef]
- Holeček, M.; Vodeničarovová, M. Effects of beta-hydroxy-beta-methylbutyrate supplementation on skeletal muscle in healthy and cirrhotic rats. Int. J. Exp. Pathol. 2019, 100, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.; Kumar, A.; Welch, N.; Sekar, J.; Mishra, S.; Bellar, A.; Gangadhariah, M.; Attaway, A.; Al Khafaji, H.; Wu, X.; et al. Multiomics-Identified Intervention to Restore Ethanol-Induced Dysregulated Proteostasis and Secondary Sarcopenia in Alcoholic Liver Disease. Cell. Physiol. Biochem. 2021, 55, 91–116. [Google Scholar] [CrossRef] [PubMed]
- Detsky, A.S.; McLaughlin, J.R.; Baker, J.P.; Johnston, N.; Whittaker, S.; Mendelson, R.A.; Jeejeebhoy, K.N. What is subjective global assessment of nutritional status? J. Parenter. Enter. Nutr. 1987, 11, 8–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pugh, R.N.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef]
- Cholongitas, E.; Papatheodoridis, G.; Vangeli, M.; Terreni, N.; Patch, D.; Burroughs, A.K. Systematic review: The model for end-stage liver disease—should it replace Child-Pugh’s classification for assessing prognosis in cirrhosis? Aliment. Pharmacol. Ther. 2005, 22, 1079–1089. [Google Scholar] [CrossRef]
- Kamath, P.S.; Wiesner, R.H.; Malinchoc, M.; Kremers, W.; Therneau, T.M.; Kosberg, C.L.; d’Amico, G.; Dickson, F.R.; Kim, W.R. A model to predict survival in patients with end-stage liver disease. Hepatology 2001, 33, 464–470. [Google Scholar] [CrossRef]
- Csapó, J.; Albert, C.; Lóki, K.; Csapó-Kiss, Z. Separation and determination of the amino acids by ion exchange column chromatography applying postcolumn derivatization. Acta Univ. Sapientiae Aliment. 2008, 1, 5–29. [Google Scholar]
- Sharer, J.D.; de Biase, I.; Matern, D.; Young, S.; Bennett, M.J.; Tolun, A.A. Laboratory analysis of amino acids, 2018 revision: A technical standard of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2018, 20, 1499–1507. [Google Scholar] [CrossRef] [Green Version]
- Fischer, J.; Yoshimura, N.; Aguirre, A.; James, J.; Cummings, M.; Aybel, R.; Deindoerfer, F. Plasma amino acids in patients with hepatic encephalopathy: Effects of amino acid infusions. Am. J. Surg. 1974, 127, 40–47. [Google Scholar] [CrossRef]
- Morgan, M.Y.; Marshall, A.W.; Milsom, J.P.; Sherlock, S. Plasma amino-acid patterns in liver disease. Gut 1982, 23, 362–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabr, S.A.; Alghadir, A.H.; Sherif, Y.E.; Ghfar, A.A. Hydroxyproline as a Biomarker in Liver Disease. In Biomarkers in Liver Disease; Predy, V.R., Ed.; Springer: Dordrecht, The Netherlands, 2016; pp. 1–21. [Google Scholar] [CrossRef]
- Jayakumar, A.; Norenberg, M.D. Hyperammonemia in Hepatic Encephalopathy. J. Clin. Exp. Hepatol. 2018, 8, 272–280. [Google Scholar] [CrossRef]
- Fischer, J.; Baldessarini, R. False neurotransmitters and hepatic failure. Lancet 1971, 298, 75–80. [Google Scholar] [CrossRef]
- Holeček, M.; Mráz, J.; Tilšer, I. Plasma amino acids in four models of experimental liver injury in rats. Amino Acids 1996, 10, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.; Berasain, C.; Torres, L.; Martín-Duce, A.; Corrales, F.J.; Yang, H.; Prieto, J.; Lu, S.C.; Caballería, J.; Rodés, J.; et al. Reduced mRNA abundance of the main enzymes involved in methionine metabolism in human liver cirrhosis and hepatocellular carcinoma. J. Hepatol. 2000, 33, 907–914. [Google Scholar] [CrossRef] [Green Version]
- Mattson, M.P.; Shea, T.B. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disor-ders. Trends Neurosci. 2003, 26, 137–146. [Google Scholar] [CrossRef]
- Koretz, R.L.; Avenell, A.; O Lipman, T. Nutritional support for liver disease. Cochrane Database Syst. Rev. 2012, 5, CD008344. [Google Scholar] [CrossRef] [PubMed]
- Gluud, L.L.; Dam, G.; Les, I.; Marchesini, G.; Borre, M.; Aagaard, N.K.; Vilstrup, H. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst. Rev. 2017, 5, CD001939. [Google Scholar] [CrossRef] [PubMed]
- Dam, G.; Keiding, S.; Munk, O.L.; Ott, P.; Buhl, M.; Vilstrup, H.; Bak, L.K.; Waagepetersen, H.S.; Schousboe, A.; Moller, N.; et al. Branched-chain amino acids increase arterial blood ammonia in spite of enhanced intrinsic muscle ammonia metabolism in patients with cirrhosis and healthy subjects. Am. J. Physiol. Liver Physiol. 2011, 301, G269–G277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanchi, N.E.; Gerlinger-Romero, F.; Guimarães-Ferreira, L.; Filho, M.A.D.S.; Felitti, V.; Lira, F.S.; Seelaender, M.; Lancha, A.H. HMB supplementation: Clinical and athletic performance-related effects and mechanisms of action. Amino Acids 2010, 40, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.R.; Belozo, F.L.; Micheletti, T.O.; Conrado, M.; Stout, J.R.; Pimentel, G.D.; Gonzalez, A.M. β-hydroxy-β-methylbutyrate free acid supplementation may improve recovery and muscle adaptations after resistance training: A systematic review. Nutr. Res. 2017, 45, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Martinez, J.; Santos-Lozano, A.; Garcia-Hermoso, A.; Sadarangani, K.P.; Cristi-Montero, C. Effects of beta-hydroxy-beta-methylbutyrate supplementation on strength and body composition in trained and competitive athletes: A meta-analysis of randomized controlled trials. J. Sci. Med. Sport 2018, 21, 727–735. [Google Scholar] [CrossRef]
- Gepner, Y.; Varanoske, A.N.; Boffey, D.; Hoffman, J.R. Benefits of β-hydroxy-β-methylbutyrate supplementation in trained and untrained individuals. Res. Sports Med. 2018, 27, 204–218. [Google Scholar] [CrossRef]
- Wu, H.; Xia, Y.; Jiang, J.; Du, H.; Guo, X.; Liu, X.; Li, C.; Huang, G.; Niu, K. Effect of beta-hydroxy-beta-methylbutyrate supplementation on muscle loss in older adults: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2015, 61, 168–175. [Google Scholar] [CrossRef]
- Oktaviana, J.; Zanker, J.; Vogrin, S.; Duque, G. The Effect of β-Hydroxy-β-Methylbutyrate (HMB) on Sarcopenia and Functional Frailty in Older Persons: A Systematic Review. J. Nutr. Health Aging 2018, 23, 145–150. [Google Scholar] [CrossRef]
- Holecek, M.; Muthny, T.; Kovarik, M.; Sispera, L. Effect of beta-hydroxy-beta-methylbutyrate (HMB) on protein metabolism in whole body and in selected tissues. Food Chem. Toxicol. 2009, 47, 255–259. [Google Scholar] [CrossRef]
- Madeira, C.; Alheira, F.V.; Calcia, M.; Silva, T.C.S.; Tannos, F.M.; Vargas-Lopes, C.; Fisher, M.; Goldenstein, N.; Brasil, M.A.; Vinogradov, S.; et al. Blood Levels of Glutamate and Glutamine in Recent Onset and Chronic Schizophrenia. Front. Psychiatry 2018, 9, 713. [Google Scholar] [CrossRef] [PubMed]
- Cavallucci, V.; Pani, G. The Leucine Catabolite and Dietary Supplement β-Hydroxy-β-Methyl Butyrate (HMB) as an Epigenetic Regulator in Muscle Progenitor Cells. Metabolites 2021, 11, 512. [Google Scholar] [CrossRef] [PubMed]

| Cirrhosis | Healthy | p | |
|---|---|---|---|
| n = 43 | n = 31 | ||
| Sex (men/women) | 27 (62%)/16 (38%) | 15 (48%)/16 (52%) | 0.362 |
| Age (years) | 64.4 [55.3;68.6] | 57.0 [47.5;66.5] | 0.054 |
| Amino acid (µM) | |||
| phospho-Ser | 3.10 [2.16;4.44] | 12.2 [8.75;16.9] | <0.001 |
| Taurine | 64.1 [52.7;83.7] | 39.8 [35.4;55.5] | <0.001 |
| Asp | 27.2 [21.9;37.0] | 1.75 [1.27;2.51] | <0.001 |
| Thr | 132 [105;172] | 120 [110;147] | 0.429 |
| Ser | 151 [125;177] | 92.0 [78.4;102] | <0.001 |
| Asn | 74.6 [58.3;83.2] | 100 [77.9;112] | <0.001 |
| Glu | 156 [120;221] | 30.3 [20.1;50.1] | <0.001 |
| Gln | 317 [249;393] | 631 [557;685] | <0.001 |
| Aminoadipic acid | 3.67 [3.14;4.54] | 0.73 [0.00;2.85] | <0.001 |
| Gly | 276 [228;322] | 195 [169;221] | <0.001 |
| Ala | 305 [246;391] | 333 [288;378] | 0.354 |
| Citrulline | 42.3 [30.7;50.8] | 36.2 [31.1;50.1] | 0.629 |
| Aminobutyric acid | 15.2 [11.6;19.4] | 14.7 [9.99;18.7] | 0.600 |
| Val | 166 [128;221] | 202 [190;255] | 0.010 |
| Cystine | 1.77 [0.71;4.92] | 46.2 [33.3;58.2] | <0.001 |
| Met | 34.1 [25.9;42.6] | 17.2 [13.9;21.9] | <0.001 |
| Ile | 54.1 [47.2;66.5] | 47.8 [40.1;59.5] | 0.072 |
| Leu | 103 [83.6;123] | 103 [89.3;121] | 0.899 |
| Tyr | 104 [75.6;129] | 56.4 [47.6;65.3] | <0.001 |
| β-Ala | 2.73 [1.25;4.34] | 0.00 [0.00;0.00] | <0.001 |
| Phe | 89.7 [72.1;104] | 44.7 [41.0;50.7] | <0.001 |
| Ethanolamine | 18.5 [13.9;23.4] | 0.00 [0.00;0.00] | <0.001 |
| Ornithine | 93.0 [74.0;120] | 77.6 [69.0;87.3] | 0.009 |
| Lys | 163 [123;187] | 173 [155;190] | 0.159 |
| 1-Methylhistidine | 11.9 [8.04;25.3] | 10.3 [5.24;15.4] | 0.060 |
| His | 89.3 [74.0;97.3] | 57.6 [47.3;63.6] | <0.001 |
| Trp | 42.7 [30.4;52.8] | 30.4 [25.0;37.7] | 0.015 |
| 3-Methylhistidine | 5.58 [3.94;8.07] | 1.37 [0.00;3.40] | <0.001 |
| Arg | 120 [102;151] | 54.1 [40.6;69.3] | <0.001 |
| Hyp | 16.4 [10.8;22.9] | 9.87 [2.46;15.6] | 0.021 |
| Pro | 205 [161;259] | 174 [136;216] | 0.092 |
| HMB Group (n = 22) | HP Group (n = 21) | p | |
|---|---|---|---|
| Age (Years) | 60.4 ± 8.61 | 61.4 ± 9.27 | 0.711 |
| Sex (men/women) | 14 (64%)/8 (36%) | 13 (62%)/8 (38%) | 1.000 |
| Etiology n (%) | |||
| Alcohol | 17 (77.3%) | 11 (52.4%) | 0.624 |
| HCV | 2 (9.09%) | 3 (14.3%) | |
| MAFLD | 1 (4.55%) | 3 (14.3%) | |
| Others | 2 (9.09%) | 4 (19%) | |
| Active alcoholism | 4 (18.1%) | 4 (19%) | 0.683 |
| Ascites | 12 (54.5%) | 9 (42.9%) | 0.645 |
| Refractory ascites | 4 (18.3%) | 0 (0%) | 0.108 |
| TIPS | 1 (4.55%) | 1 (4.76%) | 1.000 |
| Previous encephalopathy | 2 (9.09%) | 3 (14.3%) | 0.664 |
| MHE (PHES) * | 8 (36.4%) | 4 (19%) | 0.355 |
| Child-Pugh | 0.398 | ||
| Class A | 9 (40.9%) | 10 (47.6%) | |
| Class B | 11 (50%) | 11 (52.3%) | |
| Class C | 2 (9.09%) | 0 (0%) | |
| MELD | 12.7 ± 5.31 | 13 ± 4.7 | 0.835 |
| SGA | 0.355 | ||
| Class B | 14 (63.6%) | 17 (81.0%) | |
| Class C | 8 (36.4%) | 4 (19.0%) | |
| Ammonia (µM) | 56.0 [40.0;83.0] | 54.0 [39.8;78.0] | 0.923 |
| Plasma Amino Acid (µM) | HMB Group | HP Group | plong | plong*treatment | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 6 wk. | 12 wk. | Baseline | 6 wk. | 12 wk. | |||
| Leu | 92.4 [76.7;117] | 120 [105;132] | 117 [105;130] | 117 [95.5;137] | 116 [102;134] | 118 [93.9;140] | 0.01 | 0.035 |
| Ile | 53.3 [47.7;59.9] | 63.1 [56.7;72.8] | 61.9 [52.8;70.9] | 62.6 [49.6;71.9] | 57.9 [50.2;66.2] | 56.3 [49.3;75.5] | 0.532 | 0.084 |
| Val | 144 [109;191] | 199 [180;245] | 194 [161;210] | 191 [160;253] | 220 [173;244] | 215 [167;237] | 0.004 | 0.055 |
| BCAA | 300 [244;361] | 382 [348;428] | 376 [320;404] | 370 [297;462] | 385 [325;425] | 380 [306;456] | 0.012 | 0.046 |
| Phe | 81.5 [72.8;94.2] | 118 [81,4;128] | 111 [96.6;118] | 102 [78.2;122] | 115 [86.9;129] | 102 [92.6;119] | 0.001 | 0.057 |
| Tyr | 101 [73.1;129] | 119 [74.0;150] | 118 [80.5;137] | 104 [91.5;130] | 131 [110;158] | 106 [98.3;119] | 0.001 | 0.408 |
| Trp | 35.6 [30.8;45.6] | 47.7 [39.4;59.6] | 48.2 [34.2;61.8] | 51.3 [33.2;67.2] | 59.1 [46.4;62.2] | 56.7 [40.8;64.8] | 0.012 | 0.066 |
| AAA | 234 [188;265] | 264 [196;342] | 284 [226;310] | 270 [190;324] | 316 [229;344] | 266 [247;304] | 0.003 | 0.103 |
| Fischer´s ratio | 1.59 [1.32;2.43] | 1.58 [1.37;2.11] | 1.70 [1.39;2.29] | 1.80 [1.43;2.21] | 1.57 [1.25;1.84] | 1.64 [1.39;2.03] | 0.013 | 0.586 |
| Glu | 134 [118;184] | 223 [170;302] | 180 [142;235] | 182 [151;269] | 190 [118;252] | 212 [178;249] | 0.214 | 0.164 |
| Gln | 317 [224;390] | 284 [227;348] | 322 [215;344] | 308 [262;372] | 315 [264;400] | 332 [275;381] | 0.932 | 0.862 |
| Gln/Glu ratio | 2.16 [1.15;3.32] | 1.78 [0.72;2.24] | 1.69 [1.31;2.46] | 1.63 [1.10;2.79] | 2.07 [1.17;2.64] | 1.52 [1.24;2.18] | 0.31 | 0.272 |
| Ala | 267 [216;340] | 371 [268;464] | 360 [332;427] | 331 [283;442] | 392 [332;476] | 391 [302;428] | 0.004 | 0.093 |
| Ammonia (µM) | 56.0 [40.0;83.0] | 59.0 [50.5;85.2] | 67.0 [49.8;74.8] | 54.0 [39.8;78.0] | 62.0 [53.2;87.2] | 67.0 [55.5;75.5] | 0.11 | 0.689 |
| Plasma Amino Acid Concentration (µM) | MHE − n = 82 | MHE + n = 26 | p |
|---|---|---|---|
| Phospho-Ser | 3.37 [2.46;4.33] | 2.37 [1.83;3.93] | 0.061 |
| Taurine | 76.0 [57.0;94.2] | 56.6 [44.1;66.9] | 0.004 |
| Asp | 36.2 [28.3;43.0] | 20.2 [14.4;26.5] | <0.001 |
| Thr | 143 [117;175] | 135 [119;174] | 0.882 |
| Ser | 167 [146;184] | 128 [103;160] | <0.001 |
| Asn | 73.6 [64.3;84.8] | 78.0 [64.1;90.5] | 0.402 |
| Glu | 209 [161;270] | 135 [95.5;177] | <0.001 |
| Gln | 319 [242;382] | 309 [287;419] | 0.722 |
| Aminoadipic acid | 3.96 [2.98;4.77] | 3.28 [2.65;4.09] | 0.150 |
| Gly | 299 [266;331] | 261 [230;283] | 0.002 |
| Ala | 377 [311;468] | 267 [246;353] | <0.001 |
| Citrulline | 44.7 [37.3;53.8] | 47.3 [39.0;58.4] | 0.327 |
| Aminobutyric acid | 15.5 [12.4;20.4] | 14.7 [8.06;17.5] | 0.121 |
| Val | 202 [171;246] | 146 [114;181] | <0.001 |
| Cystine | 1.23 [0.00;3.38] | 1.52 [0.71;4.95] | 0.267 |
| Met | 36.1 [27.5;47.6] | 37.5 [28.9;40.7] | 0.840 |
| Ile | 62.1 [52.6;73.6] | 48.5 [44.1;59.9] | 0.001 |
| Leu | 118 [103;139] | 91.2 [68.0;104] | <0.001 |
| Tyr | 113 [87.8;138] | 112 [91.5;141] | 0.735 |
| β-Ala | 3.85 [0.89;5.94] | 3.62 [1.21;5.00] | 0.622 |
| Phe | 105 [88.9;125] | 86.7 [76.7;113] | 0.045 |
| Ethanolamine | 17.2 [13.8;21.2] | 20.8 [16.7;28.5] | 0.121 |
| Ornithine | 105 [84.6;131] | 98.1 [81.8;109] | 0.241 |
| Lys | 179 [140;201] | 147 [133;182] | 0.098 |
| 1-Methylhistidine | 12.4 [8.19;23.0] | 11.6 [6.24;22.9] | 0.482 |
| His | 89.0 [77.8;99.0] | 87.1 [76.2;92.6] | 0.216 |
| Trp | 51.9 [38.2;63.4] | 45.4 [27.3;56.9] | 0.089 |
| 3-Methylhistidine | 7.00 [4.31;11.5] | 6.86 [5.14;9.01] | 0.758 |
| Arg | 133 [114;151] | 113 [85.3;144] | 0.032 |
| Hyp | 17.2 [10.5;23.2] | 16.8 [13.4;24.8] | 0.639 |
| Pro | 232 [178;278] | 239 [174;329] | 0.591 |
| BCAA | 383 [325;457] | 289 [220;336] | <0.001 |
| AAA | 269 [220;320] | 237 [193;297] | 0.211 |
| Fischer ratio | 1.80 [1.38;2.42] | 1.45 [1.18;1.60] | 0.001 |
| Gln/Glu ratio | 1.72 [1.11;2.19] | 2.38 [1.48;3.93] | 0.022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espina, S.; Gonzalez-Irazabal, Y.; Sanz-Paris, A.; Lopez-Yus, M.; Garcia-Sobreviela, M.P.; del Moral-Bergos, R.; Garcia-Rodriguez, B.; Fuentes-Olmo, J.; Bernal-Monterde, V.; Arbones-Mainar, J.M. Amino Acid Profile in Malnourished Patients with Liver Cirrhosis and Its Modification with Oral Nutritional Supplements: Implications on Minimal Hepatic Encephalopathy. Nutrients 2021, 13, 3764. https://doi.org/10.3390/nu13113764
Espina S, Gonzalez-Irazabal Y, Sanz-Paris A, Lopez-Yus M, Garcia-Sobreviela MP, del Moral-Bergos R, Garcia-Rodriguez B, Fuentes-Olmo J, Bernal-Monterde V, Arbones-Mainar JM. Amino Acid Profile in Malnourished Patients with Liver Cirrhosis and Its Modification with Oral Nutritional Supplements: Implications on Minimal Hepatic Encephalopathy. Nutrients. 2021; 13(11):3764. https://doi.org/10.3390/nu13113764
Chicago/Turabian StyleEspina, Silvia, Yolanda Gonzalez-Irazabal, Alejandro Sanz-Paris, Marta Lopez-Yus, Maria Pilar Garcia-Sobreviela, Raquel del Moral-Bergos, Beatriz Garcia-Rodriguez, Javier Fuentes-Olmo, Vanesa Bernal-Monterde, and Jose M. Arbones-Mainar. 2021. "Amino Acid Profile in Malnourished Patients with Liver Cirrhosis and Its Modification with Oral Nutritional Supplements: Implications on Minimal Hepatic Encephalopathy" Nutrients 13, no. 11: 3764. https://doi.org/10.3390/nu13113764
APA StyleEspina, S., Gonzalez-Irazabal, Y., Sanz-Paris, A., Lopez-Yus, M., Garcia-Sobreviela, M. P., del Moral-Bergos, R., Garcia-Rodriguez, B., Fuentes-Olmo, J., Bernal-Monterde, V., & Arbones-Mainar, J. M. (2021). Amino Acid Profile in Malnourished Patients with Liver Cirrhosis and Its Modification with Oral Nutritional Supplements: Implications on Minimal Hepatic Encephalopathy. Nutrients, 13(11), 3764. https://doi.org/10.3390/nu13113764







