The Vessels-Bone Axis: Iliac Artery Calcifications, Vertebral Fractures and Vitamin K from VIKI Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Laboratory Assays
2.2.1. Parathyroidhormone (PTH)
2.2.2. 25-OH Vitamin D
2.2.3. Total BGP
2.2.4. Undercarboxylated BGP (ucBGP)
2.2.5. Total Matrix GLA Protein (MGP)
2.2.6. Undercarboxylated MGP (ucMGP)
2.3. Assessment of Vertebral Fracture and Vascular Calcification
2.4. Statistical Analysis
3. Results
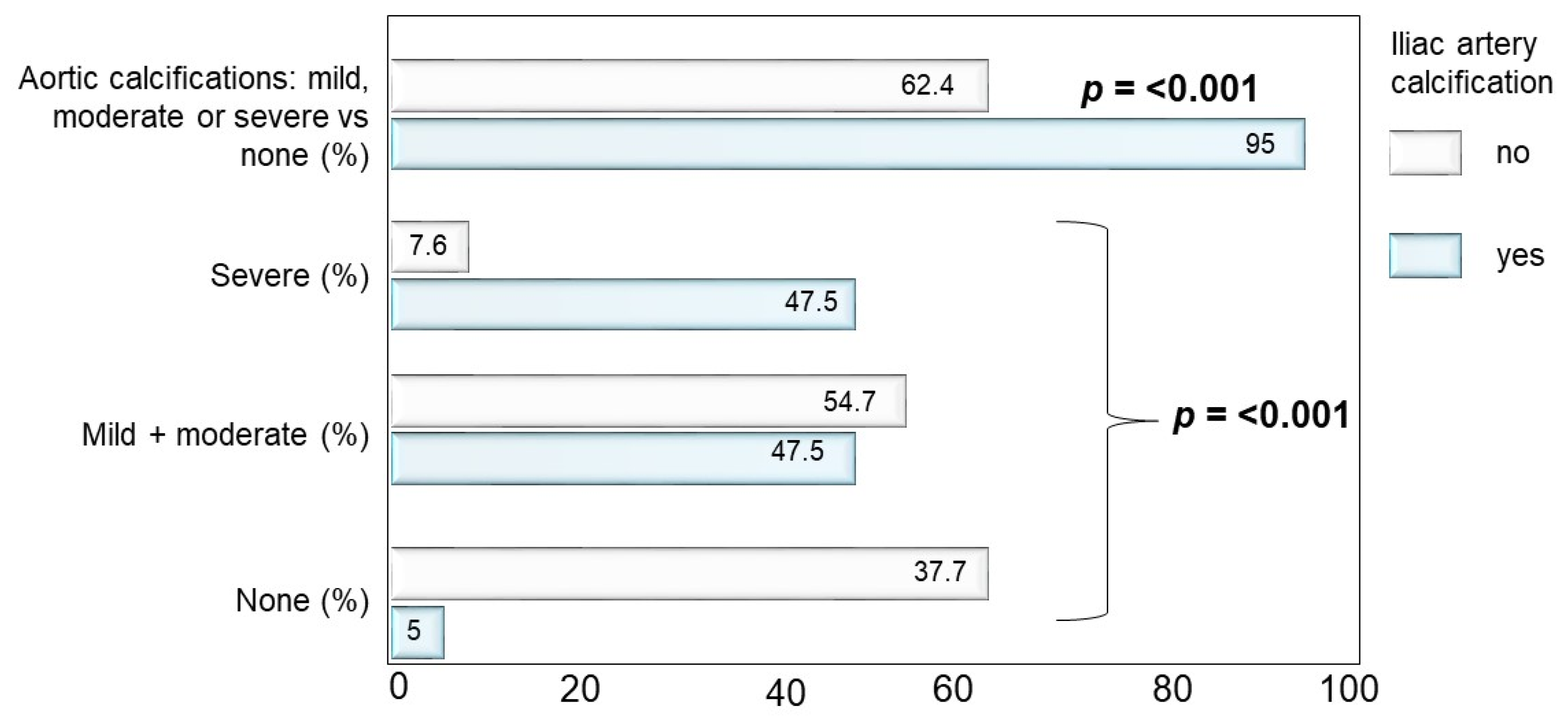
3.1. Interrelationship between Aortic and Iliac Calcifications
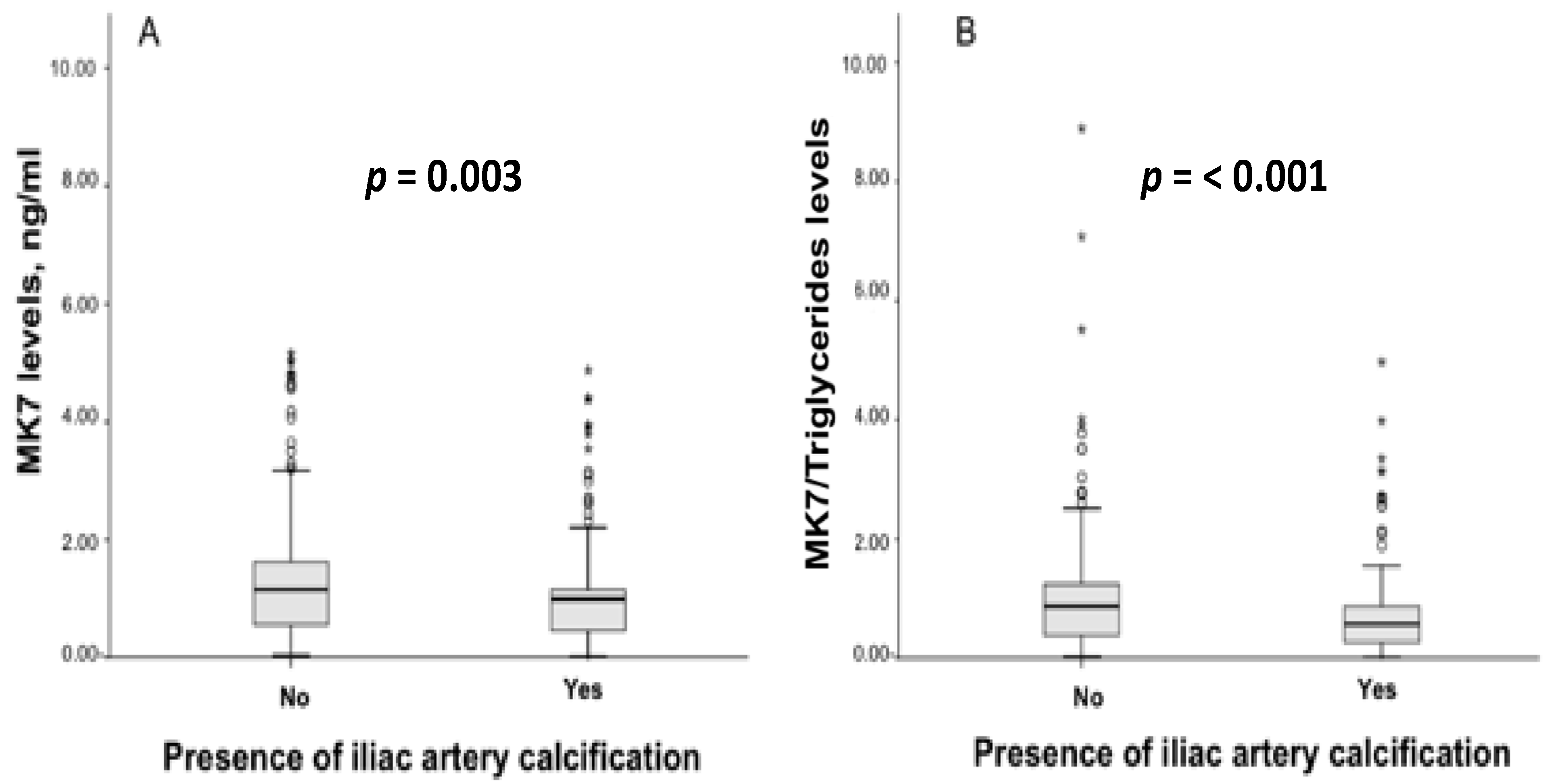
3.2. Vitamin K Levels and Pharmacological Treatment According to Iliac Artery Calcifications
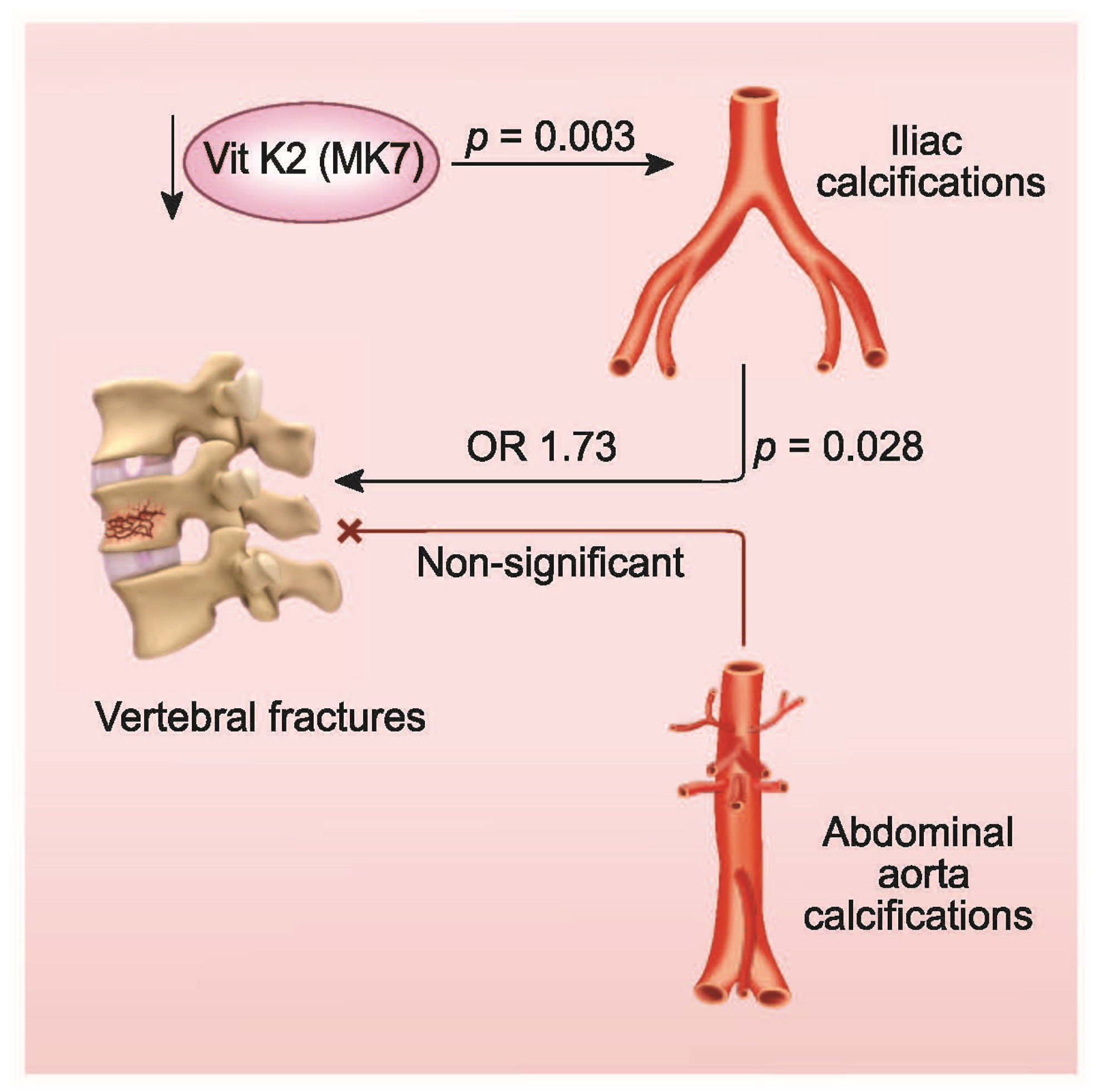
3.3. Multiple Logistic Regression Model of Vertebral Fractures
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, J.; Mao, Y.; Nieves, J.W. Identification of prevalent vertebral fractures using Vertebral Fracture Assessment (VFA) in asymptomatic postmenopausal women: A systematic review and meta-analysis. Bone 2020, 136, 115358. [Google Scholar] [CrossRef] [PubMed]
- Gehlbach, S.H.; Bigelow, C.; Heimisdottir, M.; May, S.; Walker, M.; Kirkwood, J.R. Recognition of Vertebral Fracture in a Clinical Setting. Osteoporos. Int. 2000, 11, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Delmas, P.D.; van de Langerijt, L.; Watts, N.B.; Eastell, R.; Genant, H.; Grauer, A.; Cahall, D.L. Underdiagnosis of Vertebral Fractures Is a Worldwide Problem: The IMPACT Study. J. Bone Miner. Res. 2004, 20, 557–563. [Google Scholar] [CrossRef]
- Arboleya, L.; Díaz-Curiel, M.; Del Río, L.; Blanch, J.; Díez-Pérez, A.; Guañabens, N.; Quesada, J.M.; Sosa, M.; Gómez, C.; Muñoz-Torres, M.; et al. Prevalence of vertebral fracture in postmenopausal women with lumbar osteopenia using MorphoXpressSR (OSTEOXPRESS Study). Aging Clin. Exp. Res. 2010, 22, 419–426. [Google Scholar] [CrossRef]
- Lindsay, R. Risk of New Vertebral Fracture in the Year Following a Fracture. JAMA 2001, 285, 320. [Google Scholar] [CrossRef]
- Tentori, F.; McCullough, K.; Kilpatrick, R.D.; Bradbury, B.D.; Robinson, B.M.; Kerr, P.G.; Pisoni, R.L. High rates of death and hospitalization follow bone fracture among hemodialysis patients. Kidney Int. 2014, 85, 166–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fusaro, M.; Tripepi, G.; Noale, M.; Vajente, N.; Plebani, M.; Zaninotto, M.; Guglielmi, G.; Miotto, D.; Carbonare, L.D.; D’Angelo, A.; et al. High Prevalence of Vertebral Fractures Assessed by Quantitative Morphometry in Hemodialysis Patients, Strongly Associated with Vascular Calcifications. Calcif. Tissue Int. 2013, 93, 39–47. [Google Scholar] [CrossRef]
- Giannini, S.; Sella, S.; Silva Netto, F.; Cattelan, C.; Dalle Carbonare, L.; Lazzarin, R.; Marchini, F.; Rigotti, P.; Marcocci, C.; Cetani, F.; et al. Persistent secondary hyperparathyroidism and vertebral fractures in kidney transplantation: Role of calcium-sensing receptor polymorphisms and vitamin D deficiency. J. Bone Miner. Res. 2010, 25, 841–848. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, A.; Wei, F.; Chen, H. Cardiac valve calcification and risk of cardiovascular or all-cause mortality in dialysis patients: A meta-analysis. BMC Cardiovasc. Disord. 2018, 18, 12. [Google Scholar] [CrossRef] [Green Version]
- Kwon, Y.E.; Choi, H.Y.; Oh, H.J.; Ahn, S.Y.; Ryu, D.R.; Kwon, Y.J. Vertebral fracture is associated with myocardial infarction in incident hemodialysis patients: A Korean nationwide population-based study. Osteoporos. Int. 2020, 31, 1965–1973. [Google Scholar] [CrossRef]
- Naves, M.; Rodríguez-García, M.; Díaz-López, J.B.; Gómez-Alonso, C.; Cannata-Andía, J.B. Progression of vascular calcifications is associated with greater bone loss and increased bone fractures. Osteoporos. Int. 2008, 19, 1161–1166. [Google Scholar] [CrossRef]
- Cannata-Andia, J.B.; Roman-Garcia, P.; Hruska, K. The connections between vascular calcification and bone health. Nephrol. Dial. Transplant. 2011, 26, 3429–3436. [Google Scholar] [CrossRef] [PubMed]
- Vassalle, C.; Mazzone, A. Bone loss and vascular calcification: A bi-directional interplay? Vasc. Pharmacol. 2016, 86, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Demer, L.; Tintut, Y. The bone–vascular axis in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2010, 19, 349–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evenepoel, P.; Opdebeeck, B.; David, K.; D’Haese, P.C. Bone-Vascular Axis in Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2019, 26, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, M.; Gallieni, M.; Porta, C.; Nickolas, T.L.; Khairallah, P. Vitamin K effects in human health: New insights beyond bone and cardiovascular health. J. Nephrol. 2020, 33, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Harshman, S.G.; Shen, X.; Haytowitz, D.B.; Karl, J.P.; Wolfe, B.E.; Booth, S.L. Multiple Vitamin K Forms Exist in Dairy Foods. Curr. Dev. Nutr. 2017, 1, e000638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fusaro, M.; Cianciolo, G.; Brandi, M.L.; Ferrari, S.; Nickolas, T.L.; Tripepi, G.; Plebani, M.; Zaninotto, M.; Iervasi, G.; La Manna, G.; et al. Vitamin K and Osteoporosis. Nutrients 2020, 12, 3625. [Google Scholar] [CrossRef]
- Fusaro, M.; Noale, M.; Viola, V.; Galli, F.; Tripepi, G.; Vajente, N.; Plebani, M.; Zaninotto, M.; Guglielmi, G.; Miotto, D.; et al. Vitamin K, vertebral fractures, vascular calcifications, and mortality: VItamin K Italian (VIKI) dialysis study. J. Bone Miner. Res. 2012, 27, 2271–2278. [Google Scholar] [CrossRef] [PubMed]
- Bover, J.; Górriz, J.L.; Ureña-Torres, P.; Lloret, M.J.; Ruiz-García, C.; daSilva, I.; Chang, P.; Rodríguez, M.; Ballarín, J. Detección de las calcificaciones cardiovasculares: ¿una herramienta útil para el nefrólogo? Nefrología 2016, 36, 587–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adragao, T.; Pires, A.; Lucas, C.; Birne, R.; Magalhaes, L.; Goncalves, M.; Negrao, A.P. A simple vascular calcification score predicts cardiovascular risk in haemodialysis patients. Nephrol. Dial. Transplant. 2004, 19, 1480–1488. [Google Scholar] [CrossRef] [Green Version]
- Górriz, J.L.; Molina, P.; Cerverón, M.J.; Vila, R.; Bover, J.; Nieto, J.; Barril, G.; Martínez-Castelao, A.; Fernández, E.; Escudero, V.; et al. Vascular Calcification in Patients with Nondialysis CKD over 3 Years. Clin. J. Am. Soc. Nephrol. 2015, 10, 654–666. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.R.; Eggermont, C.J.; Schousboe, J.T.; Lim, W.H.; Wong, G.; Khoo, B.; Sim, M.; Yu, M.; Ueland, T.; Bollerslev, J.; et al. Association Between Abdominal Aortic Calcification, Bone Mineral Density, and Fracture in Older Women. J. Bone Miner. Res. 2019, 34, 2052–2060. [Google Scholar] [CrossRef] [PubMed]
- Lamon-Fava, S.; Sadowski, J.A.; Davidson, K.W.; O’Brien, M.E.; McNamara, J.R.; Schaefer, E.J. Plasma lipoproteins as carriers of phylloquinone (vitamin K1) in humans. Am. J. Clin. Nutr. 1998, 67, 1226–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cranenburg, E.C.; Vermeer, C.; Koos, R.; Boumans, M.L.; Hackeng, T.M.; Bouwman, F.G.; Kwaijtaal, M.; Brandenburg, V.M.; Ketteler, M.; Schurgers, L.J. The circulating inactive form of matrix Gla Protein (ucMGP) as a biomarker for cardiovascular calcification. J Vasc Res 2008, 45, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Genant, H.K.; Jergas, M.; Palermo, L.; Nevitt, M.; Valentin, R.S.; Black, D.; Cummings, S.R. Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis. J. Bone Miner. Res. 2009, 11, 984–996. [Google Scholar] [CrossRef] [PubMed]
- Witteman, J. J-shaped relation between change in diastolic blood pressure and progression of aortic atherosclerosis. Lancet 1994, 343, 504–507. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Zhou, H.; Cui, M.; Chen, L.; Xu, J. The Association between Aortic Calcification and Fracture Risk in Postmenopausal Women in China: The Prospective Chongqing Osteoporosis Study. PLoS ONE 2014, 9, e93882. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, M.; Gomez-Alonso, C.; Naves-Diaz, M.; Diaz-Lopez, J.B.; Diaz-Corte, C.; Cannata-Andia, J.B.; the Asturias Study, G. Vascular calcifications, vertebral fractures and mortality in haemodialysis patients. Nephrol. Dial. Transplant. 2008, 24, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Bandeira, E.; Neves, A.P.; Costa, C.; Bandeira, F. Association Between Vascular Calcification and Osteoporosis in Men With Type 2 Diabetes. J. Clin. Densitom. 2012, 15, 55–60. [Google Scholar] [CrossRef]
- Issever, A.S.; Kentenich, M.; Köhlitz, T.; Diederichs, G.; Zimmermann, E. Osteoporosis and atherosclerosis: A post-mortem MDCT study of an elderly cohort. Eur. Radiol. 2013, 23, 2823–2829. [Google Scholar] [CrossRef]
- Hyder, J.A.; Allison, M.A.; Criqui, M.H.; Wright, C.M. Association between Systemic Calcified Atherosclerosis and Bone Density. Calcif. Tissue Int. 2007, 80, 301–306. [Google Scholar] [CrossRef]
- Giachelli, C.M. Vascular Calcification Mechanisms. J. Am. Soc. Nephrol. 2004, 15, 2959–2964. [Google Scholar] [CrossRef] [Green Version]
- Pustlauk, W.; Westhoff, T.H.; Claeys, L.; Roch, T.; Geißler, S.; Babel, N. Induced osteogenic differentiation of human smooth muscle cells as a model of vascular calcification. Sci. Rep. 2020, 10, 5951. [Google Scholar] [CrossRef]
- Iseri, K.; Carrero, J.J.; Evans, M.; Felländer-Tsai, L.; Berg, H.; Runesson, B.; Stenvinkel, P.; Lindholm, B.; Qureshi, A.R. Major fractures after initiation of dialysis: Incidence, predictors and association with mortality. Bone 2020, 133, 115242. [Google Scholar] [CrossRef]
- Hong, D.; Wu, S.; Pu, L.; Wang, F.; Wang, J.; Wang, Z.; Gao, H.; Zhang, Y.; Deng, F.; Li, G.; et al. Abdominal aortic calcification is not superior over other vascular calcification in predicting mortality in hemodialysis patients: A retrospective observational study. BMC Nephrol. 2013, 14, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orlando, P.; Silvestri, S.; Marcheggiani, F.; Cirilli, I.; Tiano, L. Menaquinone 7 Stability of Formulations and Its Relationship with Purity Profile. Molecules 2019, 24, 829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geleijnse, J.M.; Vermeer, C.; Grobbee, D.E.; Schurgers, L.J.; Knapen, M.H.J.; van der Meer, I.M.; Hofman, A.; Witteman, J.C.M. Dietary Intake of Menaquinone Is Associated with a Reduced Risk of Coronary Heart Disease: The Rotterdam Study. J. Nutr. 2004, 134, 3100–3105. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, M.; Crepaldi, G.; Maggi, S.; Galli, F.; D’Angelo, A.; Calò, L.; Giannini, S.; Miozzo, D.; Gallieni, M. Vitamin K, bone fractures, and vascular calcifications in chronic kidney disease: An important but poorly studied relationship. J. Endocrinol. Investig. 2011, 34, 317–323. [Google Scholar] [CrossRef]
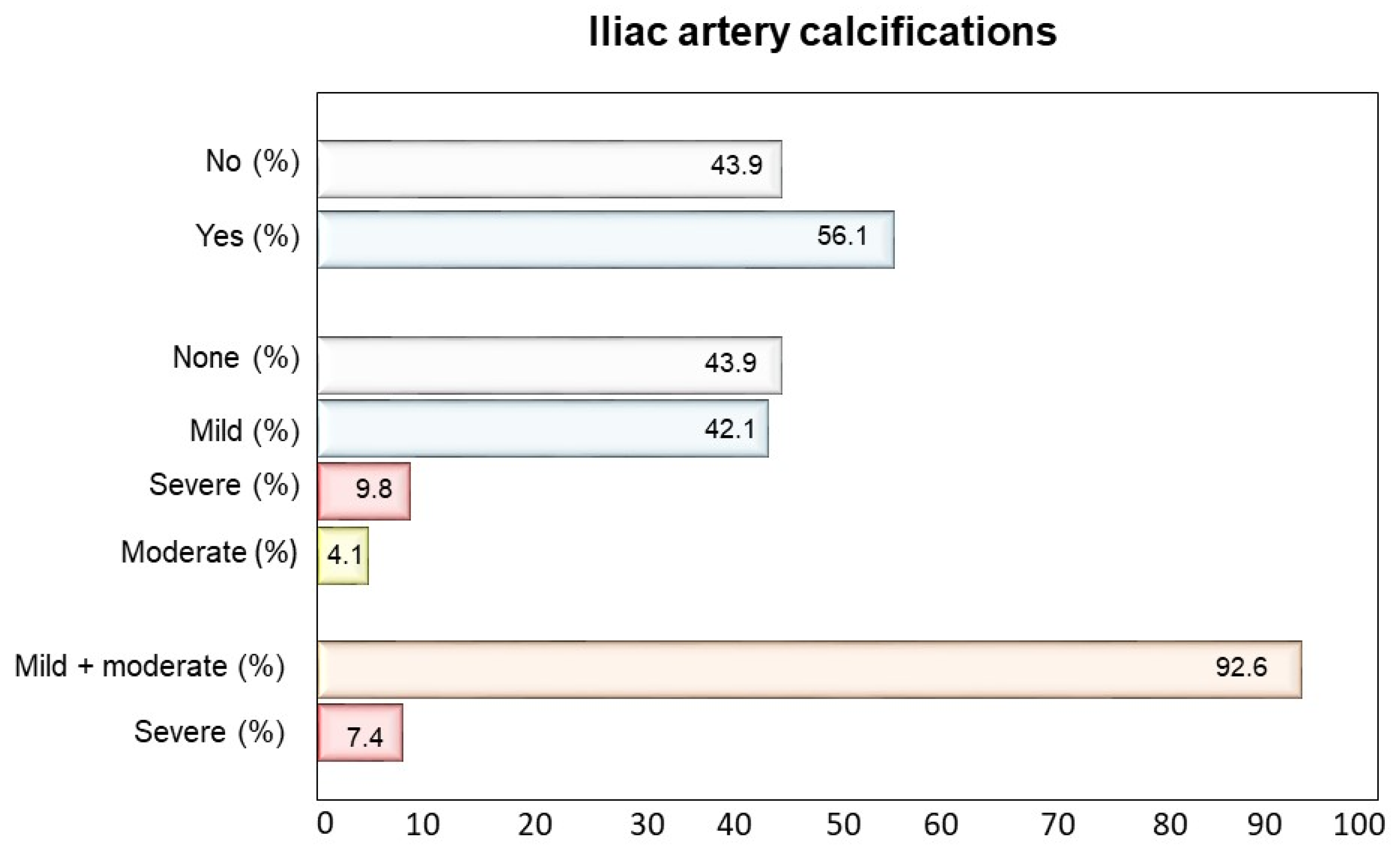
| Variable | Iliac Calcification (Yes) | Iliac Calcification (No) | p-Value | |
|---|---|---|---|---|
| N = 217, 56.1% | N = 170, 43.9% | |||
| Demographic variables and risk factors | ||||
| Sex, female, n (%) | 79 (36.4%) | 66(38.8%) | 0.626 | |
| Age, years | 70 (61.5, 76) | 63 (47, 72) | <0.001 | |
| Weight, kg | 70.53 ± 13.56 | 69.74 ± 15.83 | 0.601 | |
| Height, meters | 1.67 (1.60, 1.73) | 1.68 (1.60. 1.75) | 0.633 | |
| BMI, kg/cm2 | 24.68 (22.39, 28.12) | 24.2 (21.37. 27.73) | 0.157 | |
| Smoker, n (%) (n = 370) | 0.475 | |||
| Yes | 24 (11.8%) | 27 (16.2%) | ||
| No | 132 (65.0%) | 102 (61.0%) | ||
| Ex | 47 (23.2%) | 38 (22.8%) | ||
| Current or former alcohol drinker | 48 (23.8%) | 34 (21.4%) | 0.592 | |
| n (%) (n = 361) | ||||
| Medical history | ||||
| Dialysis vintage, months, median | 49 (29.5, 101.5) | 49.5 (26, 85) | 0.452 | |
| Type of dialysis, n (%) | 0.577 | |||
| Bicarbonate dialysis | 113 (52.1%) | 76 (44.7%) | ||
| Hemofiltration (HF) | 17 (7.8%) | 15 (8.8%) | ||
| Hemodiafiltration (HDF) | 51 (23.5%) | 51 (30.1%) | ||
| Acetate free biofiltration (AFB) | 31 (14.3%) | 23 (13.5%) | ||
| Other types of dialysis | 5 (2.3%) | 5 (2.9%) | ||
| Previous kidney transplant, n (%) | 39 (13.8%) | 24 (14.1%) | 0.934 | |
| Hypertension, n (%) | 168(77.4%) | 136 (80%) | 0.539 | |
| Angina, n (%) | 40 (18.4%) | 24 (14.1%) | 0.257 | |
| Myocardial infarction, n (%) | 50 (23.0%) | 23 (13.5%) | 0.018 | |
| Atrial fibrillation, n (%) | 40(18.4%) | 11 (6.5%) | 0.001 | |
| Heart failure, n (%) | 26 (12.0%) | 13 (7.6%) | 0.16 | |
| Diabetes mellitus, n (%) | 54 (24.9%) | 31 (18.2%) | 0.117 | |
| Peripheral vascular disease, n (%) | 0.014 | |||
| No | 129 (59.4%) | 124 (72.9%) | ||
| Asymptomatic | 65 (30.0%) | 33 (19.4%) | ||
| Intermittent claudication | 20 (9.2%) | 8 (4.8%) | ||
| Amputation | 3 (1.4%) | 5 (2.9%) | ||
| Cerebrovascular accident, n (%) | 0.209 | |||
| No | 191 (88.0%) | 155 (91.2%) | ||
| Stroke | 15 (6.9%) | 5 (2.9%) | ||
| Other type | 11 (5.1%) | 10 (5.9%) | ||
| Vertebral fractures, n (%) | 139 (64.1%) | 75 (44.1%) | <0.001 | |
| Routine biochemical profile | ||||
| Ca, mg/dL | 9.20 ± 0.71 | 9.10 ± 0.64 | 0.141 | |
| P, mg/dL | 4.72 ± 1.32 | 4.83 ± 1.21 | 0.366 | |
| Mg, mg/dL (n = 139) | 2.40 ± 0.56 (n = 61) | 2.44 ± 0.59 (n = 78) | 0.644 | |
| Alkaline phosphatase, U/L | 85 (65, 111) | 80 (63, 110) | ||
| PTH, pg/mL | 240 (134, 384) | 244 (143. 379) | ||
| Albumin, d/dL | 3.81 ± 0.49 | 3.85 ± 0.40 | ||
| CRP, mg/L | 1.9 (0.58, 5.50) | 1.02 (0.38. 4.50) | ||
| KT/V | 1.24 ± 0.26 | 1.26 ± 0.28 | ||
| Aluminium, mcg/L | (n = 60)13.6 (8.5, 22.0) | (n = 107) 10.2 (7.2, 17.8) | ||
| Total cholesterol, mg/dL | 170 (146.25, 194) | 157.5 (131.75, 191.25) | 0.025 | |
| Tryglicerides, mg/dL | 155.5 (116.5, 225) | 134(100, 185) | 0.002 | |
| HDL Cholesterol, mg/dL | 40.00 (32.00, 49.25) | 40 (33, 50) | 0.981 | |
| LDL Cholesterol, mg/dL | 93 (70.75, 116) | 89 (66, 118) | 0.455 | |
| 25(OH) vitamin D, ng/mL | 28.8 (19.25, 43.85) | 28.95 (19.08, 46.14) | 0.382 | |
| BGP total, mcg/L | 164 (84.65, 266.5) | 206 (106, 373.75) | 0.011 | |
| BGP undecarboxylated, ng/mL | 10.08 (4.31, 17.10) | 12.03 (4.69, 18.65) | 0.148 | |
| MGP total, nmol/L | 18.67 (12.13, 30.34) | 18.97 (13.20, 31.64) | 0.598 | |
| MGP decarboxylated, nmol/L | 583 (318, 1030) | 533 (259, 878) | 0.183 | |
| Variables * | Odds Ratio | 95% CI | p Value |
|---|---|---|---|
| Deficit of vitamin K1 (yes/no) | 2.929 | (1.324, 6.482) | 0.008 |
| Gender (female vs. male) | 0.533 | (0.332, 0.854) | 0.009 |
| Iliac Calcifications (yes/no) | 1.73 | (1.060, 2.818) | 0.028 |
| Age (years) | 1.02 | (1.00, 1.04) | 0.043 |
| Oral Calcitriol (yes/no) | 0.598 | (0.363, 0.985) | 0.043 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fusaro, M.; Tripepi, G.; Plebani, M.; Politi, C.; Aghi, A.; Taddei, F.; Schileo, E.; Zaninotto, M.; La Manna, G.; Cianciolo, G.; et al. The Vessels-Bone Axis: Iliac Artery Calcifications, Vertebral Fractures and Vitamin K from VIKI Study. Nutrients 2021, 13, 3567. https://doi.org/10.3390/nu13103567
Fusaro M, Tripepi G, Plebani M, Politi C, Aghi A, Taddei F, Schileo E, Zaninotto M, La Manna G, Cianciolo G, et al. The Vessels-Bone Axis: Iliac Artery Calcifications, Vertebral Fractures and Vitamin K from VIKI Study. Nutrients. 2021; 13(10):3567. https://doi.org/10.3390/nu13103567
Chicago/Turabian StyleFusaro, Maria, Giovanni Tripepi, Mario Plebani, Cristina Politi, Andrea Aghi, Fulvia Taddei, Enrico Schileo, Martina Zaninotto, Gaetano La Manna, Giuseppe Cianciolo, and et al. 2021. "The Vessels-Bone Axis: Iliac Artery Calcifications, Vertebral Fractures and Vitamin K from VIKI Study" Nutrients 13, no. 10: 3567. https://doi.org/10.3390/nu13103567
APA StyleFusaro, M., Tripepi, G., Plebani, M., Politi, C., Aghi, A., Taddei, F., Schileo, E., Zaninotto, M., La Manna, G., Cianciolo, G., Gallieni, M., Cosmai, L., Messa, P., Ravera, M., Nickolas, T. L., Ferrari, S., Ketteler, M., Iervasi, G., Mereu, M. C., ... De Caterina, R. (2021). The Vessels-Bone Axis: Iliac Artery Calcifications, Vertebral Fractures and Vitamin K from VIKI Study. Nutrients, 13(10), 3567. https://doi.org/10.3390/nu13103567












