Reduction of Major Adverse Cardiovascular Events (MACE) after Bariatric Surgery in Patients with Obesity and Cardiovascular Diseases: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
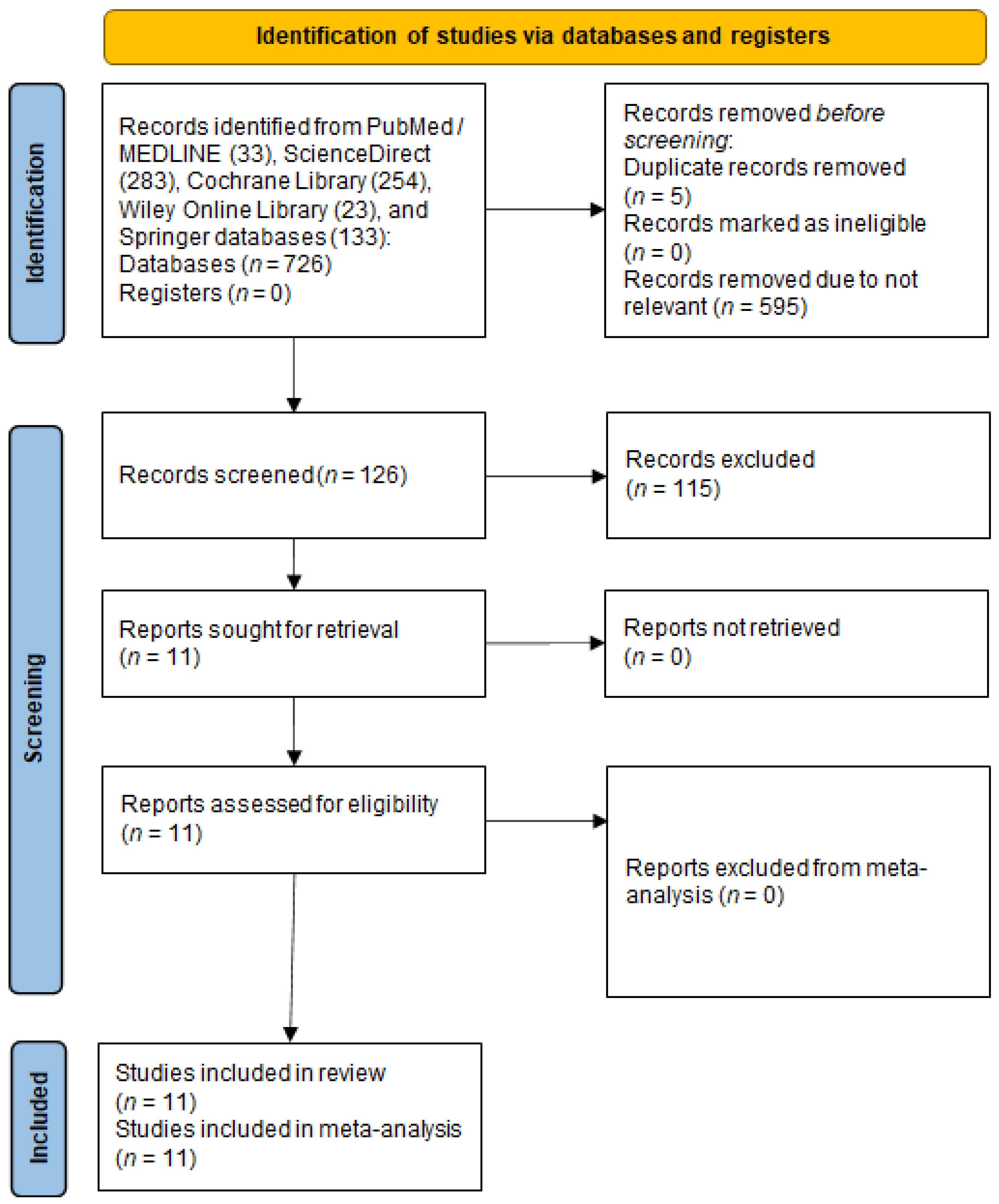
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility
2.3. Data Collection and Extraction
2.4. Data Synthesis
3. Results
3.1. Study Characteristics
3.2. Highlights of the Included Studies
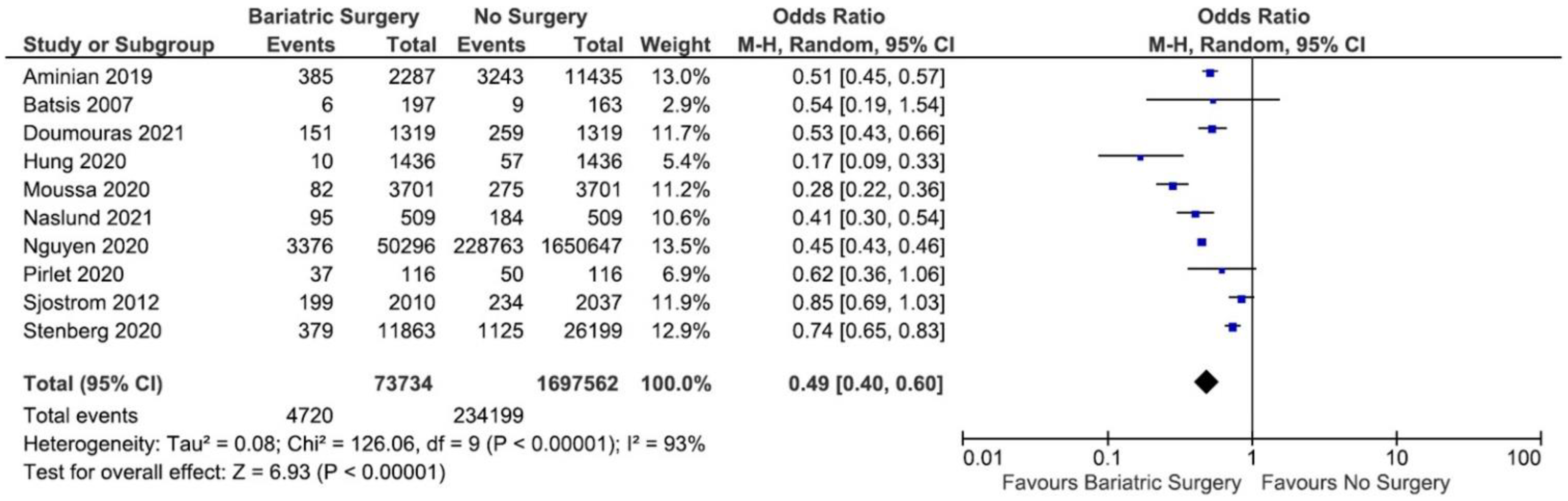
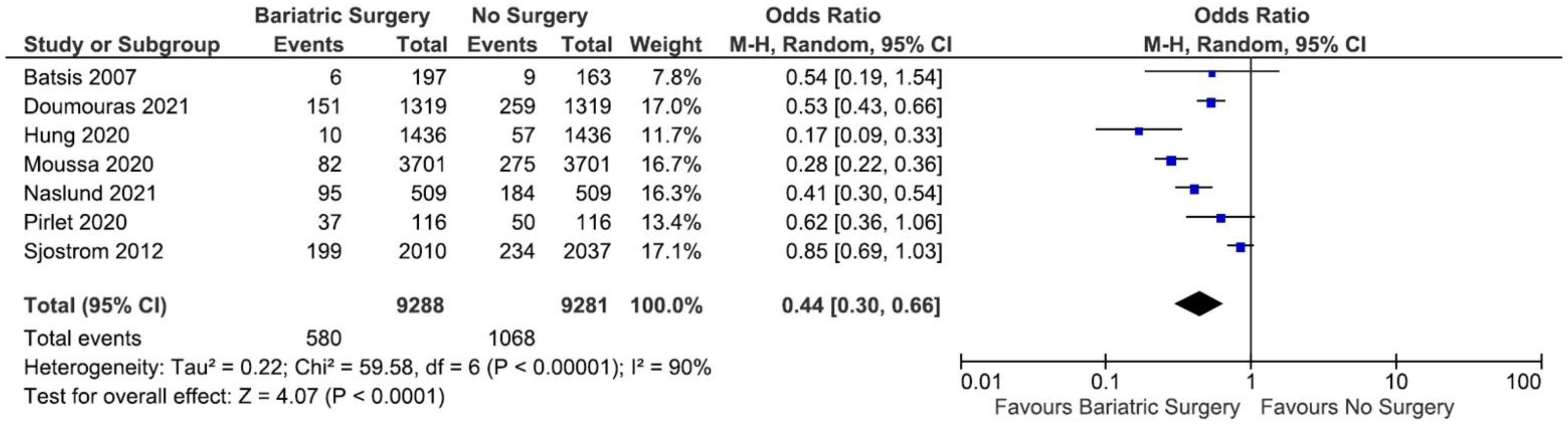
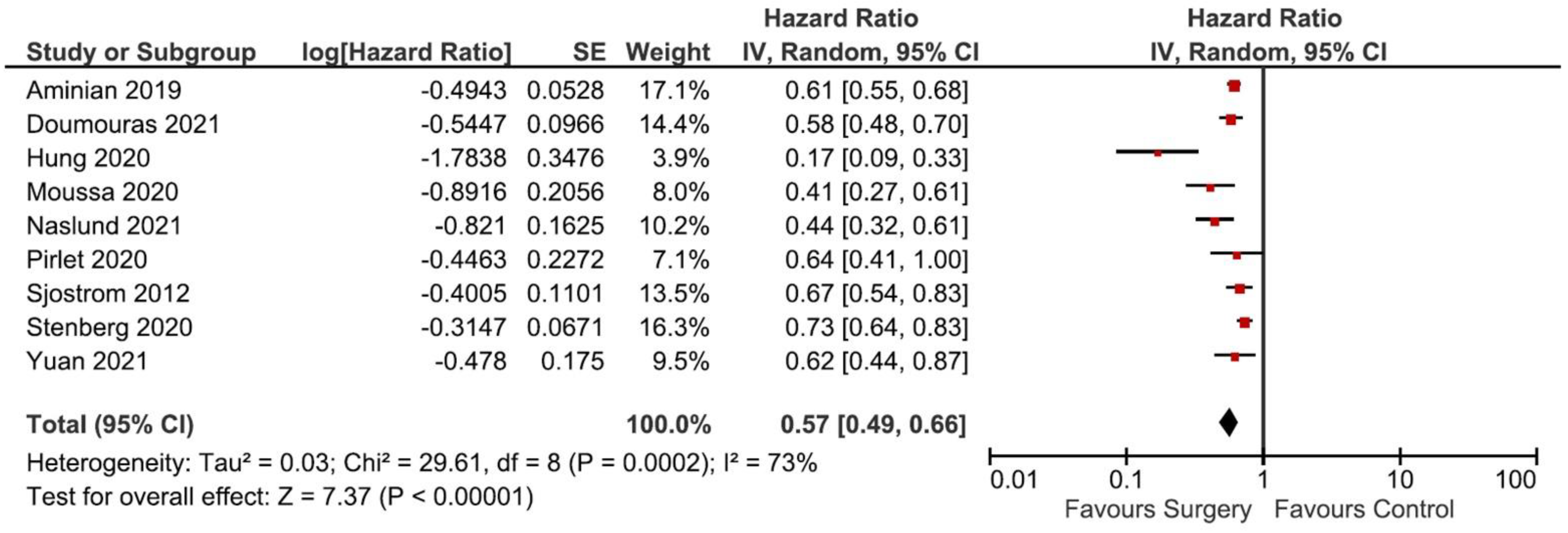
3.3. The Incidence of MACE
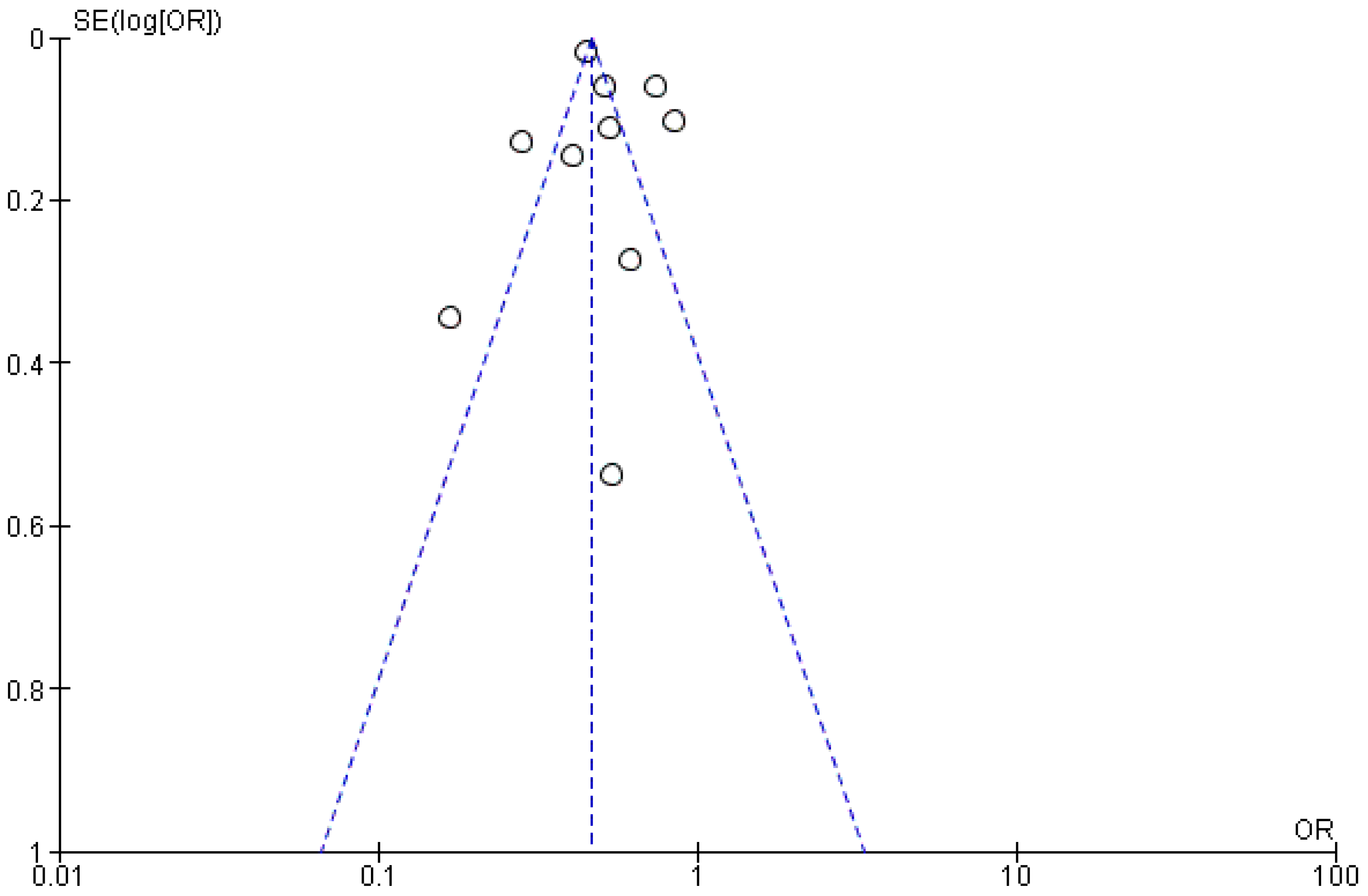
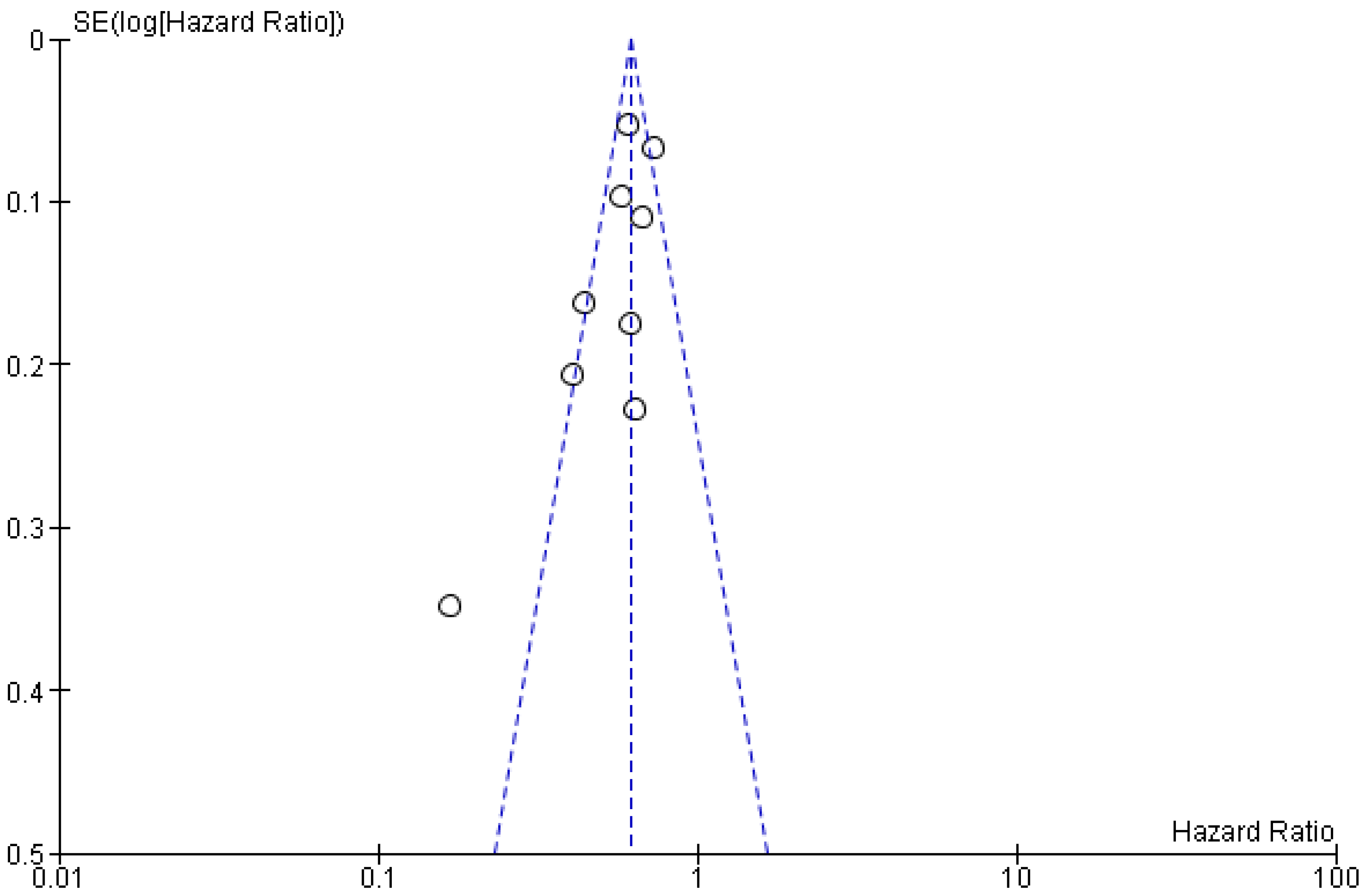
3.4. Publication Bias
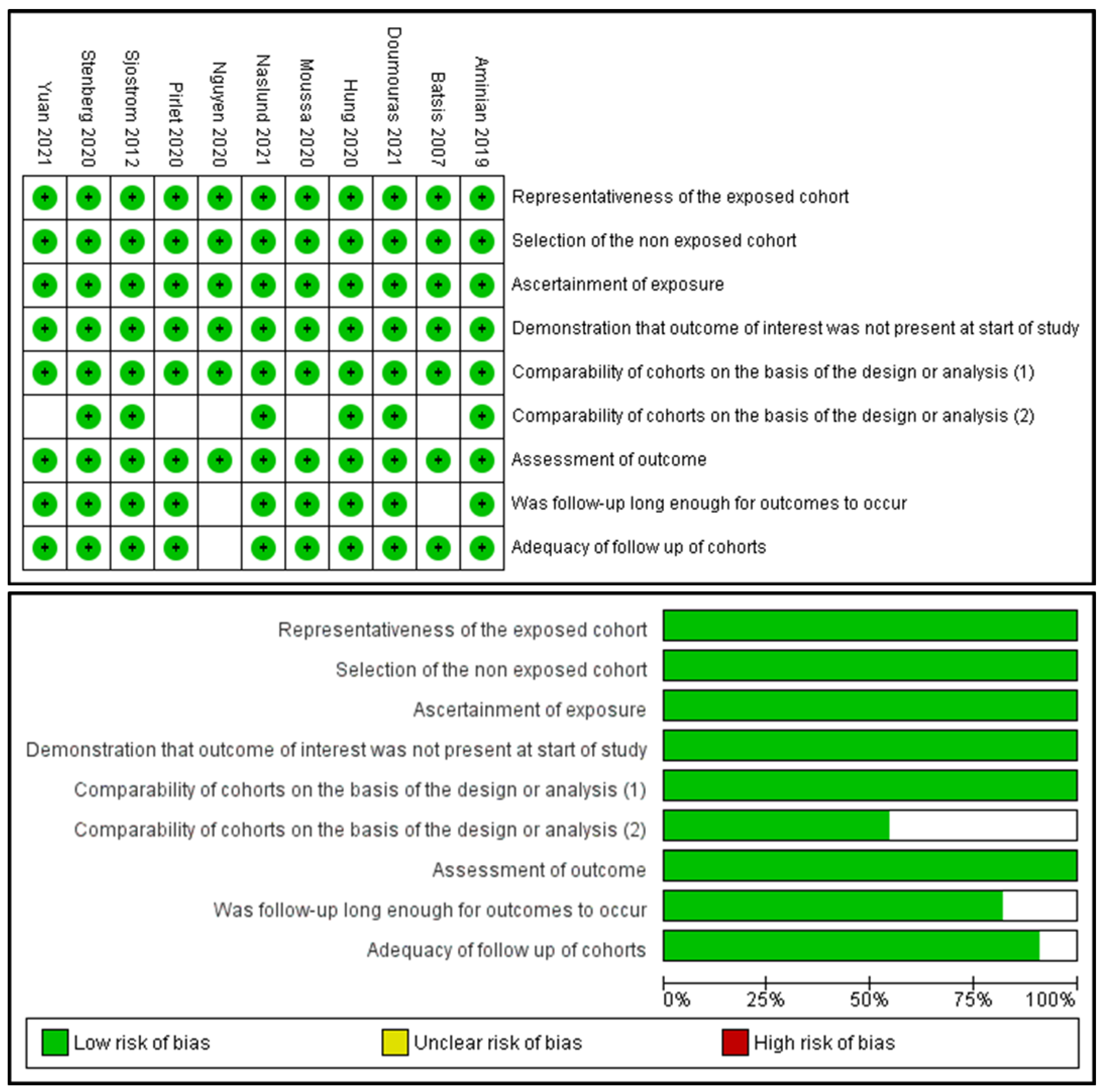
3.5. Sensitivity Analysis
3.6. Confounder Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Reddy, S.; Ounpuu, S.; Anand, S. Global burden of cardiovascular diseases: Part I: General considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation 2001, 104, 2746–2753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Despres, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Pabon, M.A.; Manocha, K.; Cheung, J.W.; Lo, J.C. Linking Arrhythmias and Adipocytes: Insights, Mechanisms, and Future Directions. Front. Physiol. 2018, 9, 1752. [Google Scholar] [CrossRef] [PubMed]
- Fock, K.M.; Khoo, J. Diet and exercise in management of obesity and overweight. J. Gastroenterol. Hepatol. 2013, 28 (Suppl. 4), 59–63. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, B.M.; Kvach, E.; Eckel, R.H. Treatment of Obesity: Weight Loss and Bariatric Surgery. Circ. Res. 2016, 118, 1844–1855. [Google Scholar] [CrossRef]
- Elder, K.A.; Wolfe, B.M. Bariatric surgery: A review of procedures and outcomes. Gastroenterology 2007, 132, 2253–2271. [Google Scholar] [CrossRef]
- Rubino, F.; Nathan, D.M.; Eckel, R.H.; Schauer, P.R.; Alberti, K.G.; Zimmet, P.Z.; Del Prato, S.; Ji, L.; Sadikot, S.M.; Herman, W.H.; et al. Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: A Joint Statement by International Diabetes Organizations. Diabetes Care 2016, 39, 861–877. [Google Scholar] [CrossRef] [Green Version]
- Cummings, D.E.; Cohen, R.V. Bariatric/Metabolic Surgery to Treat Type 2 Diabetes in Patients with a BMI < 35 kg/m2. Diabetes Care 2016, 39, 924–933. [Google Scholar] [CrossRef] [Green Version]
- Martin-Timon, I.; Sevillano-Collantes, C.; Segura-Galindo, A.; Del Canizo-Gomez, F.J. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J. Diabetes 2014, 5, 444–470. [Google Scholar] [CrossRef]
- Miao, B.; Hernandez, A.V.; Alberts, M.J.; Mangiafico, N.; Roman, Y.M.; Coleman, C.I. Incidence and Predictors of Major Adverse Cardiovascular Events in Patients With Established Atherosclerotic Disease or Multiple Risk Factors. J. Am. Heart Assoc. 2020, 9, e014402. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.G.; Rha, S.W.; Yoon, S.G.; Choi, C.U.; Lee, M.W.; Kim, S.W. Association of Major Adverse Cardiac Events up to 5 Years in Patients with Chest Pain without Significant Coronary Artery Disease in the Korean Population. J. Am. Heart Assoc. 2019, 8, e010541. [Google Scholar] [CrossRef] [PubMed]
- Elagizi, A.; Kachur, S.; Lavie, C.J.; Carbone, S.; Pandey, A.; Ortega, F.B.; Milani, R.V. An Overview and Update on Obesity and the Obesity Paradox in Cardiovascular Diseases. Prog. Cardiovasc. Dis. 2018, 61, 142–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horwich, T.B.; Fonarow, G.C.; Clark, A.L. Obesity and the Obesity Paradox in Heart Failure. Prog. Cardiovasc. Dis. 2018, 61, 151–156. [Google Scholar] [CrossRef]
- Lavie, C.J.; Laddu, D.; Arena, R.; Ortega, F.B.; Alpert, M.A.; Kushner, R.F. Healthy Weight and Obesity Prevention: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018, 72, 1506–1531. [Google Scholar] [CrossRef]
- Hainer, V.; Aldhoon-Hainerova, I. Obesity paradox does exist. Diabetes Care 2013, 36 (Suppl. 2), S276–S281. [Google Scholar] [CrossRef] [Green Version]
- Kuno, T.; Tanimoto, E.; Morita, S.; Shimada, Y.J. Effects of Bariatric Surgery on Cardiovascular Disease: A Concise Update of Recent Advances. Front. Cardiovasc. Med. 2019, 6, 94. [Google Scholar] [CrossRef]
- Smith, M.D.; Patterson, E.; Wahed, A.S.; Belle, S.H.; Berk, P.D.; Courcoulas, A.P.; Dakin, G.F.; Flum, D.R.; Machado, L.; Mitchell, J.E.; et al. Thirty-day mortality after bariatric surgery: Independently adjudicated causes of death in the longitudinal assessment of bariatric surgery. Obes. Surg. 2011, 21, 1687–1692. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Grinberg, R.; Johnson, S.; Afthinos, J.N.; Gibbs, K.E. Perioperative risk factors for 30-day mortality after bariatric surgery: Is functional status important? Surg. Endosc. 2013, 27, 1772–1777. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quailty of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 28 August 2021).
- Sjostrom, L.; Peltonen, M.; Jacobson, P.; Sjostrom, C.D.; Karason, K.; Wedel, H.; Ahlin, S.; Anveden, A.; Bengtsson, C.; Bergmark, G.; et al. Bariatric surgery and long-term cardiovascular events. JAMA 2012, 307, 56–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aminian, A.; Zajichek, A.; Arterburn, D.E.; Wolski, K.E.; Brethauer, S.A.; Schauer, P.R.; Kattan, M.W.; Nissen, S.E. Association of Metabolic Surgery with Major Adverse Cardiovascular Outcomes in Patients with Type 2 Diabetes and Obesity. JAMA 2019, 322, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Stenberg, E.; Cao, Y.; Marsk, R.; Sundbom, M.; Jernberg, T.; Naslund, E. Association between metabolic surgery and cardiovascular outcome in patients with hypertension: A nationwide matched cohort study. PLoS Med. 2020, 17, e1003307. [Google Scholar] [CrossRef] [PubMed]
- Pirlet, C.; Voisine, P.; Poirier, P.; Cieza, T.; Ruzsa, Z.; Bagur, R.; Julien, F.; Hould, F.S.; Biertho, L.; Bertrand, O.F. Outcomes in Patients with Obesity and Coronary Artery Disease with and without Bariatric Surgery. Obes. Surg. 2020, 30, 2085–2092. [Google Scholar] [CrossRef]
- Moussa, O.; Ardissino, M.; Heaton, T.; Tang, A.; Khan, O.; Ziprin, P.; Darzi, A.; Collins, P.; Purkayastha, S. Effect of bariatric surgery on long-term cardiovascular outcomes: A nationwide nested cohort study. Eur. Heart J. 2020, 41, 2660–2667. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.L.; Chen, C.Y.; Chin, W.L.; Lee, C.H.; Chen, J.H. The long-term risk of cardiovascular events in patients following bariatric surgery compared to a non-surgical population with obesity and the general population: A comprehensive national cohort study. Langenbecks Arch. Surg. 2021, 406, 189–196. [Google Scholar] [CrossRef]
- Doumouras, A.G.; Wong, J.A.; Paterson, J.M.; Lee, Y.; Sivapathasundaram, B.; Tarride, J.E.; Thabane, L.; Hong, D.; Yusuf, S.; Anvari, M. Bariatric Surgery and Cardiovascular Outcomes in Patients with Obesity and Cardiovascular Disease: A Population-Based Retrospective Cohort Study. Circulation 2021, 143, 1468–1480. [Google Scholar] [CrossRef]
- Naslund, E.; Stenberg, E.; Hofmann, R.; Ottosson, J.; Sundbom, M.; Marsk, R.; Svensson, P.; Szummer, K.; Jernberg, T. Association of Metabolic Surgery with Major Adverse Cardiovascular Outcomes in Patients with Previous Myocardial Infarction and Severe Obesity: A Nationwide Cohort Study. Circulation 2021, 143, 1458–1467. [Google Scholar] [CrossRef]
- Batsis, J.A.; Romero-Corral, A.; Collazo-Clavell, M.L.; Sarr, M.G.; Somers, V.K.; Brekke, L.; Lopez-Jimenez, F. Effect of weight loss on predicted cardiovascular risk: Change in cardiac risk after bariatric surgery. Obesity 2007, 15, 772–784. [Google Scholar] [CrossRef]
- Nguyen, T.; Alzahrani, T.; Mandler, A.; Alarfaj, M.; Panjrath, G.; Krepp, J. Relation of Bariatric Surgery to Inpatient Cardiovascular Outcomes (from the National Inpatient Sample). Am. J. Cardiol. 2021, 144, 143–147. [Google Scholar] [CrossRef]
- Yuan, H.; Medina-Inojosa, J.R.; Lopez-Jimenez, F.; Miranda, W.R.; Collazo-Clavell, M.L.; Sarr, M.G.; Chamberlain, A.M.; Hodge, D.O.; Bailey, K.R.; Wang, Y.; et al. The Long-Term Impact of Bariatric Surgery on Development of Atrial Fibrillation and Cardiovascular Events in Obese Patients: An Historical Cohort Study. Front. Cardiovasc. Med. 2021, 8, 647118. [Google Scholar] [CrossRef] [PubMed]
- Riaz, H.; Khan, M.S.; Siddiqi, T.J.; Usman, M.S.; Shah, N.; Goyal, A.; Khan, S.S.; Mookadam, F.; Krasuski, R.A.; Ahmed, H. Association between Obesity and Cardiovascular Outcomes: A Systematic Review and Meta-analysis of Mendelian Randomization Studies. JAMA Netw. Open 2018, 1, e183788. [Google Scholar] [CrossRef] [PubMed]
- Ades, P.A.; Savage, P.D. The obesity paradox: Perception vs knowledge. Mayo Clin. Proc. 2010, 85, 112–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singla, P.; Bardoloi, A.; Parkash, A.A. Metabolic effects of obesity: A review. World J. Diabetes 2010, 1, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Rheinheimer, J.; de Souza, B.M.; Cardoso, N.S.; Bauer, A.C.; Crispim, D. Current role of the NLRP3 inflammasome on obesity and insulin resistance: A systematic review. Metabolism 2017, 74, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [Green Version]
- Sutanto, H.; Dobrev, D.; Heijman, J. Angiotensin Receptor.-Neprilysin Inhibitor (ARNI) and Cardiac Arrhythmias. Int. J. Mol. Sci. 2021, 22, 8994. [Google Scholar] [CrossRef]
- .Munoz Torres, M.; Munoz Garach, A. Results from Cardiovascular Outcome Trials in Diabetes. Endocrinol. Nutr. 2016, 63, 317–319. [Google Scholar] [CrossRef]
- Haase, C.L.; Lopes, S.; Olsen, A.H.; Satylganova, A.; Schnecke, V.; McEwan, P. Weight loss and risk reduction of obesity-related outcomes in 0.5 million people: Evidence from a UK primary care database. Int. J. Obes. 2021, 45, 1249–1258. [Google Scholar] [CrossRef]
- Cunningham, J.W.; Wiviott, S.D. Modern obesity pharmacotherapy: Weighing cardiovascular risk and benefit. Clin. Cardiol. 2014, 37, 693–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batsis, J.A.; Romero-Corral, A.; Collazo-Clavell, M.L.; Sarr, M.G.; Somers, V.K.; Lopez-Jimenez, F. Effect of bariatric surgery on the metabolic syndrome: A population-based, long-term controlled study. Mayo Clin. Proc. 2008, 83, 897–907. [Google Scholar] [CrossRef]
- Arterburn, D.E.; Telem, D.A.; Kushner, R.F.; Courcoulas, A.P. Benefits and Risks of Bariatric Surgery in Adults: A Review. JAMA 2020, 324, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Knop, F.K.; Taylor, R. Mechanism of metabolic advantages after bariatric surgery: It’s all gastrointestinal factors versus it’s all food restriction. Diabetes Care 2013, 36 (Suppl. 2), S287–S291. [Google Scholar] [CrossRef] [Green Version]
- Spivak, H.; Sakran, N.; Dicker, D.; Rubin, M.; Raz, I.; Shohat, T.; Blumenfeld, O. Different effects of bariatric surgical procedures on dyslipidemia: A registry-based analysis. Surg. Obes. Relat. Dis. 2017, 13, 1189–1194. [Google Scholar] [CrossRef]
- Beamish, A.J.; Olbers, T.; Kelly, A.S.; Inge, T.H. Cardiovascular effects of bariatric surgery. Nat. Rev. Cardiol. 2016, 13, 730–743. [Google Scholar] [CrossRef]
- Tsilingiris, D.; Koliaki, C.; Kokkinos, A. Remission of Type 2 Diabetes Mellitus after Bariatric Surgery: Fact or Fiction? Int. J. Environ. Res. Public Health 2019, 16, 3171. [Google Scholar] [CrossRef] [Green Version]
- Aarts, F.; Geenen, R.; Gerdes, V.E.; van de Laar, A.; Brandjes, D.P.; Hinnen, C. Attachment anxiety predicts poor adherence to dietary recommendations: An indirect effect on weight change 1 year after gastric bypass surgery. Obes. Surg. 2015, 25, 666–672. [Google Scholar] [CrossRef]
- Bonfils, P.K.; Taskiran, M.; Damgaard, M.; Goetze, J.P.; Floyd, A.K.; Funch-Jensen, P.; Kristiansen, V.B.; Stockel, M.; Bouchelouche, P.N.; Gadsboll, N. Roux-en-Y gastric bypass alleviates hypertension and is associated with an increase in mid-regional pro-atrial natriuretic peptide in morbid obese patients. J. Hypertens. 2015, 33, 1215–1225. [Google Scholar] [CrossRef]
- Gandolfini, M.P.; Coupaye, M.; Bouaziz, E.; Dehoux, M.; Hajage, D.; Lacorte, J.M.; Ledoux, S. Cardiovascular Changes after Gastric Bypass Surgery: Involvement of Increased Secretions of Glucagon-Like Peptide-1 and Brain Natriuretic Peptide. Obes. Surg. 2015, 25, 1933–1939. [Google Scholar] [CrossRef]
- Arora, P.; Reingold, J.; Baggish, A.; Guanaga, D.P.; Wu, C.; Ghorbani, A.; Song, Y.; Chen-Tournaux, A.; Khan, A.M.; Tainsh, L.T.; et al. Weight loss, saline loading, and the natriuretic peptide system. J. Am. Heart Assoc. 2015, 4, e001265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woelnerhanssen, B.; Peterli, R.; Steinert, R.E.; Peters, T.; Borbely, Y.; Beglinger, C. Effects of postbariatric surgery weight loss on adipokines and metabolic parameters: Comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy--a prospective randomized trial. Surg. Obes. Relat. Dis. 2011, 7, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Illan-Gomez, F.; Gonzalvez-Ortega, M.; Orea-Soler, I.; Alcaraz-Tafalla, M.S.; Aragon-Alonso, A.; Pascual-Diaz, M.; Perez-Paredes, M.; Lozano-Almela, M.L. Obesity and inflammation: Change in adiponectin, C-reactive protein, tumour necrosis factor-alpha and interleukin-6 after bariatric surgery. Obes. Surg. 2012, 22, 950–955. [Google Scholar] [CrossRef]
- Ma, I.T.; Madura, J.A., 2nd. Gastrointestinal Complications after Bariatric Surgery. Gastroenterol. Hepatol. 2015, 11, 526–535. [Google Scholar]
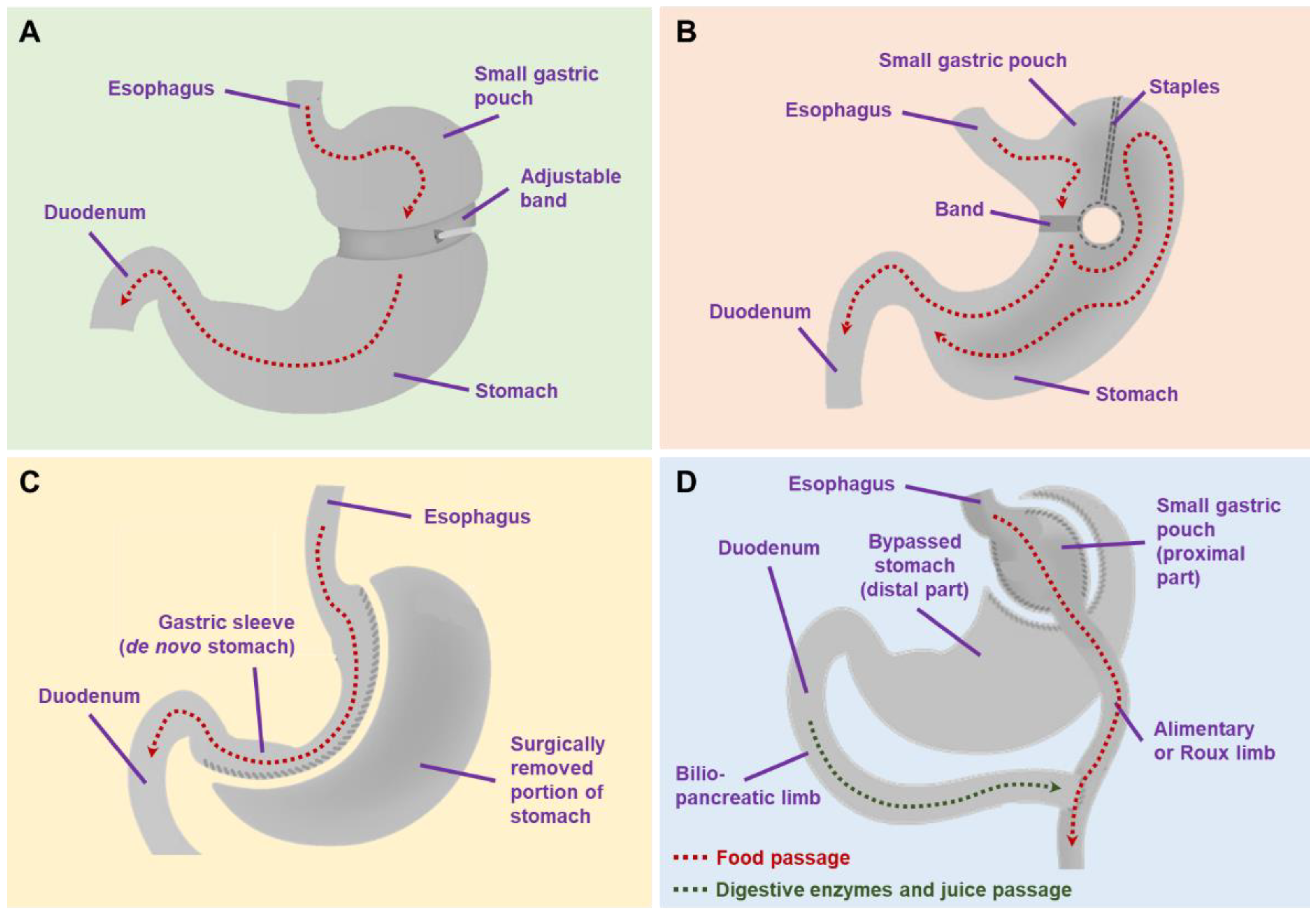
| Study | Design | Study Location | Sample Size | Surgical Procedure (%) | Follow-Up Period (Mean ± SD/Median (IQR), y) | Population | REF |
|---|---|---|---|---|---|---|---|
| Sjostrom et al., 2012 | Non-RCT | Sweden | 2010 surgery | Gastric Bypass: 265 (13.2) Gastric Banding: 376 (18.7) Vertical Banded Gastroplasty: 1360 (68.1) | 14.7 | Patients aged 37 to 60 years and with BMI of at least 34 for men and at least 38 for women. | [23] |
| 2037 control | - | 14.7 | |||||
| Aminian et al., 2019 | Matched Cohort Study | Florida, Ohio, USA | 2287 surgery | Roux-en-Y Gastric Bypass: 1443 (63) Sleeve Gastrectomy: 730 (32) Gastric Banding: 109 (5) Duodenal Switch: 5 (0.2) | 3.3 (1.2–6.3) | Patients with age 18–80 years, BMI ≥ 30, and diabetes. | [24] |
| 11,435 control | 4.0 (2.1–6.1) | ||||||
| Stenberg et al., 2020 | Matched Cohort Study | Sweden | 11,863 surgery | Gastric Bypass: 10,692 (90.1) Sleeve Gastrectomy: 1171 (9.9) | 5.09 ± 2.53 | Patients with morbid obesity and hypertension | [25] |
| 26,199 control | - | 5.06 ± 2.55 | |||||
| Pirlet et al., 2020 | Matched Cohort Study | Quebec, Canada | 116 surgery | Gastric Bypass: 3 (2.6) Biliopancreatic diversion with duodenal switch: 44 (38) Sleeve gastrectomy: 67 (58) Duodenal Switch only: 2 (1.7) | 8.9 (6.3–14.2) | Patients with history of coronary artery disease (CAD) and obesity | [26] |
| 116 control | - | 8.9 (6.3–14.2) | |||||
| Moussa et al., 2020 | Matched Cohort Study | UK | 3701 surgery | N/A | 11.2 | Patients with BMI ≥ 35 with exclusion of previous MACE | [27] |
| 3701 control | - | 11.2 | |||||
| Hung et al., 2020 | Matched Cohort Study | Taiwan | 1436 surgery | N/A | 7.5 | Patients with BMI > 35 with co-morbidities or >40 | [28] |
| 1436 control | - | 7.5 | |||||
| Doumouras et al., 2021 | Matched Cohort Study | Ontario, Canada | 1319 surgery | Gastric Bypass: 1049 (79.5) Sleeve Gastrectomy: 270 (20.5) | 4.65 (3.09–6.28) | Patients with BMI > 35 with a comorbidity or BMI ≥ 40 | [29] |
| 1319 control | - | 4.38 (2.83–6.09) | |||||
| Naslund et al., 2021 | Matched Cohort Study | Sweden | 509 surgery | Roux-en-Y Gastric Bypass: 465 (91) Sleeve Gastrectomy: 44 (9) | 4.6 (2.7–7.1) | Patients with severe obesity and history of MI | [30] |
| 509 control | - | 4.6 (2.7–7.1) | |||||
| Batsis et al., 2007 | Retrospective Cohort | Olmsted, Minnesota, USA | 197 surgery | Roux-en-Y Gastric Bypass: 197 (100) | 3.3 ± 2.6 | Patients with BMI > 35 | [31] |
| 163 control | 3.3 ± 2.6 | ||||||
| Nguyen et al., 2020 | Retrospective Cohort | USA | 50,296 surgery | N/A | N/A | Adult patients with class II (BMI 35.0 to 39.9) or class III obesity (BMI > 40) | [32] |
| 1650,647 control | - | N/A | |||||
| Yuan et al., 2021 | Retrospective Cohort | Minnesota, USA | 308 surgery | Roux-en-Y Gastric Bypass: 308 (100) | 4.6 (2.7–7.1) | Patients with class II-III obesity (BMI > 35) | [33] |
| 701 control | - | 4.6 (2.7–7.1) |
| Study | Group | Age (Mean ± SD/Median (IQR), y) | BMI (Mean ± SD/Median (IQR), y) | Ischemic Heart Disease (%) | HF (%) | AF (%) | Hypertension (%) | Dyslipidemia (%) | Diabetes Mellitus (%) |
|---|---|---|---|---|---|---|---|---|---|
| Sjostrom et al., 2012 [23] | Surgery | ≤47.8: 55% ˃47.8: 45% | ≤40.8: 40% ˃40.8: 60% | N/A | N/A | N/A | 991 (49.3) | N/A | 345 (17.2) |
| Control | ≤47.8: 45% ˃47.8: 55% | ≤40.8: 60% ˃40.8: 40% | N/A | N/A | N/A | 725 (35.6) | N/A | 262 (12.8) | |
| Aminian et al., 2019 [24] | Surgery | 52.5 (43.7–60.5) | 45.1 (40–51.8) | 237 (10.4) | 238 (10.4) | 152 (6.6) | 1953 (85.4) | 1686 (73.7) | 2287 (100) |
| Control | 54.8 (46.2–62.5) | 42.6 (39.4–47.2) | 1104 (9.7) | 1342 (11.7) | 701 (6.1) | 8565 (74.9) | 7457 (65.2) | 11,435 (100) | |
| Stenberg et al., 2020 [25] | Surgery | 52.1 ± 7.46 | 41.9 ± 5.43 | N/A | N/A | N/A | 11,863 (100) | 4437 (37.4) | 3328 (28.1) |
| Control | 54.6 ± 7.12 | N/A | N/A | N/A | N/A | 26,199 (100) | 7802 (29.8) | 2690 (10.3) | |
| Pirlet et al., 2020 [26] | Surgery | 52.9 ± 7.2 | 42.0 ± 6.1 | 116 (100) | 6 (5.2) | 3 (2.6) | 94 (81) | 96 (83) | 57 (49) |
| Control | 52.1 ± 8.4 | 41.2 ± 6.7 | 116 (100) | 9 (7.8) | 5 (4.3) | 94 (81) | 101 (87) | 59 (51) | |
| Moussa et al., 2020 [27] | Surgery | 36 (29–44) | 40.3 (36.6–43.9) | N/A | N/A | N/A | 1928 (52.1) | 50 (1.4) | 922 (25) |
| Control | 36 (29–44) | 40.5 (37.1–45.5) | N/A | N/A | N/A | 1822 (49.2) | 39 (1.1) | 881 (23.9) | |
| Hung et al., 2020 [28] | Surgery | 32.39 ± 8.6 | N/A | N/A | N/A | N/A | 109 (7.59) | 50 (3.48) | 74 (5.15) |
| Control | 32.27 ± 9.3 | N/A | N/A | N/A | N/A | 116 (8.08) | 57 (3.97) | 76 (5.29) | |
| Doumouras et al., 2021 [29] | Surgery | 55.4 ± 7.43 | 48.0 ± 8.04 | 1202 (91.1) | 274 (20.8) | 105 (8) | 1098 (83.2) | N/A | 775 (58.8) |
| Control | 56.5 ± 7.85 | 46.7 ± 13.8 | 1201 (91.1) | 274 (20.8) | 80 (6.1) | 1061 (80.4) | N/A | 745 (56.5) | |
| Naslund et al., 2021 [30] | Surgery | 53.0 ± 7.0 | 40.6 ± 4.4 | 509 (100) | 53 (10.4) | 29 (5.7) | 332 (65.5) | N/A | 209 (41.1) |
| Control | 53.2 ± 7.4 | 39.7 ± 4.7 | 509 (100) | 97 (19.1) | 49 (9.6) | 365 (71.7) | N/A | 229 (45) | |
| Batsis et al., 2007 [31] | Surgery | 44.0 ± 9.9 | 49.5 ± 8.9 | N/A | N/A | N/A | 105 (53.3) | 114 (57.9) | 61 (31) |
| Control | 43.4 ± 11.2 | 44.0 ± 5.7 | N/A | N/A | N/A | 80 (49.1) | 97 (59.5) | 41 (25.2) | |
| Nguyen et al., 2020 [32] | Surgery | 52.9 ± 12.1 | N/A | 6776 (13.47) | 6743 (13.41) | 6552 (13.03) | 26,793 (53.27) | 14,886 (29.6) | 17,059 (33.92) |
| Control | 54.1 ± 15.6 | N/A | 345,014 (20.9) | 389,677 (23.61) | 249,228 (15.1) | 804,920 (48.76) | 614,021 (37.2) | 748,484 (45.34) | |
| Yuan et al., 2021 [33] | Surgery | 44.2 ± 10.5 | 46.4 ± 6.5 | 15 (4.9) | 1 (0.3) | N/A | 136 (44.2) | 134 (43.5) | 65 (21.1) |
| Control | 43.6 ± 12.6 | 44.8 ± 6.9 | 64 (9.1) | 25 (3.6) | N/A | 393 (56.1) | 372 (53.1) | 271 (38.7) |
| Studies | Matching Method | Adjusted Criteria (Confounder Exclusion) | Results |
|---|---|---|---|
| Sjostrom, et al., 2012 [23] | A matched control group of participants was created by an automatic matching program using 18 matching variables. | Adjusted for sex, age, history of stroke or MI, diabetes, insulin level, smoking history, BMI, waist circumference, hip circumference, systolic BP, total cholesterol, HDL cholesterol, triglycerides, lipid-lowering medication, antihypertensive medication. | aHR = 0.67; 95% CI 0.54–0.83; p < 0.001 |
| Aminian, et al., 2019 [24] | Each surgical patient was matched with a propensity score by the nearest-neighbor method to 5 nonsurgical patients from a logistic regression model with a logit link function based on 7 a priori–identified potential confounders including the index date, age at index date, sex, BMI at index date (categorized as 30–34.9, 35–39.9, ≥40), location, insulin use, and presence of diabetes-related end-organ complications. | Adjusted for index date, sex, age, BMI, weight, race, annual income, smoking status, location of patients, medical history (hypertension, dyslipidemia, peripheral neuropathy, HF, coronary artery disease, chronic obstructive pulmonary disease, nephropathy, atrial fibrillation, peripheral artery disease, MI, cerebrovascular disease, ischemic stroke and dialysis). | aHR = 0.61; 95%CI 0.55–0.69; p < 0.001 |
| Stenberg, et al., 2020 [25] | 1:10 matched group of non-operated–on individuals, based on age, sex, and regional area of residence in Sweden. | Adjusted for duration of hypertension, comorbidities, and education. | aHR = 0.73; 95% CI 0.64–0.84; p < 0.001 |
| Pirlet et al., 2020 [26] | Matched based on propensity score based on age, sex, BMI, weight, status of dyslipidemia, hypertension, diabetes, history of smoking, atrial fibrillation, HF, cancer, stroke, chronic obstructive pulmonary disease, chronic kidney disease, history of MI, history of ercutaneous coronary intervention, revascularization for MI, revascularization for unstable angina. | Adjusted from baseline characteristic not balanced in propensity score matching (History of MI and weight). | aHR = 0.64; 95% CI 0.41–0.99; p = 0.046 |
| Moussa et al., 2020 [27] | Matched based on age, gender, and baseline BMI. | Adjusted for hypertension, hyperlipidaemia, diabetes, smoking, alcohol use, cocaine use, exercise and use of medications, such as beta-blockers, calcium channel blockers, angiotension converting enzyme inhibitors or angiotensin receptor blockers, statins, aspirin and hormone replacement therapy. | aHR = 0.410, 95% CI 0.274–0.615; p < 0.001 |
| Hung et al., 2020 [28] | Matched 1:4 with patients in the surgical group by propensity-score matching, based on age and sex. | Adjusted for age, sex, Charlson Comorbidity Index (CCI), comorbidities (i.e., diabetes, hypertension, hyperlipidemia and gout). | aHR = 0.168; 95% CI 0.085–0.328; p < 0.001 |
| Doumouras et al., 2021 [29] | Matched based on demographic status, history of smoking, history of diabetes mellitus, cardiac disease, stroke, hypertension, substance abuse, eating and mood disorder, liver and renal disease. | Adjusted for age, BMI, sex, immigrant status, income, rurality, diabetes status, overall cardiac history, stroke, chronic obstructive pulmonary disease, hypertension, sleep apnea, renal disease, smoking status, previous malignancy, substance abuse, self-harm, mood disorder, cancer screening (colon, breast, cervical) and health care use in previous year (family physician, hospital inpatient, emergency room visit, specialist visit). | aHR = 0.58; 95% CI 0.48–0.71; p < 0.001 |
| Naslund et al., 2021 [30] | Matched 1:1 based on sex, age ( ± 3 years), year of MI ( ± 3 years), and BMI ( ± 3) to a control with MI registered in SWEDEHEART. | Adjusted for BMI, smoking, hypertension, chronic kidney disease, peripheral artery disease, HF, atrial fibrillation, chronic obstructive pulmonary disease, cancer disease within 3 years, and treatment with aspirin, P2Y12 receptor blockers, and statins. | aHR = 0.44; 95% CI 0.32–0.61 |
| Yuan et al., 2021 [33] | Matched based on age, sex and BMI. | Adjusted for age and sex. | aHR = 0.62; 95% CI 0.44–0.88; p = 0.008 |
| Batsis et al., 2007 [31] | Not matched | No adjustment | OR = 0.54; 95% CI 0.19–1.54 |
| Nguyen et al., 2020 [32] | Not matched | Adjusted for gender, hospital region, all patients refined diagnosis related groups severity and risk of mortality, diabetes, hypertension, hyperlipidemia, chronic kidney disease, prior MI, peripheral arterial disease, chronic obstructive pulmonary disease, pulmonary arterial hypertension, atrial fibrillation and smoking. | aOR = 0.62; 95% CI 0.60–0.65; p < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutanto, A.; Wungu, C.D.K.; Susilo, H.; Sutanto, H. Reduction of Major Adverse Cardiovascular Events (MACE) after Bariatric Surgery in Patients with Obesity and Cardiovascular Diseases: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3568. https://doi.org/10.3390/nu13103568
Sutanto A, Wungu CDK, Susilo H, Sutanto H. Reduction of Major Adverse Cardiovascular Events (MACE) after Bariatric Surgery in Patients with Obesity and Cardiovascular Diseases: A Systematic Review and Meta-Analysis. Nutrients. 2021; 13(10):3568. https://doi.org/10.3390/nu13103568
Chicago/Turabian StyleSutanto, Andryanto, Citrawati Dyah Kencono Wungu, Hendri Susilo, and Henry Sutanto. 2021. "Reduction of Major Adverse Cardiovascular Events (MACE) after Bariatric Surgery in Patients with Obesity and Cardiovascular Diseases: A Systematic Review and Meta-Analysis" Nutrients 13, no. 10: 3568. https://doi.org/10.3390/nu13103568
APA StyleSutanto, A., Wungu, C. D. K., Susilo, H., & Sutanto, H. (2021). Reduction of Major Adverse Cardiovascular Events (MACE) after Bariatric Surgery in Patients with Obesity and Cardiovascular Diseases: A Systematic Review and Meta-Analysis. Nutrients, 13(10), 3568. https://doi.org/10.3390/nu13103568







