Impacts of Fructose on Intestinal Barrier Function, Inflammation and Microbiota in a Piglet Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Piglets Feeding Experiment
2.2. Quantitative RT-PCR
2.3. Detections of Antioxidant Indices and Immune Factors
2.4. 16S rDNA Sequencing and Data Analysis
2.5. Statistical Analyses
3. Results
3.1. The Effect of Fructose on Growth Performance in Weaned Piglets
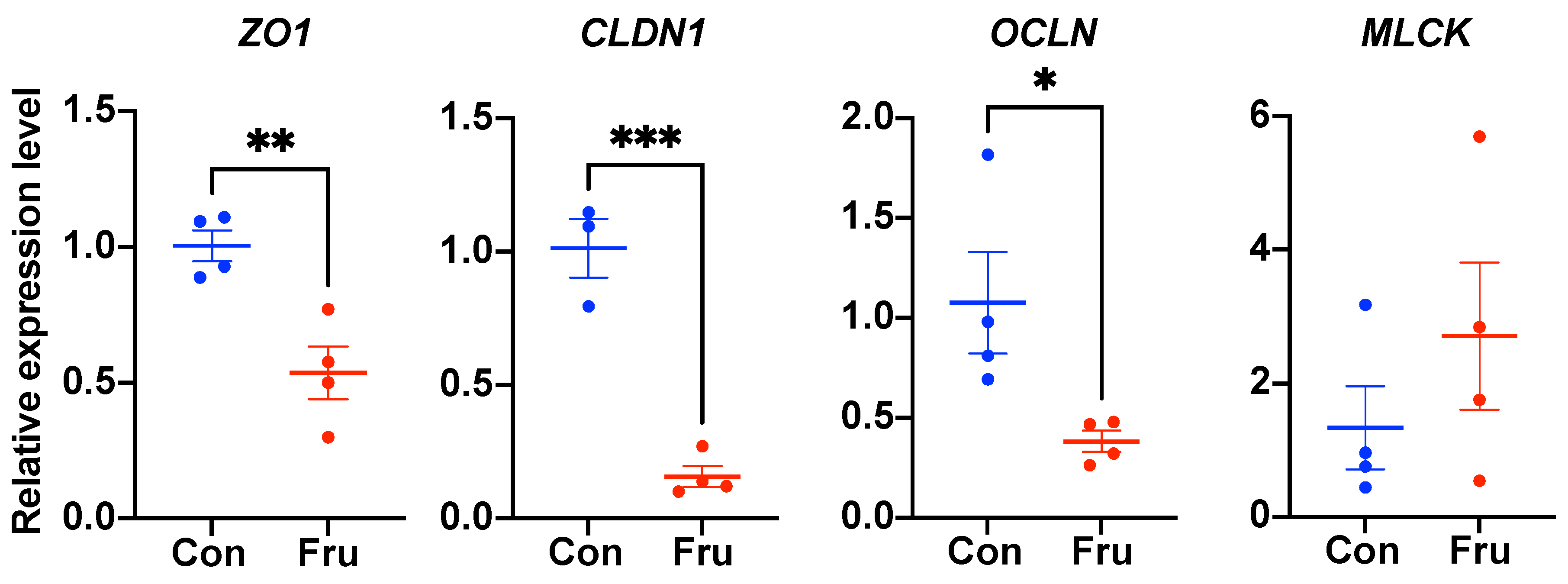
3.2. The Effect of Fructose on Ileal Tight Junction Gene Expressions in Weaned Piglets
3.3. The Effects of Fructose on Serous and Ileal Immunologic Function and Oxidation Resistance in Weaned Piglets
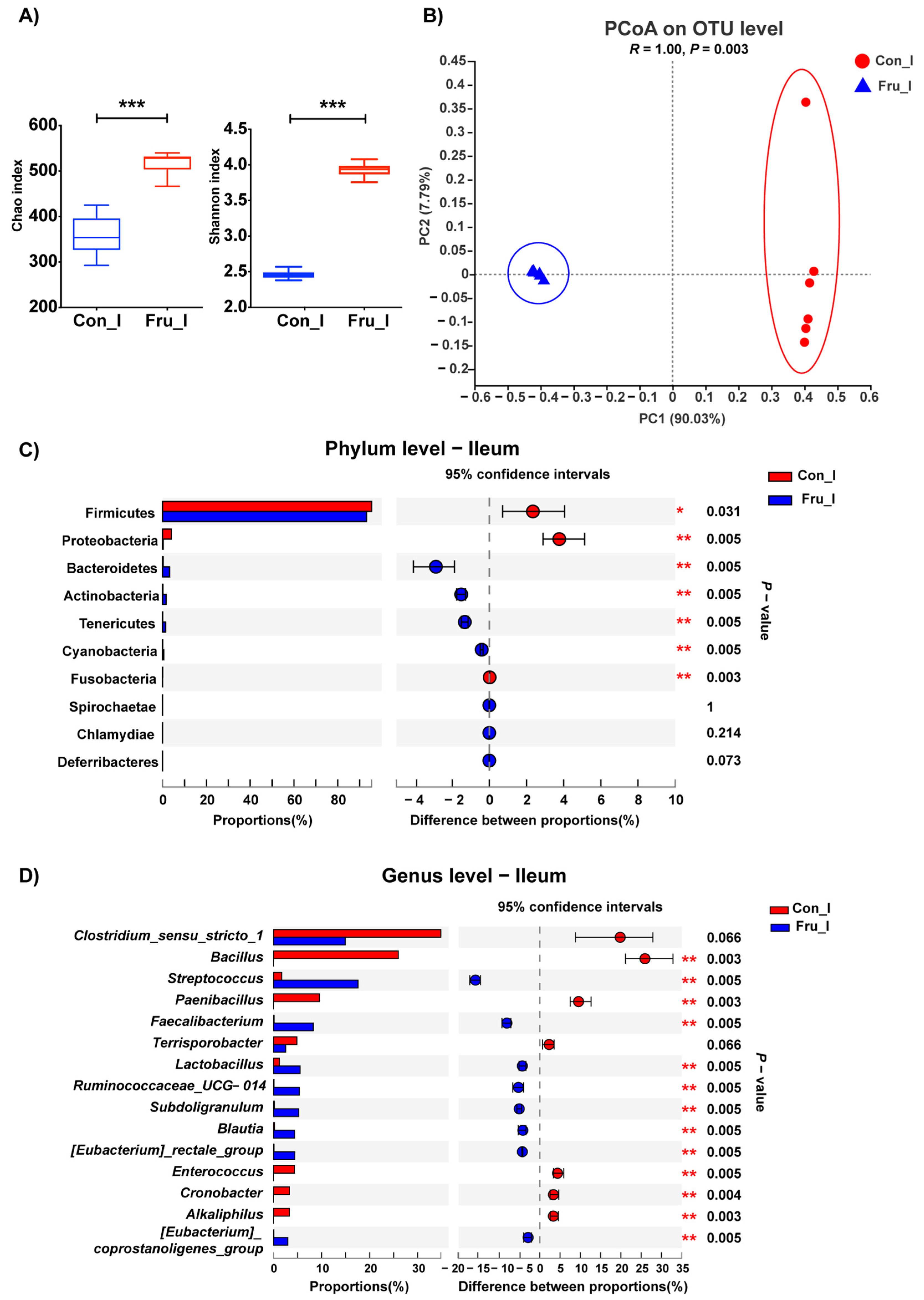
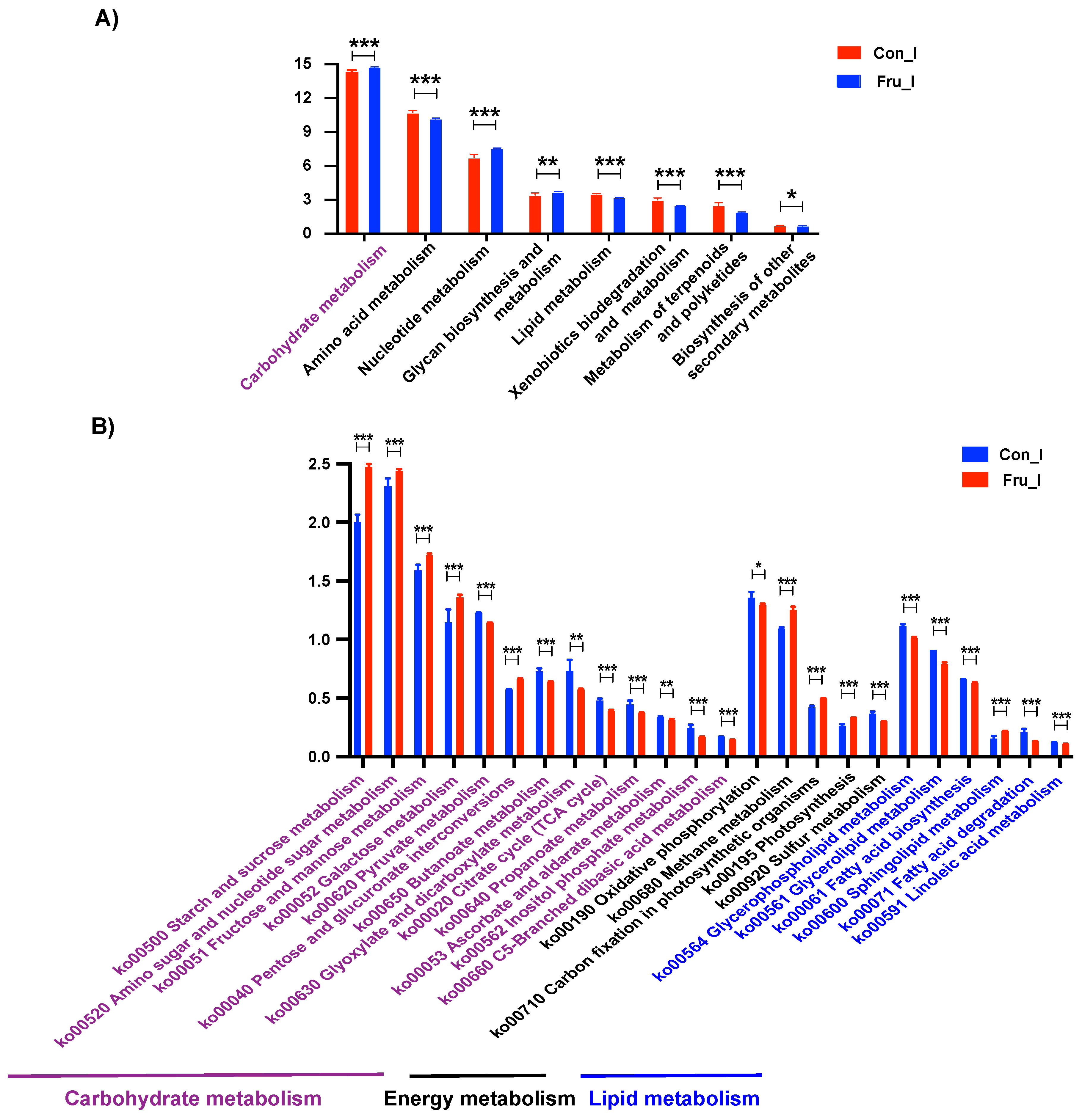
3.4. The Effect of Fructose on Ileal Microbiota Structure of Weaned Piglets
3.5. The Effect of Fructose on Colonic Microbiota Structure of Weaned Piglets
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hilary, F.J.; Ross, N.B.; David, J.M.; Doug, A.B. Developmental changes and fructose absorption in children: Effect on malabsorption testing and dietary management. Nutr. Rev. 2013, 71, 300–309. [Google Scholar]
- Cho, Y.E.; Kim, D.K.; Seo, W.; Gao, B.; Yoo, S.H.; Song, B.J. Fructose Promotes Leaky Gut, Endotoxemia, and Liver Fibrosis Through Ethanol-Inducible Cytochrome P450-2E1-Mediated Oxidative and Nitrative Stress. Hepatology 2021, 73, 2180–2195. [Google Scholar] [CrossRef]
- Herman, M.A.; Samuel, V.T. The Sweet Path to Metabolic Demise: Fructose and Lipid Synthesis. Trends Endocrinol. Metab. 2016, 27, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Lopez-Otin, C.; Madeo, F.; de Cabo, R. Carbotoxicity-Noxious Effects of Carbohydrates. Cell 2018, 175, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Leong, I. Metabolism: The small intestine—A new player in fructose metabolism. Nat. Rev. Endocrinol. 2018, 14, 190. [Google Scholar] [CrossRef] [PubMed]
- Chakaroun, R.M.; Massier, L.; Kovacs, P. Gut Microbiome, Intestinal Permeability, and Tissue Bacteria in Metabolic Disease: Perpetrators or Bystanders? Nutrients 2020, 12, 1082. [Google Scholar] [CrossRef] [PubMed]
- Lam, Y.Y.; Ha, C.W.; Campbell, C.R.; Mitchell, A.J.; Dinudom, A.; Oscarsson, J.; Cook, D.I.; Hunt, N.H.; Caterson, I.D.; Holmes, A.J.; et al. Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS ONE 2012, 7, e34233. [Google Scholar] [CrossRef]
- Miele, L.; Valenza, V.; La Torre, G.; Montalto, M.; Cammarota, G.; Ricci, R.; Masciana, R.; Forgione, A.; Gabrieli, M.L.; Perotti, G.; et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009, 49, 1877–1887. [Google Scholar] [CrossRef]
- Do, M.H.; Lee, E.; Oh, M.J.; Kim, Y.; Park, H.Y. High-Glucose or -Fructose Diet Cause Changes of the Gut Microbiota and Metabolic Disorders in Mice without Body Weight Change. Nutrients 2018, 10, 761. [Google Scholar] [CrossRef] [PubMed]
- Kage, H.; Flodby, P.; Zhou, B.; Borok, Z. Dichotomous roles of claudins as tumor promoters or suppressors: Lessons from knockout mice. Cell. Mol. Life Sci. 2019, 76, 4663–4672. [Google Scholar] [CrossRef]
- Bohringer, M.; Pohlers, S.; Schulze, S.; Albrecht-Eckardt, D.; Piegsa, J.; Weber, M.; Martin, R.; Hunniger, K.; Linde, J.; Guthke, R.; et al. Candida albicans infection leads to barrier breakdown and a MAPK/NF-kappaB mediated stress response in the intestinal epithelial cell line C2BBe1. Cell. Microbiol. 2016, 18, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Wang, P.; Sun, C.; He, W.; Wang, F. Amelioration of IFN-gamma and TNF-alpha-induced intestinal epithelial barrier dysfunction by berberine via suppression of MLCK-MLC phosphorylation signaling pathway. PLoS ONE 2013, 8, e61944. [Google Scholar]
- Gangwar, R.; Meena, A.S.; Shukla, P.K.; Nagaraja, A.S.; Dorniak, P.L.; Pallikuth, S.; Waters, C.M.; Sood, A.; Rao, R. Calcium-mediated oxidative stress: A common mechanism in tight junction disruption by different types of cellular stress. Biochem. J. 2017, 474, 731–749. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Ye, D.; Said, H.M.; Ma, T.Y. IL-1beta-induced increase in intestinal epithelial tight junction permeability is mediated by MEKK-1 activation of canonical NF-kappaB pathway. Am. J. Pathol. 2010, 177, 2310–2322. [Google Scholar] [CrossRef]
- Casteleyn, C.; Rekecki, A.; Van der Aa, A.; Simoens, P.; Van den Broeck, W. Surface area assessment of the murine intestinal tract as a prerequisite for oral dose translation from mouse to man. Lab. Anim. 2010, 44, 176–183. [Google Scholar] [CrossRef]
- Swindle, M.M. Comparative anatomy and physiology of the pig. Scand. J. Lab. Anim. Sci. 1998, 25, 11–21. [Google Scholar]
- Kidder, D.E.; Manners, M.J.; McCrea, M.R. The digestion of sucrose by the piglet. Res. Vet. Sci. 1963, 4, 131–144. [Google Scholar] [CrossRef]
- Aherne, F.; Hays, V.W.; Ewan, R.C.; Speer, V.C. Absorption and utilization of sugars by the baby pigs. J. Anim. Sci. 1969, 29, 444–450. [Google Scholar] [CrossRef]
- Bird, P.H.; Hartmann, P.E. Changes in the concentration of fructose in the blood of piglets of different ages after doses of fructose, fructose plus glucose, and sucrose. Brit. J. Nutr. 1996, 76, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Zhang, R.; Wang, X.; He, P.; Tan, L.; Ma, X. Dietary grape-seed procyanidins decreased postweaning diarrhea by modulating intestinal permeability and suppressing oxidative stress in rats. J. Agric. Food Chem. 2011, 59, 6227–6232. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, J.; Wang, W.; Guo, P.; Lu, W.; Wang, C.; Liu, L.; Johnston, L.J.; Zhao, Y.; Wu, X.; et al. Dietary corn bran altered the diversity of microbial communities and cytokine production in weaned pigs. Front. Microbiol. 2018, 9, 2090. [Google Scholar] [CrossRef]
- Basciano, H.; Federico, L.; Adeli, K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr. Metab. 2005, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Havel, P.J. Dietary fructose: Implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr. Rev. 2005, 63, 133–157. [Google Scholar] [CrossRef]
- Gonzalez, J.T.; Betts, J.A. Dietary Fructose Metabolism by Splanchnic Organs: Size Matters. Cell Metab. 2018, 27, 483–485. [Google Scholar] [CrossRef]
- Li, J.M.; Yu, R.; Zhang, L.P.; Wen, S.Y.; Wang, S.J.; Zhang, X.Y.; Xu, Q.; Kong, L.D. Dietary fructose-induced gut dysbiosis promotes mouse hippocampal neuroinflammation: A benefit of short-chain fatty acids. Microbiome 2019, 7, 98. [Google Scholar] [CrossRef]
- Mastrocola, R.; Ferrocino, I.; Liberto, E.; Chiazza, F.; Cento, A.S.; Collotta, D.; Querio, G.; Nigro, D.; Bitonto, V.; Cutrin, J.C.; et al. Fructose liquid and solid formulations differently affect gut integrity, microbiota composition and related liver toxicity: A comparative in vivo study. J. Nutr. Biochem. 2018, 55, 185–199. [Google Scholar] [CrossRef]
- Fasano, A. Intestinal zonulin: Open sesame! Gut 2001, 49, 159–162. [Google Scholar] [CrossRef][Green Version]
- Drolia, R.; Tenguria, S.; Durkes, A.C.; Turner, J.R.; Bhunia, A.K. Listeria Adhesion Protein Induces Intestinal Epithelial Barrier Dysfunction for Bacterial Translocation. Cell Host Microbe 2018, 23, 470–484. [Google Scholar] [CrossRef]
- Graham, W.V.; He, W.; Marchiando, A.M.; Zha, J.; Singh, G.; Li, H.S.; Biswas, A.; Ong, M.; Jiang, Z.H.; Choi, W.; et al. Intracellular MLCK1 diversion reverses barrier loss to restore mucosal homeostasis. Nat. Med. 2019, 25, 690–700. [Google Scholar] [CrossRef] [PubMed]
- He, W.Q.; Wang, J.; Sheng, J.Y.; Zha, J.M.; Graham, W.V.; Turner, J.R. Contributions of Myosin Light Chain Kinase to Regulation of Epithelial Paracellular Permeability and Mucosal Homeostasis. Int. J. Mol. Sci. 2020, 21, 993. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Bang, J.Y.; Park, G.W.; Choi, D.S.; Kang, J.S.; Kim, H.J.; Park, K.S.; Lee, J.O.; Kim, Y.K.; Kwon, K.H.; et al. Global proteomic profiling of native outer membrane vesicles derived from Escherichia coli. Proteomics 2007, 7, 3143–3153. [Google Scholar] [CrossRef]
- Di Luccia, B.; Crescenzo, R.; Mazzoli, A.; Cigliano, L.; Venditti, P.; Walser, J.C.; Widmer, A.; Baccigalupi, L.; Ricca, E.; Iossa, S. Rescue of Fructose-Induced Metabolic Syndrome by Antibiotics or Faecal Transplantation in a Rat Model of Obesity. PLoS ONE 2015, 10, e134893. [Google Scholar] [CrossRef] [PubMed]
- Hokayem, M.; Blond, E.; Vidal, H.; Lambert, K.; Meugnier, E.; Feillet-Coudray, C.; Coudray, C.; Pesenti, S.; Luyton, C.; Lambert-Porcheron, S.; et al. Grape polyphenols prevent fructose-induced oxidative stress and insulin resistance in first-degree relatives of type 2 diabetic patients. Diabetes Care 2013, 36, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Crescenzo, R.; Mazzoli, A.; Di Luccia, B.; Bianco, F.; Cancelliere, R.; Cigliano, L.; Liverini, G.; Baccigalupi, L.; Iossa, S. Dietary fructose causes defective insulin signalling and ceramide accumulation in the liver that can be reversed by gut microbiota modulation. Food Nutr. Res. 2017, 61, 1331657. [Google Scholar] [CrossRef]
- Rutledge, A.C.; Adeli, K. Fructose and the metabolic syndrome: Pathophysiology and molecular mechanisms. Nutr. Rev. 2007, 65, S13–S23. [Google Scholar] [CrossRef] [PubMed]
- Sapp, V.; Gaffney, L.; EauClaire, S.F.; Matthews, R.P. Fructose leads to hepatic steatosis in zebrafish that is reversed by mechanistic target of rapamycin (mTOR) inhibition. Hepatology 2014, 60, 1581–1592. [Google Scholar] [CrossRef]
- Zhao, L.; Lou, H.; Peng, Y.; Chen, S.; Fan, L.; Li, X. Elevated levels of circulating short-chain fatty acids and bile acids in type 2 diabetes are linked to gut barrier disruption and disordered gut microbiota. Diabetes Res. Clin. Pract. 2020, 169, 108418. [Google Scholar] [CrossRef] [PubMed]
- Michaudel, C.; Sokol, H. The Gut Microbiota at the Service of Immunometabolism. Cell Metab. 2020, 32, 514–523. [Google Scholar] [CrossRef]
- Liao, J.; Li, Q.; Lei, C.; Yu, W.; Deng, J.; Tang, Z. Toxic effects of copper on jejunum and colon of pigs mechanisms related to gut barrier dysfunction and inflammation influenced by gut microbiota. Food Funct. 2021, in press. [Google Scholar]
- Morkl, S.; Lackner, S.; Meinitzer, A.; Mangge, H.; Lehofer, M.; Halwachs, B.; Gorkiewicz, G.; Kashofer, K.; Painold, A.; Holl, A.K.; et al. Gut microbiota, dietary intakes and intestinal permeability reflected by serum zonulin in women. Eur. J. Nutr. 2018, 57, 2985–2997. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Eren, A.M.; Sogin, M.L.; Morrison, H.G.; Vineis, J.H.; Fisher, J.C.; Newton, R.J.; McLellan, S.L. A single genus in the gut microbiome reflects host preference and specificity. ISME J. 2015, 9, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Bernalier, A.; Willems, A.; Leclerc, M.; Rochet, V.; Collins, M.D. Ruminococcus hydrogenotrophicus sp. nov., a new H2/CO2-utilizing acetogenic bacterium isolated from human feces. Arch. Microbiol. 1996, 166, 176–183. [Google Scholar] [CrossRef] [PubMed]

| Ingredients | Content (%) | Nutrient Levels | Content |
|---|---|---|---|
| Extruded maize meal | 54.19 | Gross energy (MJ/kg) | 16.95 |
| Dehulled soybean meal | 20.70 | Dry matter (%) | 91.41 |
| Extruded soybean | 11.00 | Crude protein (%) | 20.26 |
| Whey power | 4.00 | Ether extract (%) | 8.11 |
| Fish meal | 3.00 | Calcium (%) | 0.87 |
| Wheat bran | 1.50 | Total Phosphorus (%) | 0.71 |
| Dicalcium phosphate | 2.20 | ||
| Glucose | 1.00 | ||
| Limestone | 0.80 | ||
| L-Lysine·HCl | 0.35 | ||
| L-Threonine | 0.18 | ||
| DL-Methionine | 0.05 | ||
| Tryptophan | 0.03 | ||
| Premix 1 | 1.00 | ||
| Total | 100.00 |
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| ACTB | ACACGGTGCCCATCTACGAG | GCTTCTCCTTGATGTCCCGC |
| ZO1 | AGCCATCCACTCCTGCCTAT | GACGGGACCTGCTCATAACT |
| OCLN | CTTTCTCAGCCAGCGTATTC | AGGCAAGCGTGGAGGCAACA |
| CLDN1 | CATTGCTATCTTTGCCTGTG | GCCATAACCGTAGCCATAAC |
| MLCK | CCTGTCCTGGTATGGCTCCT | CTGCGGCATGTGGCTAGTTC |
| Items | Con | Fru | p-Value |
|---|---|---|---|
| 1–14 days | |||
| ADG, g | 224 ± 9 | 236 ± 12 | 0.439 |
| ADFI, g | 490 ± 20 | 471 ± 20 | 0.534 |
| F/G | 2.19 ± 0.05 | 2.01 ± 0.07 | 0.076 |
| 15–35 days | |||
| ADG, g | 479 ± 4 | 473 ± 16 | 0.694 |
| ADFI, g | 1064 ± 20 | 1043 ± 36 | 0.619 |
| F/G | 2.22 ± 0.03 | 2.21 ± 0.03 | 0.780 |
| 1–35 days | |||
| ADG, g | 377 ± 4 | 378 ± 11 | 0.947 |
| ADFI, g | 834 ± 19 | 814 ± 27 | 0.556 |
| F/G | 2.21 ± 0.03 | 2.15 ± 0.02 | 0.110 |
| Items | Con | Fru | p-Value |
|---|---|---|---|
| Serum | |||
| C3, g/L | 0.92 ± 0.02 | 0.93 ± 0.03 | 0.738 |
| IL-1β, ng/mL | 0.21 ± 0.03 | 0.19 ± 0.02 | 0.762 |
| IL-2, ng/mL | 4.85 ± 0.30 | 6.86 ± 0.67 | 0.052 |
| IFN-γ, pg/mL | 46.31 ± 4.92 | 32.71 ± 5.74 | 0.146 |
| TNF-α, ng/mL | 1.03 ± 0.15 | 1.54 ± 0.31 | 0.214 |
| Ileum | |||
| C3, g/g TP | 0.18 ± 0.01 | 0.20 ± 0.01 | 0.222 |
| IL-1β, ng/mg TP | 0.02 ± 0.00 | 0.03 ± 0.01 | 0.094 |
| IL-2, ng/mg TP | 0.55 ± 0.10 | 0.63 ± 0.08 | 0.548 |
| IFN-γ, pg/mg TP | 3.92 ± 0.54 | 4.43 ± 0.72 | 0.597 |
| TNF-α, ng/mg TP | 0.10 ± 0.01 | 0.07 ± 0.02 | 0.319 |
| Items | Con | Fru | p-Value |
|---|---|---|---|
| Serum | |||
| MDA, nM/mL | 5.65 ± 0.24 | 5.60 ± 0.35 | 0.900 |
| GSH, μM/L | 3.76 ± 0.09 | 3.88 ± 0.18 | 0.573 |
| SOD, U/mL | 82.67 ± 5.83 | 69.45 ± 4.35 | 0.143 |
| GSH-Px, U/mL | 1010 ± 143 | 1086 ± 124 | 0.707 |
| Ileum | |||
| MDA, nM/mg TP | 0.51 ± 0.07 | 0.67 ± 0.12 | 0.315 |
| GSH, μM/mg TP | 0.31 ± 0.03 | 0.40 ± 0.04 | 0.139 |
| SOD, U/mg TP | 8.07 ± 0.59 | 7.51 ± 0.28 | 0.422 |
| GSH-Px, U/mg TP | 66.88 ± 8.06 | 78.89 ± 11.76 | 0.432 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, P.; Wang, H.; Ji, L.; Song, P.; Ma, X. Impacts of Fructose on Intestinal Barrier Function, Inflammation and Microbiota in a Piglet Model. Nutrients 2021, 13, 3515. https://doi.org/10.3390/nu13103515
Guo P, Wang H, Ji L, Song P, Ma X. Impacts of Fructose on Intestinal Barrier Function, Inflammation and Microbiota in a Piglet Model. Nutrients. 2021; 13(10):3515. https://doi.org/10.3390/nu13103515
Chicago/Turabian StyleGuo, Pingting, Haichao Wang, Linbao Ji, Peixia Song, and Xi Ma. 2021. "Impacts of Fructose on Intestinal Barrier Function, Inflammation and Microbiota in a Piglet Model" Nutrients 13, no. 10: 3515. https://doi.org/10.3390/nu13103515
APA StyleGuo, P., Wang, H., Ji, L., Song, P., & Ma, X. (2021). Impacts of Fructose on Intestinal Barrier Function, Inflammation and Microbiota in a Piglet Model. Nutrients, 13(10), 3515. https://doi.org/10.3390/nu13103515






