1. Introduction
Gastric ulceration is the most prevalent gastrointestinal disorder accounting for an estimated mortality of 15 out of every 15,000 complications yearly [
1]. The most prevalent causes of gastric ulcers are
Helicobacter pylori infection (infecting almost half of the world’s population, causes acute gastritis, chronic atrophic gastritis, gastro-esophageal reflux, ulcers of the stomach, and duodenum, esophageal cancer, gastric adenocarcinoma, MALT lymphomas, and gastric adenocarcinoma), nonsteroidal anti-inflammatory drugs (NSAIDs), and persistent drinking with gastric malignancy and chronic gastric ischemia being the less common causes. However, the clinical outcome of the infection may be influenced by a combination of bacterial factors, host factors, and environmental variables [
2,
3,
4,
5]. With regards to NSAIDs, indomethacin is a nonsteroidal anti-inflammatory drug that was introduced in 1963 to treat inflammatory diseases [
6]. It is readily absorbed from the gastrointestinal tract almost entirely after oral ingestion and is metabolized by the liver and converted to active metabolites [
7]. The clinical use of indomethacin is associated with potentially life-threatening deleterious effects as gastrointestinal ulceration, bleeding [
8], renal toxicity [
9], hepatic injury [
10], intestinal damage, anemia, and the loss of protein [
11]. In addition, the administration of indomethacin results in serious adverse effects on the cardiovascular system [
12], initiation of lipid peroxidation, the elevation of oxidative stress [
13], and infiltration of inflammatory cells [
14].
Spirulina platensis (SP) is a blue-green alga found in many lakes. It contains approximately 70% easily digestible protein, where 18 out of 22 amino acids and all essential amino acids are available, making it a unique, complete protein source. Carotenoids, vitamins, minerals, and essential fatty acids are available in SP. It is an excellent source of B vitamins, particularly vitamin B
12. This nutritious food also contains vitamin E, a highly bioavailable source of iron, 14 naturally chelated minerals, and numerous trace elements [
15]. SP is claimed in folk medicine to be a potent wound-healing inducer of external and gastrointestinal wounds. Indeed, pre-clinical and clinical studies suggest it has various therapeutic effects, such as reduction in blood cholesterol; protection against some cancers [
16]; enhancement of the immune system; an increase of intestinal lactobacilli, a decrease of nephrotoxicity by heavy metals and drugs; radiation protection; and reduction of hyperlipidemia and obesity [
17].
It is evident from the scientific literature that kiwifruit has potentially beneficial actions in improving health in several domains. In that regard, ultimate biological activities toward specific diseases could be approached to recommend dietetic therapy application. There are rare or no available publications about using kiwifruit as a gastro-protective agent despite efficient application in the treatment of diabetic foot ulcers [
18,
19]. Kiwifruit had higher total flavonoids, total chlorophyll, carotenoids, and vitamin C [
20,
21,
22,
23]. It prevents tissue damage induced by indomethacin toxicity and protects gastric and hepatic tissues [
24].
Stomach ulcers are often treated with antibiotics or medications to reduce, block, or neutralize stomach acid. The commercially available synthetic anti-ulcer-drugs are often expensive, have many side effects, and do not prevent ulcer recurrence. There has been growing interest in alternative therapies and natural products in recent years, especially those derived from plants. Foods containing the antioxidant polyphenols can protect you from ulcers and help ulcers heal, such as polyphenol-rich foods. Interestingly, there is no available information on the antiulcerogenic activity of SP and kiwifruit individually or in combination against peptic ulcers. However, even though the literature showed promising potentialities related to the use of SP and kiwifruit [
18] separately, the gastroprotective potential of SP and kiwifruit both individually or in combination needs to be carefully investigated. Moreover, literature has mainly reviewed the antiulcerogenic efficiency of golden kiwifruit in Swiss albino mice [
24], but the antiulcerogenic and gastroprotective potential of golden kiwifruit, their peels, and SP individually and/or in combination has not been studied so far, thus motivating this work. Therefore, the current study aims to investigate the possible antiulcerogenic and gastroprotective potential of golden kiwifruit (flesh and peel) and SP extracts against an indomethacin-induced gastric ulcer in rats model, which will be be further investiaged for potential application in functional supplements or beverages as well as in dietetic therapy for peptic and duodenum ulcers.
4. Discussion
Because NSAIDs are widely used worldwide due to their outstanding efficacy in managing pain, fever, and inflammation, the indomethacin model was chosen as the NSAIDs medicine. However, NSAIDs use was associated with severe adverse effects in the upper gastrointestinal tract and the small intestine, cardiovascular system, and liver; inhibited DNA synthesis; and accelerated oxidative stress in vivo [
7,
24,
32]. Overdose, inappropriate administration, or extended usage might result in severe stomach ulcers and gastroduodenal disorders. [
33]. It is believed that indomethacin inhibited prostaglandins (PGs) synthesis and blocked their therapeutic actions via inhibition of cyclooxygenase (COX) enzymes [
34]. PGs biosynthesis inhibition is linked to decreased gastric mucosal blood flow; disruption of microcirculation; and decreased mucus secretion, lipid peroxidation, and neutrophil activation; all of which are implicated in the pathophysiology of gastrointestinal mucosal disorders [
35]. There is a rising interest in natural antioxidants that are non-toxic, safe, and affordable, particularly those derived from plants. Natural antioxidants derived from fruits and vegetables are generally regarded as safe by most consumers [
36]. Therefore, the current study compared the gastroprotective effect of SP, KF, KP, and SFP on indomethacin-toxicity in rats utilizing biochemicals and histological investigation to the commonly used Lansoprazole at equal dosages. Rats that orally received indomethacin recorded the most significant volume, congested appearance, and severe bleeding in the stomach compared to other treated rat groups after 2 weeks due to indomethacin-induced ulceration. The generation of inflammatory mediators is an essential key factor in the development of mucosal lesions. Furthermore, gastric blood flow stasis and microvascular disruption are implicated in the processes of bleeding and necrotic tissue damage [
37] or as a result of stomach ischemia induced by a blockage of the stomach’s dual blood supply [
2].
Neutrophil infiltration also plays an essential part in the process of injury and inflammation via aggregation and release of tissue-disrupting chemicals in numerous tissues, including stomach mucosal lesions [
38]. Acute gastric mucosal lesions are caused by neutrophil infiltration into the stomach mucosal tissues [
39]. Administration of SP, KF, KP, and SFP extracts markedly reduced gastric exudation and volume expansion induced by indomethacin. The best amelioration effect was observed for SP treatment (GIII). In addition, the inflation was attenuated by 83.5, 80.0, 92.9, and 62.4% when KF, KP, and SFP extracts, and Lansoprazole were given (calculated based on GJV). SP, KF, KP, and SFP showed significant inhibition of this infiltration, suggesting they possess gastroprotective properties. Similar anti-inflammatory effects were recorded with
Rhus tripartita stem extract [
29],
Ficus indica roots [
40], and
Rosmarinus officinalis leaf extracts [
41]. Giving KP, KF and SFP extracts increased the gastric juice pH of rats when compared to GII rats but not usually when compared to GI. Indeed, the significant increase of gastric pH after SP, KF, KP, and SFP extracts could be due to its inhibitory action on hydrochloric acid secretion, as similarly indicated by Barka et al. [
29] and Giridharan et al. [
42]. In GVII, giving Lansoprazole orally to rats recorded a significant (
p < 0.05) increase of gastric juice pH (4.68,
Table 1), which may be due to inhibition of acid secretion and protection against NSAID-induced gastric damage that depends on a reduction in mucosal oxidative injury as explained by Blandizzi et al. [
43]. Inhibition of acid secretion may occur because Lansoprazole inhibits acid-secreting enzymes located in the gastric parietal cells (H+, K+-ATPase), as mentioned by Matsukawa et al. [
44].
Regarding ulcer index and protection %, treating rats with SP and SFP exuded a more or less similar amelioration effect as observed by using Lansoprazole as treating medicine [
43,
44,
45]. Our study demonstrated, following the literature [
46], that kiwifruits have antioxidant biomolecules such as phenolics and flavonoids and exhibited antiulcer and beneficial gut health effects in vitro. In recent years, researchers indicated that various plants such as
F. indica and
R. tripartitum are known for their antioxidant and antiulcer therapeutic virtues [
40,
47]. SP exhibited gastroprotective activity against acetic acid and ethanol-induced ulcers in rats [
48]. The suppressive effect of SP was similarly observed for
S. fusiformis at 400 mg kg
−1 as gastrointestinal ulcer treatment [
42]. Recently, Guzman-Gomez et al. [
49] suggested that a significant gastroprotective effect of SP was relevant to its phycobiliproteins, which repair gastric damage; its antioxidant properties by activating some enzymatic antioxidant mechanisms (SOD, CAT, and GPx); diminishing lipid peroxidation; and attenuating the inflammatory response, improving defences against the erosive lesion that characterizes the development of gastric ulcers.
Mucosal surfaces are exposed to the external environment and pathogens. Therefore, they are protected by a secreted layer of mucus rich in mucin glycoproteins, which are the main components of mucus. Gastric mucus is an important protective factor for gastric mucosa. It consists of a viscous, elastic, adherent, and transparent gel formed by 95% water and 5% glycoproteins covering the entire gastrointestinal mucosa. It provides physical protection and hydration, excludes pathogens, and is a reservoir for antimicrobial molecules. Underlying mucus, further protection is provided by epithelial cell surface mucins, which limit microbial adherence and regulate growth and apoptosis [
4]. Experimental deficiencies in mucins lead to infectious and inflammatory diseases [
50]. Rat gastric mucosal damage has widely been used to investigate the gastroprotective effect of medicinal plants [
51]. Ulceration induction by indomethacin decreased gastric mucin content, which significantly recovered in treated groups with SP and/or SFP better than treated with Lansoprazole. In addition to the protection function of mucus, it can act as an antioxidant and thus reduce mucosal damage mediated by oxygen-free radicals [
52]. SP, KF, KP, and SFP extracts, and Lansoprazole improved the reform of gastric mucosal. The increased secreted mucus may be due to the antioxidant capacity of kiwifruit [
21,
22,
24] and SP [
42,
48,
49]. Gastrin is a gastrointestinal hormone that regulates gastric acid secretion, releases histamine, and regulates gastric endocrine cell proliferation [
53]. Hiruma-Lima et al. [
54] indicated that ulcer induction increases the gastrin level in the plasma. It is secreted by antral G cells and is the principal stimulant of gastric acid secretion, which decreases stomach pH (
Table 1). On the contrary, SPE, KFE, KPE, SFP, and Lansoprazole substantially reduced plasma gastrin levels in rats [
44]. Hiruma-Lima et al. [
54] marked a decrease in serum gastrin level by administrating an enriched flavonoids matrix of
Alchornea castaneaefolia hydroethanolic extract, which possesses an antiulcer mechanism. These results corroborate our present finding of an ameliorative action of SP, KF, KP, and SKP extracts on indomethacin-induced gastric ulceration, possibly due to its relatively high content of antioxidants [
55]. Phytochemical analysis of SP, KF, and KP extracts indicated high phenolic acids and flavonoids content and high antioxidant capacity. Interestingly, literature reported antiulcer activity of flavonoids [
54], phenolic substances [
21], and bioactive compounds from herbal plants [
1,
40,
56,
57].
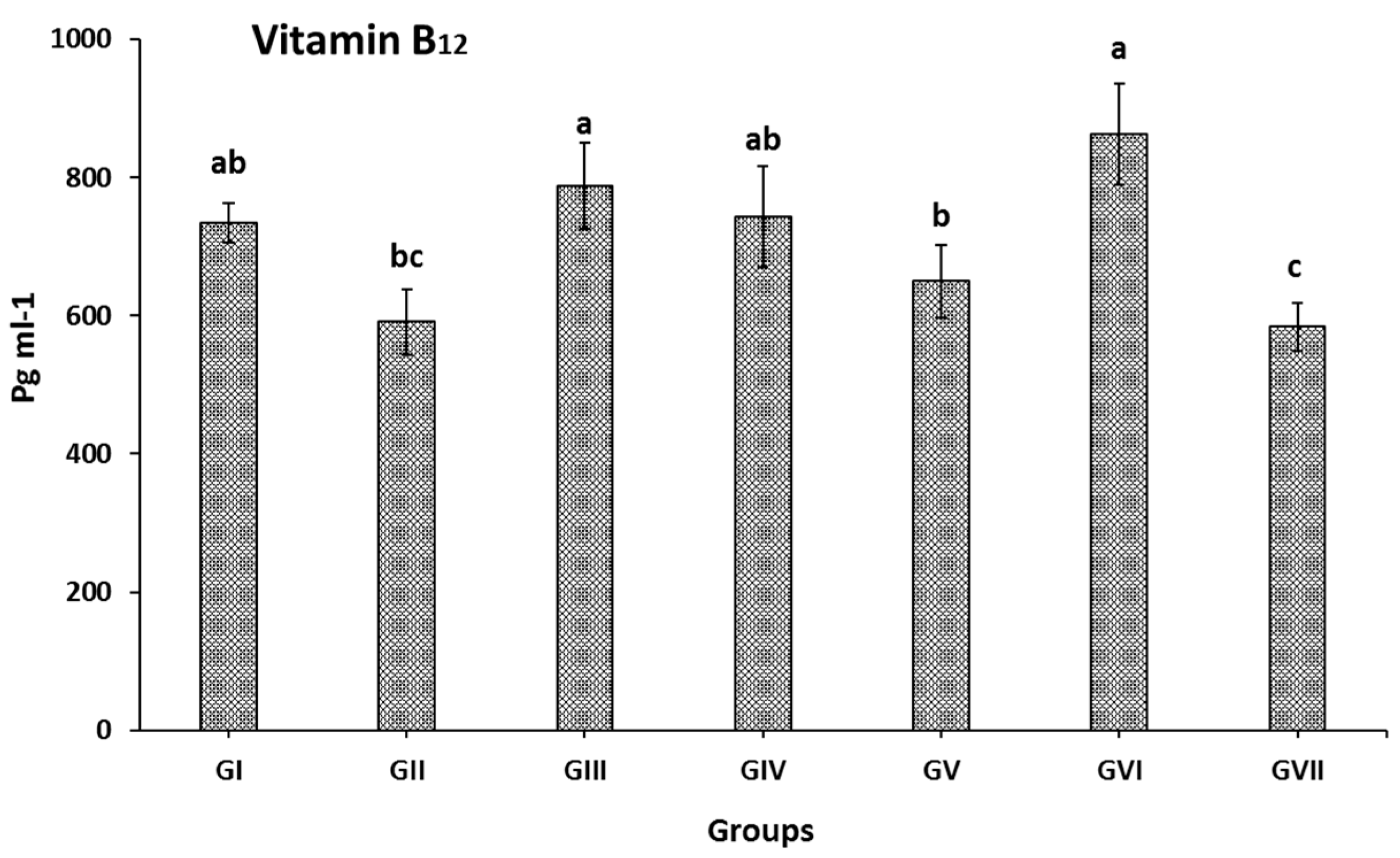
Treating rats with KF, KP, and SFP extracts attenuated vit. B
12 by 25.8, 10.0, and 46.0%; SFP mix was better than both KP and KF extracts due to rich vit. C content in kiwifruit that has a positive relation to iron absorption [
58] and high vitamin E. Aa highly bioavailable source of iron is SP [
15]. On the other hand, administrating Lansoprazole does not significantly attenuate vit. B
12 level in ulcerated rats as observed in GVII rats. Human studies found that oral omeprazole (treat acid reflux and ulcers) for up to 2 weeks significantly decreased vitamin B
12 levels [
59,
60]. However, Kittang et al. [
61] showed that an intravenous infusion of omeprazole did not change absorption of vit. B
12 but longer treatment evidently reduced vit. B
12 [
62]. The decrease of vit B
12 in lansoprazole-treated rats is a side effect of using such treatment. It is worth mentioning that most dietary vit. B
12 is tightly protein bound. It is released in the stomach by gastric acid and pepsin, where it binds to salivary R proteins and intrinsic factors. This complex remains intact until it binds to specific receptors in the terminal ileum, where vit. B
12 is absorbed [
63,
64]. Indomethacin significantly reduced the blood iron and hemoglobin levels. After 14 days of administrating SP and kiwifruit extracts individually or in combination, the iron level was attenuated; the result was not shown with Lansoprazole. It could be due to the efficiency of SP, KF, and SFP as an iron source (SP) and vit. C source (KF), which efficiently helps improving plasma iron level in ulcerated rats. This could explain that giving Lansoprazole did not increase iron levels similarly as noticed in SP and Kiwifruit extracts even if it treated ulcers efficiently [
43,
44,
45]. The result is also positively correlated with obtained results in
Figure 2. In the same context, administrating SP and fruit extracts mix with SP improved the Hb levels in ulcerated rats (
Table 4). The lowest improving rat was observed when Lansoprazole was administered to rats, which may be related to Lansoprazole’s inefficiency in assisting the cell in absorbing iron and vitamin B
12 as the key material for generating blood hemoglobin [
59,
60].
Indomethacin is an NSAIDS drug used as an analgesic and anti-inflammatory agent causing gastric ulcer, hepatotoxicity, and cellular damage [
8,
65,
66]. In the present study, administration of indomethacin resulted in an increase in stomach MDA and a decrease in GSH and SOD levels significantly compared with GI rats. MDA is a secondary product of polyunsaturated fatty acids to peroxidation and is the primary marker for estimating lipid peroxidation levels [
67]. Indomethacin was remarked for the initiation of lipid peroxidation [
9], the elevation of oxidative stress [
13], and the infiltration of inflammatory cells [
14]. Interestingly, MDA levels were dramatically decreased when compared with GII rats. SP, KF, KP, and SFP extracts prevented lipid peroxidation levels, which could be attributed to the radical scavenging activity of antioxidant constituents [
68]. Administrating SP individually or combined with KF and KP extracts was expressively better than Lansoprazole (GVII). However, previous studies have shown that Spirulina possesses a significant anticancer activity [
69]. Giridharan et al. [
42] stated that
S. fusiformis at 400 mg/kg as gastrointestinal ulcer treatment successfully recovered peptic ulceration in rats. Spirulina contains C-phycocyanin, which is considered one of the major biliproteins. This water-soluble protein pigment is shown to have gastroprotective activity against ulcered rats with acetic acid and ethanol [
48]. Recently, Guzman-Gomez et al. [
49] suggested a significant gastroprotective effect of its phycobiliproteins against ethanol-induced gastric damage. This protection may be related to the antioxidant properties of phycobiliproteins by activating some enzymatic antioxidant mechanisms (SOD, CAT, and GPx), diminishing lipid peroxidation, and attenuating the inflammatory response, improving defences against the erosive lesion that characterizes the development of gastric ulcers produced by ethanol. In addition, Giridharan et al. [
42] proved the hepato-renal and gastroprotective activity of
S. fusiformis in diclofenac-treated rats. Moreover, Kepekci et al. [
70] demonstrated that
S. platensis enriched in phenolic compounds have a protective effect against hepatotoxicity induced by CCl
4 in rats.
The inbuilt antioxidant systems like SOD and GSH would prevent the tissues from free radical attack. Administration of indomethacin decreased the SOD and GSH levels significantly compared with GI rats, as mentioned [
13,
14]. GSH and SOD levels were considerably reduced after treatment with SP, KF, KP, and SFP extracts. This may be due to their content of polyphenolic compounds that may attenuate the cellular toxicity by increasing expressions of antioxidant enzymes [
71], antioxidant enzymes induced by transcription factor (Nrf2) activation and other signal transduction pathways; increasing Cytochrome P450 2E1 activity (as a marker of oxidative stress);and decreasing the oxidative damage to DNA [
72]. Following the known fact, the amelioration of cellular intoxication may correlate with the overall improvement of antioxidant defence mechanisms influenced by treating rats with SP, KF, KP, and SFP extracts. This increase recovered depleted GSH level and provided significant protection against GSH reduction in rats, Chu et al. [
72]. Accordingly, glutathione deficiency is associated with oxidative stress and, therefore, may play a key role in the aging and pathogenesis of many diseases [
73]. The possible reason is that GSH allows free radicals and ROS; consequently, its concentration decreases [
69]. Therefore, supplemental ingested GSH can benefit the treatment of these diseases and increase liver GSH concentration for detoxification. Thus, it was speculated that increased GSH levels in the presence of SP, KF, KP, and SFP extracts in the face of oxidative damage enhanced the detoxification of free radical and ROS, thereby resulting in an improvement of antioxidants enzymes in rats as similarly found by various plant extracts [
45,
72,
74,
75,
76].
As shown in the treated groups, administration of SP, KF, KP, and SFP extracts significantly decreased the MDA and increased GSH and SOD levels. The most preferred effect was observed with SP and SFP, a result that was similarly confirmed previously [
69]. This suppressive effect may be due to the high content of C-phycocyanin, which is considered one of the major phycobiliproteins that have gastroprotective activity [
48]. This protection may be related to the antioxidant properties of phycobiliproteins by activating some enzymatic antioxidant mechanisms (SOD, CAT, and GPx), diminishing lipid peroxidation, and attenuating the inflammatory response, improving defenses against the erosive lesion caused by gastric ulcers [
70]. The inbuilt antioxidant systems like SOD and GSH would prevent the tissues from free radical attack. Administration of indomethacin decreased the SOD and GSH levels significantly compared with GI rats. Treatment with SP, KF, KP, and SFP extracts significantly attenuated the GSH and SOD levels. Their content of polyphenolic compounds may attenuate cellular toxicity by increasing the expressions of antioxidant enzymes [
71]. In accordance with the known fact, the amelioration of cellular intoxication may correlate with the overall improvement of antioxidant defense mechanisms influenced by the treatments of SP, KF, KP, and SFP extracts. This increase recovered depleted the GSH level and provided significant protection against GSH reduction in rats [
72]. A possible reason for this is that GSH allows free radicals and ROS; consequently, its concentration decreases [
73]. Thus, it was speculated that increased GSH levels in the presence of SPE, KFE, KPE, and SFP extracts in the face of oxidative damage enhanced the detoxification of free radicals and ROS, thereby resulting in an improvement of antioxidants enzymes in rats as similarly found by various plant extracts [
72,
74,
75,
76]. Even though Lansoprazole has efficient antiulcer activity by preventing gastric mucosal from injury [
45], it has no remarkable effect in enhancing antioxidants enzyme compared to SP and kiwifruit extracts. Similar results indicated that Lansoprazole has an unignorable effect on antioxidant enzymes and GSH [
77]. However, the inhibition of Na
+, K
+ -ATPase activity can be evidence for the possible side effects of Lansoprazole when used to treat acid-dependent diseases of the stomach [
78].
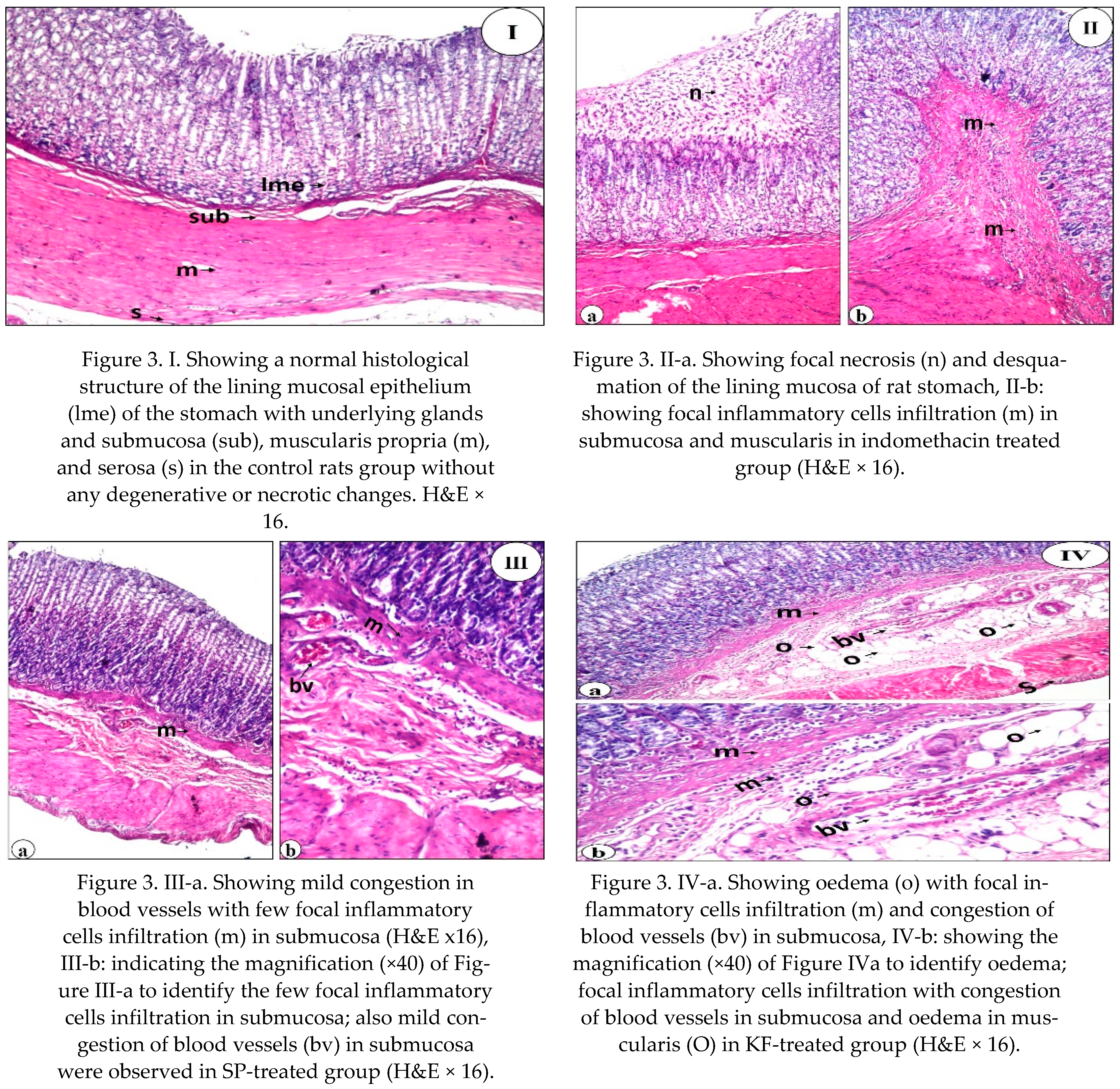
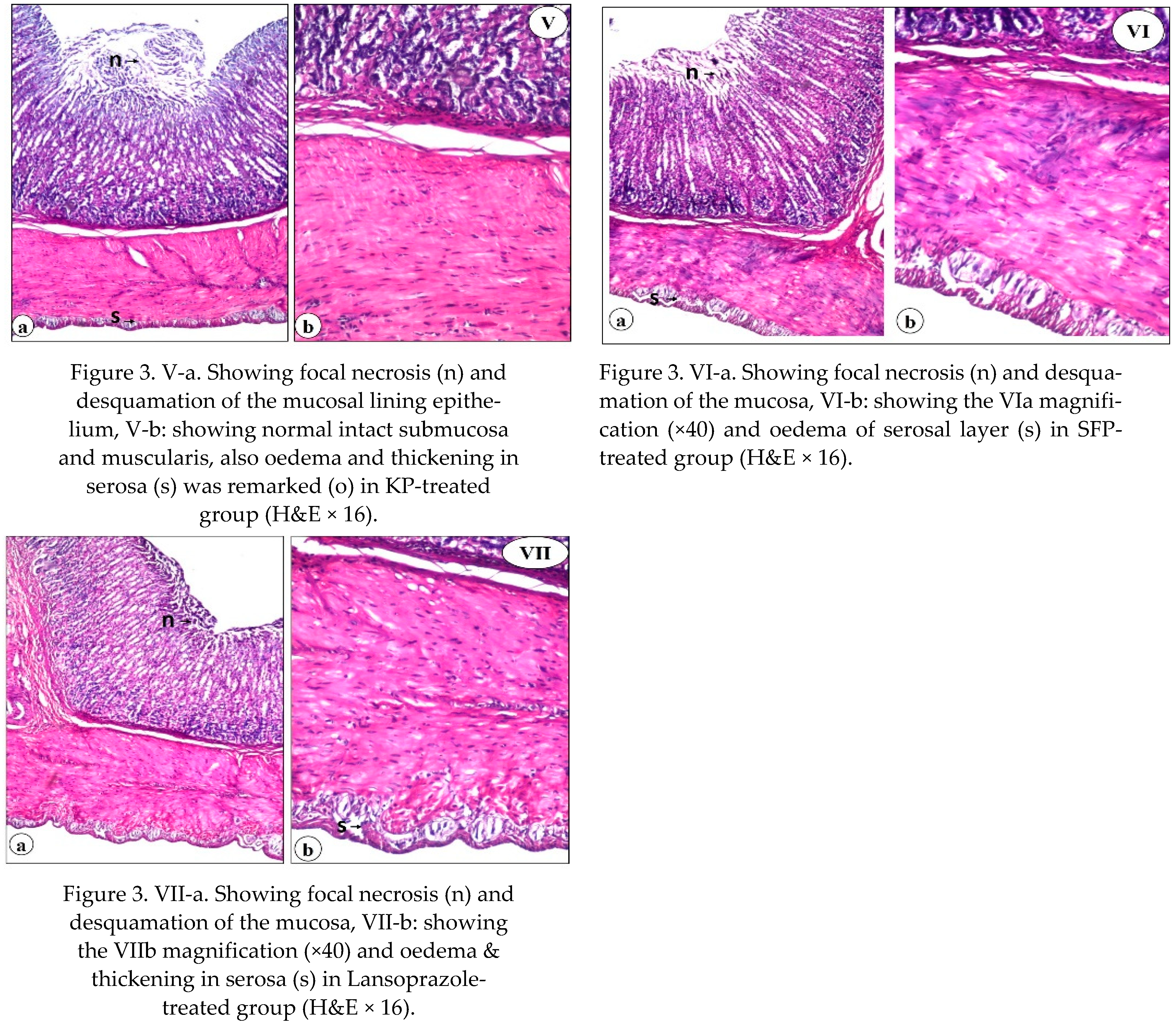
Using a histological examination, we evaluated the therapeutic effect of SP, KF, KP, and SFP compared to Lansoprazole. Our findings revealed that SP, KF, KP, SFP, and lansoprazole treatment improved the histological lesions. The ability of SP, KF, KP, and SFP to heal gastric ulcers in this study could be attributed to their antioxidant, anti-inflammatory, and free radical scavenging properties [
24,
79]. Giridharan et al. [
42] explained how SP protected DFC-treated rats from oxidative stress-induced liver and kidney damage, as well as ulcer formation. The presence of phycobiliproteins in SP may explain its significant therapeutic effect. It improves defenses against the erosive lesion that characterizes the development of gastric ulcers by activating some enzymatic antioxidant mechanisms, decreasing lipid peroxidation, and attenuating the inflammatory response. Furthermore, Somchit et al. [
48] reported that phyto-compounds in SP may improve wound/ulcer healing and protect the gastric mucosal layer from ulceration agents.