Sensitivity of Food-Based Recommendations Developed Using Linear Programming to Model Input Data in Young Kenyan Children
Abstract
:1. Introduction
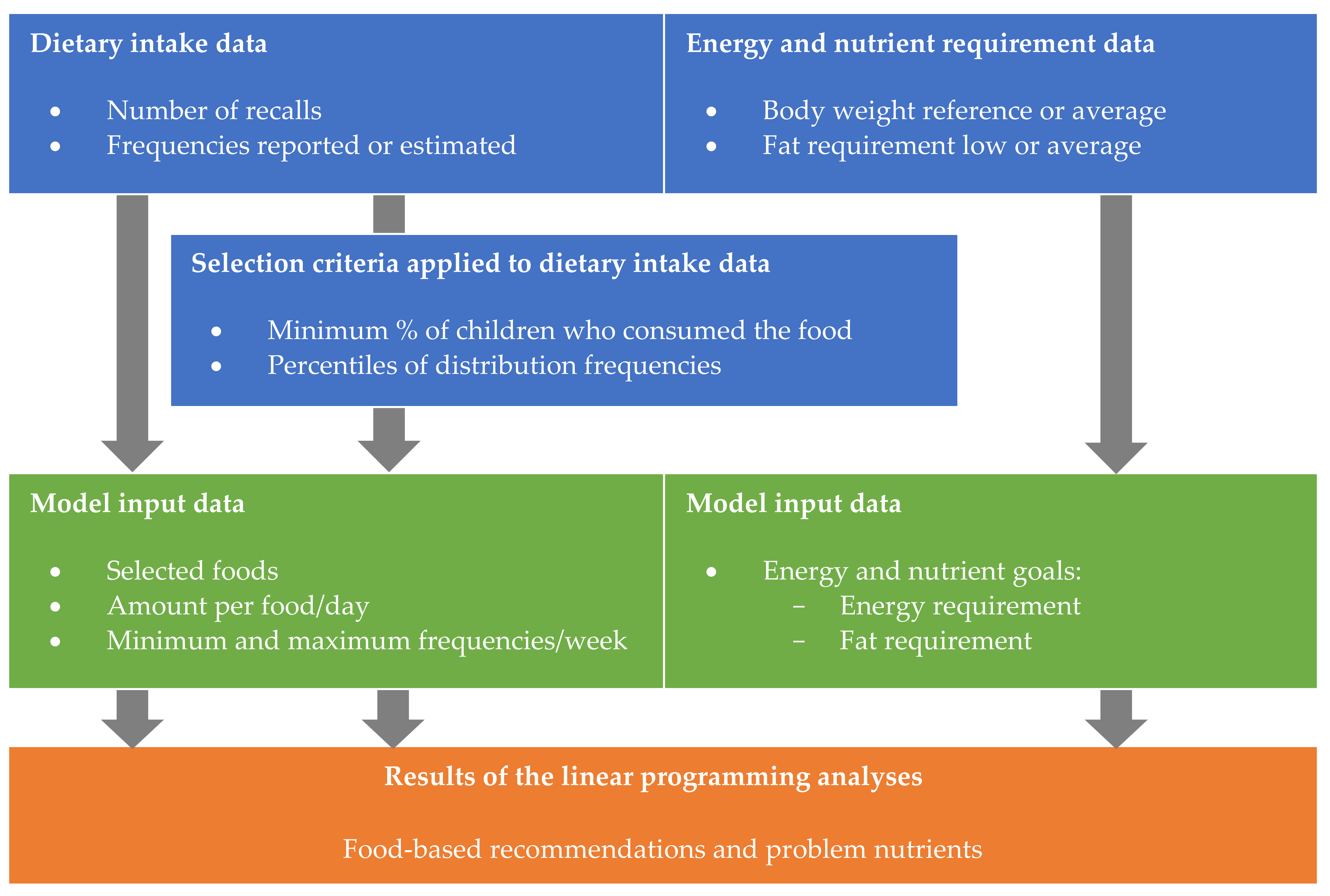
2. Materials and Methods
2.1. Study Design
2.2. Dietary Assessment and Anthropometry
2.3. Determining Diet Models for Various Scenarios
2.4. Linear Programming Analyses
3. Results
3.1. Characteristics and Dietary Intake of the Study Population
3.2. The Effects of Scenarios on FBR and Problem Nutrients
3.3. The Effects of Scenarios on Model Input Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- Development Initiatives. 2020 Global Nutrition Report: Action on Equity to End Malnutrition; Development Initiatives: Bristol, UK, 2020. [Google Scholar]
- Popkin, B.M.; Corvalan, C.; Grummer-Strawn, L.M. Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet 2020, 395, 65–74. [Google Scholar] [CrossRef]
- Beal, T.; Massiot, E.; Arsenault, J.E.; Smith, M.R.; Hijmans, R.J. Global trends in dietary micronutrient supplies and estimated prevalence of inadequate intakes. PLoS ONE 2017, 12, e0175554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez Fischer, C.; Garnett, T. Plates, Pyramids, Planet. Developments in National Healthy and Sustainable Dietary Guidelines: A State of Play Assessment; FAO: Rome, Italy; University of Oxford: Oxford, UK, 2016. [Google Scholar]
- Keller, I.; Lang, T. Food-based dietary guidelines and implementation: Lessons from four countries—Chile, Germany, New Zealand and South Africa. Public Health Nutr. 2008, 11, 867–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO; FAO. Preparation and Use of Food-Based Dietary Guidelines: Report of a Joint FAO/WHO Consultation; World Health Organisation: Geneva, Switzerland, 1998. [Google Scholar]
- Van’t Erve, I.; Tulen, C.B.M.; Jansen, J.; Van Laar, A.D.E.; Minnema, R.; Schenk, P.R.; Wolvers, D.; Van Rossum, C.T.M.; Verhagen, H. Overview of Elements within National Food-Based Dietary Guidelines. Eur. J. Nutr. Food Saf. 2017, 7, 172–227. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, E.; Chege, P.; Kimiywe, J.; Wiesmann, D.; Hotz, C. Zinc, iron and calcium are major limiting nutrients in the complementary diets of rural Kenyan children. Matern. Child Nutr. 2015, 11 (Suppl. (3), 6–20. [Google Scholar] [CrossRef]
- Levesque, S.; Delisle, H.; Agueh, V. Contribution to the development of a food guide in Benin: Linear programming for the optimization of local diets. Public Health Nutr. 2015, 18, 622–631. [Google Scholar] [CrossRef] [Green Version]
- Vossenaar, M.; Knight, F.A.; Tumilowicz, A.; Hotz, C.; Chege, P.; Ferguson, E.L. Context-specific complementary feeding recommendations developed using Optifood could improve the diets of breast-fed infants and young children from diverse livelihood groups in northern Kenya. Public Health Nutr. 2016, 20, 971–983. [Google Scholar] [CrossRef] [Green Version]
- Wessells, K.R.; Young, R.R.; Ferguson, E.L.; Ouédraogo, C.T.; Faye, M.T.; Hess, S.Y. Assessment of Dietary Intake and Nutrient Gaps, and Development of Food-Based Recommendations, among Pregnant and Lactating Women in Zinder, Niger: An Optifood Linear Programming Analysis. Nutrients 2019, 11, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chileshe, J.; Talsma, E.F.; Schoustra, S.E.; Borgonjen-van den Berg, K.J.; Handema, R.; Zwaan, B.J.; Brouwer, I.D. Potential contribution of cereal and milk based fermented foods to dietary nutrient intake of 1–5 years old children in Central province in Zambia. PLoS ONE 2020, 15, e0232824. [Google Scholar] [CrossRef]
- Samuel, A.; Osendarp, S.J.M.; Ferguson, E.; Borgonjen, K.; Alvarado, B.M.; Neufeld, L.M.; Adish, A.; Kebede, A.; Brouwer, I.D. Identifying Dietary Strategies to Improve Nutrient Adequacy among Ethiopian Infants and Young Children Using Linear Modelling. Nutrients 2019, 11, 1416. [Google Scholar] [CrossRef] [Green Version]
- Buttriss, J.L.; Briend, A.; Darmon, N.; Ferguson, E.L.; Maillot, M.; Lluch, A. Diet modelling: How it can inform the development of dietary recommendations and public health policy. Nutr. Bull. 2014, 39, 115–125. [Google Scholar] [CrossRef]
- FAO; WHO; UNU. Human Energy Requirements. Report of a Joint FAO/WHO/UNU Expert Consultation; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004. [Google Scholar]
- FAO. Fats and Fatty Acids in Human Nutrition. Report of an Expert Consultation; Food and Agriculture Organization of the United Nations: Rome, Italy, 2010. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes: Applications in Dietary Assessment; National Academy Press: Washington, DC, USA, 2000. [Google Scholar]
- European Food Safety Authority. Dietary Reference Values for Nutrients: Summary Report; EFSA Supporting Publication: Parma, Italy, 2017; Volume 14, p. e15121. [Google Scholar]
- Vila-Real, C.; Pimenta-Martins, A.; Gomes, A.M.; Pinto, E.; Maina, N.H. How dietary intake has been assessed in African countries? A systematic review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1002–1022. [Google Scholar] [CrossRef]
- Gibson, R.S.; Ferguson, E.L. An Interactive 24 h Recall for Assessing the Adequacy of Iron and Zinc Intakes in Developing Countries; HarvestPlus, International Life Sciences Institute: Washington, DC, USA, 2008. [Google Scholar]
- Hlaing, L.M.; Fahmida, U.; Htet, M.K.; Utomo, B.; Firmansyah, A.; Ferguson, E.L. Local food-based complementary feeding recommendations developed by the linear programming approach to improve the intake of problem nutrients among 12–23-month-old Myanmar children. Br. J. Nutr. 2016, 116 (Suppl. (1), S16–S26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kujinga, P.; Borgonjen-van den Berg, K.J.; Superchi, C.; Ten Hove, H.J.; Onyango, E.O.; Andang’o, P.; Galetti, V.; Zimmerman, M.B.; Moretti, D.; Brouwer, I.D. Combining food-based dietary recommendations using Optifood with zinc-fortified water potentially improves nutrient adequacy among 4- to 6-year-old children in Kisumu West district, Kenya. Matern. Child Nutr. 2017, 14, e12515. [Google Scholar] [CrossRef] [PubMed]
- Skau, J.K.; Bunthang, T.; Chamnan, C.; Wieringa, F.T.; Dijkhuizen, M.A.; Roos, N.; Ferguson, E.L. The use of linear programming to determine whether a formulated complementary food product can ensure adequate nutrients for 6- to 11-month-old Cambodian infants. Am. J. Clin. Nutr. 2014, 99, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Talsma, E.F.; van den Berg, K.J.B.; Melse-Boonstra, A.; Mayer, E.V.; Verhoef, H.; Demir, A.Y.; Ferguson, E.L.; Kok, F.J.; Brouwer, I.D. The potential contribution of yellow cassava to dietary nutrient adequacy of primary-school children in Eastern Kenya; the use of linear programming. Public Health Nutr. 2017, 21, 365–376. [Google Scholar] [CrossRef] [Green Version]
- Tharrey, M.; Olaya, G.A.; Fewtrell, M.; Ferguson, E. Adaptation of New Colombian Food-based Complementary Feeding Recommendations using Linear Programming. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 667–672. [Google Scholar] [CrossRef]
- Daelmans, B.; Ferguson, E.; Lutter, C.K.; Singh, N.; Pachon, H.; Creed-Kanashiro, H.; Woldt, M.; Mangasaryan, N.; Cheung, E.; Mir, R.; et al. Designing appropriate complementary feeding recommendations: Tools for programmatic action. Matern. Child Nutr. 2013, 9 (Suppl. (2), 116–130. [Google Scholar] [CrossRef] [Green Version]
- Kujinga-Chopera, P. Effectiveness of Zinc Fortified Drinking Water on Zinc Intake, Status and Morbidity of Rural Kenyan Pre-School Children. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2016. [Google Scholar]
- Conway, J.M.; Ingwersen, L.A.; Vinyard, B.T.; Moshfegh, A.J. Effectiveness of the US Department of Agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. Am. J. Clin. Nutr. 2003, 77, 1171–1178. [Google Scholar] [CrossRef] [Green Version]
- Sehmi, J.K. National Food Composition Tables and the Planning of Satisfactory Diets in Kenya; Government Press: Nairobi, Kenya, 1993.
- Wolmarans, P.; Danster, N.; Dalton, A.; Rossouw, K.; Schönfeldt, H. Condensed Food Composition Tables for South Africa; Medical Research Council: Cape Town, South Africa, 2010. [Google Scholar]
- Barikmo, I.; Ouattara, F.; Oshaug, A. Table de Composition des Aliments du Mali; Akerhus University College: Oslo, Norway, 2004. [Google Scholar]
- USDA; ARS. USDA National Nutrient Database for Standard Reference, Release 27; USDA: Washington, DC, USA, 2014.
- West, C.E.; Pepping, F.; Temalilwa, C.R. The Composition of Foods Commonly Eaten in East Africa; Wageningen University: Wageningen, The Netherlands, 1988. [Google Scholar]
- USDA; ARS. USDA Table of Nutrient Retention Factors, Release 6; USDA: Washington, DC, USA, 2007.
- Allen, L.; de Benoist, B.; Dary, O.; Hurrell, R. WHO/FAO Guidelines on Food Fortification with Micronutrients; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- FANTA. Summary Report: Development of Evidence-Based Dietary Recommendations for Children, Pregnant Women, and Lactating Women Living in the Western Highlands in Guatemala; FHI 360/FANTA: Washington, DC, USA, 2013. [Google Scholar]
- FAO; WHO. Human Vitamin and Mineral Requirements. Report of a Joint FAO/WHO Expert Consultation Bangkok, Thailand; Food and Agriculture Organization of the United Nations: Rome, Italy, 2001. [Google Scholar]
- FAO; WHO; UNU. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- De Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standard: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Coates, J.C.; Colaiezzi, B.A.; Bell, W.; Charrondiere, U.R.; Leclercq, C. Overcoming Dietary Assessment Challenges in Low-Income Countries: Technological Solutions Proposed by the International Dietary Data Expansion (INDDEX) Project. Nutrients 2017, 9, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subar, A.F.; Dodd, K.W.; Guenther, P.M.; Kipnis, V.; Midthune, D.; McDowell, M.; Tooze, J.A.; Freedman, L.S.; Krebs-Smith, S.M. The food propensity questionnaire: Concept, development, and validation for use as a covariate in a model to estimate usual food intake. J. Am. Diet. Assoc. 2006, 106, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Ost, C.; De Ridder, K.A.A.; Tafforeau, J.; Van Oyen, H. The added value of food frequency questionnaire (FFQ) information to estimate the usual food intake based on repeated 24 h recalls. Arch. Public Health 2017, 75, 46. [Google Scholar] [CrossRef] [Green Version]
- Gibson, R.S.; Charrondiere, U.R.; Bell, W. Measurement Errors in Dietary Assessment Using Self-Reported 24 h Recalls in Low-Income Countries and Strategies for Their Prevention. Adv. Nutr. 2017, 8, 980–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Castro, M.A.; Verly, E., Jr.; Fisberg, M.; Fisberg, R.M. Children’s nutrient intake variability is affected by age and body weight status according to results from a Brazilian multicenter study. Nutr. Res. 2014, 34, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Ollberding, N.J.; Couch, S.C.; Woo, J.G.; Kalkwarf, H.J. Within- and between-individual variation in nutrient intake in children and adolescents. J. Acad. Nutr. Diet. 2014, 114, 1749–1758.e1745. [Google Scholar] [CrossRef]
- Stote, K.S.; Radecki, S.V.; Moshfegh, A.J.; Ingwersen, L.A.; Baer, D.J. The number of 24 h dietary recalls using the US Department of Agriculture’s automated multiple-pass method required to estimate nutrient intake in overweight and obese adults. Public Health Nutr. 2011, 14, 1736–1742. [Google Scholar] [CrossRef] [Green Version]
- Kenya National Bureau of Statistics; Ministry of Health/Kenya; National AIDS Control Council/Kenya; Kenya Medical Research Institute; National Council for Population Development/Kenya. Kenya Demographic and Health Survey 2014; Kenya National Bureau of Statistics: Rockville, MD, USA, 2015.
- Willett, W. Nature of Variation in Diet. In Nutritional Epidemiology, 3rd ed.; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
| Model Input Data | Selection Criteria | Frequencies | |
|---|---|---|---|
| Reported | Estimated | ||
| Amount per food/day | 2 dietary recalls | Reference scenario | Scenario A |
| 1 dietary recall | Scenario B | Scenario C | |
| Selected foods | ≥3% of children consumed the food | Reference scenario | |
| ≥10% of children consumed the food | Scenario D | ||
| All foods consumed | Scenario E | ||
| Min and max frequencies/week per food and food (sub)group | 5–95th percentile | Reference scenario | |
| 10–90th percentile | Scenario F | ||
| Energy requirement | Based on average body weight | Reference scenario | |
| Based on reference body weight | Scenario G | ||
| Fat requirement | 30 en% (mean) and average body weight | Reference scenario | |
| 25 en% (low) and average body weight | Scenario H | ||
| Median 1 | 25–75th Perc 1 | CV%wtn 2 | CV%btn 3 | % below EAR 4 | ||
|---|---|---|---|---|---|---|
| Background | ||||||
| Sex, girls | n (%) | 36 (58) | ||||
| Age | Y | 5.3 | 4.6–6.0 | |||
| Anthropometrics 5 | ||||||
| Body weight | kg | 16.9 | 15.5–18.4 | |||
| Height for age 6 | z-score | −1.1 | −1.9–0.4 | |||
| Stunted 6 | N | 13 | ||||
| BMI for age 6 | z-score | 0.0 | −0.6–0.6 | |||
| Underweight 6 | N | 0 | ||||
| Dietary intake of nutrients 7 | ||||||
| Energy | kcal/d | 1489 | 1172–1852 | 29.8 | 22.4 | 34 |
| Protein | g/d | 35.8 | 28.3–46.5 | 37.9 | 23.7 | 2 |
| Fat | g/d | 39.4 | 29.7–54.4 | 59.1 | 15.4 | 40 |
| Thiamin | mg/d | 0.78 | 0.58–1.11 | 51.4 | 22.5 | 18 |
| Riboflavin | mg/d | 0.49 | 0.36–0.70 | 54.6 | 36.7 | 52 |
| Niacin | mg/d | 5.05 | 4.03–6.38 | 50.1 | 0 | 68 |
| Vitamin B6 | mg/d | 0.64 | 0.52–0.91 | 53.1 | 0 | 21 |
| Folate | ug/d | 112 | 74–159 | 62.5 | 32.3 | 76 |
| Vitamin B12 | ug/d | 0.88 | 0.48–1.57 | 104.2 | 48.8 | 58 |
| Vitamin C | mg/d | 29.8 | 18.5–43.1 | 90.2 | 31.0 | 37 |
| Vitamin A (RAE) | ug/d | 95.5 | 49.1–150.0 | 98.3 | 69.2 | 98 |
| Calcium | mg/d | 511 | 300–669 | 68.6 | 50.8 | 48 |
| Iron | mg/d | 10.6 | 8.8–14.4 | 48.7 | 31.8 | 63 |
| Zinc | mg/d | 5.26 | 4.04–7.10 | 48.2 | 24.0 | 82 |
| Reference Scenario 1 | Scenario A Est Freq 2 | Scenario B Rp Freq 3 1 Recall | Scenario C Est Freq 2 1 Recall | Scenario D ≥ 10% Cons 4 | Scenario E All Foods | Scenario F 10–90th Perc 5 | Scenario G Ref Weight 6 | Scenario H 25 en% Fat | |
|---|---|---|---|---|---|---|---|---|---|
| Food group 7 | Number of daily amounts per week | ||||||||
| Added fats | 7 | 4 | 6 | 4 | 7 | 7 | 7 | 7 | 5 |
| Added sugars | 0 | 4 | 1 | 7 | 0 | 0 | 4 | 0 | 0 |
| Bakery and breakfast cereals 8 | 0 | 0 | 0 | 0 | -- | 0 | -- | 2 | 2 |
| Dairy products | 8 | 11 | 12 | 14 | 7 | 8 | 7 | 8 | 8 |
| Fruits | 7 | 7 | 7 | 10 | 7 | 7 | 2 | 7 | 7 |
| Grains and grain products | 21 | 12 | 19 | 11 | 21 | 21 | 21 | 22 | 22 |
| Legumes, nuts and seeds 8 | 4 | 7 | 4 | 3 | 3 | 4 | -- | 4 | 4 |
| Meat, fish and eggs | 7 | 11 | 5 | 14 | 7 | 7 | 3 | 7 | 7 |
| Starchy roots and other starchy plant foods 8 | -- | 3 | 0 | 3 | -- | -- | -- | -- | -- |
| Vegetables | 28 | 32 | 30 | 35 | 28 | 28 | 24 | 28 | 28 |
| Maximised Diet | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reference Scenario 1 | Scenario A Est Freq 2 | Scenario B Rp Freq 3, 1 Recall | Scenario C Est Freq 2, 1 Recall | Scenario D ≥10% Cons 4 | Scenario E All Foods | Scenario F 10–90th Perc 5 | Scenario G Ref Weight 6 | Scenario H 25 en% Fat | |
| Nutrient | % RNI | ||||||||
| Protein | 371 | 450 | 411 | 466 | 364 | 371 | 286 | 356 | 371 |
| Fat (en%) 7 | 33 | 51 | 36 | 35 | 29 9 | 33 | 28 9 | 30 | 33 |
| Thiamin | 220 | 209 | 282 | 230 | 216 | 220 | 177 | 235 | 220 |
| Riboflavin | 134 | 188 | 179 | 215 | 115 | 134 | 94 8 | 136 | 134 |
| Niacin | 103 | 103 | 122 | 118 | 102 | 103 | 77 8 | 106 | 103 |
| Vitamin B6 | 177 | 183 | 196 | 207 | 177 | 177 | 149 | 192 | 177 |
| Folate | 94 8 | 130 | 96 8 | 128 | 86 8 | 94 8 | 52 8 | 96 8 | 94 8 |
| Vitamin B12 | 110 | 205 | 173 | 285 | 101 | 110 | 86 8 | 111 | 110 |
| Vitamin C | 196 | 296 | 221 | 438 | 195 | 196 | 110 | 196 | 196 |
| Vitamin A | 56 8 | 72 8 | 69 8 | 84 8 | 53 8 | 56 8 | 28 8 | 56 8 | 56 8 |
| Calcium | 136 | 225 | 229 | 342 | 126 | 136 | 103 | 136 | 136 |
| Iron | 123 | 136 | 135 | 140 | 119 | 123 | 97 8 | 129 | 123 |
| Zinc | 86 8 | 76 8 | 89 8 | 79 8 | 83 8 | 86 8 | 66 8 | 92 8 | 86 8 |
| Consumed | In Food List 1 | ||
|---|---|---|---|
| Scenario | Number of Foods | ||
| Reference scenario 2 | 64 | 37 | |
| Scenario A: | Estimated frequencies | 64 | 59 |
| Scenario B: | 1 recall | 50 | 44 |
| Scenario C: | 1 recall, Est freq 3 | 50 | 48 |
| Scenario D: | ≥10% consumed 4 | 33 | 33 |
| Scenario E: | All foods consumed | 86 | 37 |
| Scenario F: | 10–90th percentile 5 | 64 | 26 |
| Reference Scenario 1 | Scenario A Est Freq 2 | Scenario B Rp Freq 3 1 Recall | Scenario C Est Freq 2 1 Recall | Scenario D (break) ≥ 10% Cons 4 | Scenario E All Foods | Scenario F 10–90th Perc 5 | Scenario G Ref Weight 6 | Scenario H 25 en% Fat | |
|---|---|---|---|---|---|---|---|---|---|
| Draft FBR 7 | Frequencies per week | Changes in frequencies and problem nutrients compared to the reference scenario | |||||||
| Added fats | 7 | − | − | − | |||||
| Dairy products | 8 | + | + | + | |||||
| Fruits | 7 | Negligible | + | Negligible | None | − | Negligible | Negligible | |
| Grains and grain products | 21 | − | − | ||||||
| Legumes, nuts and seeds | 4 | + | − | ||||||
| Meat, fish and eggs | 7 | + | + | − | |||||
| Vegetables | 28 | + | + | − | |||||
| Added sugars | 0 | + | + | + | + | ||||
| Starchy roots and other starchy plant foods 8 | -- | + | + | ||||||
| Bakery and breakfast cereals | 0 | + | + | ||||||
| Problem nutrients | |||||||||
| Folate | ● | ● | ● | ● | ● | ● | ● | ||
| Vitamin A | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Zinc | ● | ● | ● | ● | ● | ● | ● | ● | ● |
| Riboflavin | ● | ||||||||
| Niacin | ● | ||||||||
| Vitamin B12 | ● | ||||||||
| Iron | ● | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borgonjen-van den Berg, K.J.; de Vries, J.H.M.; Chopera, P.; Feskens, E.J.M.; Brouwer, I.D. Sensitivity of Food-Based Recommendations Developed Using Linear Programming to Model Input Data in Young Kenyan Children. Nutrients 2021, 13, 3485. https://doi.org/10.3390/nu13103485
Borgonjen-van den Berg KJ, de Vries JHM, Chopera P, Feskens EJM, Brouwer ID. Sensitivity of Food-Based Recommendations Developed Using Linear Programming to Model Input Data in Young Kenyan Children. Nutrients. 2021; 13(10):3485. https://doi.org/10.3390/nu13103485
Chicago/Turabian StyleBorgonjen-van den Berg, Karin J., Jeanne H. M. de Vries, Prosper Chopera, Edith J. M. Feskens, and Inge D. Brouwer. 2021. "Sensitivity of Food-Based Recommendations Developed Using Linear Programming to Model Input Data in Young Kenyan Children" Nutrients 13, no. 10: 3485. https://doi.org/10.3390/nu13103485
APA StyleBorgonjen-van den Berg, K. J., de Vries, J. H. M., Chopera, P., Feskens, E. J. M., & Brouwer, I. D. (2021). Sensitivity of Food-Based Recommendations Developed Using Linear Programming to Model Input Data in Young Kenyan Children. Nutrients, 13(10), 3485. https://doi.org/10.3390/nu13103485






