Betulinic Acid Improves Cardiac-Renal Dysfunction Caused by Hypertrophy through Calcineurin-NFATc3 Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Meterials
2.2. Animals and Treatment
2.3. Echocardiographic Assessments
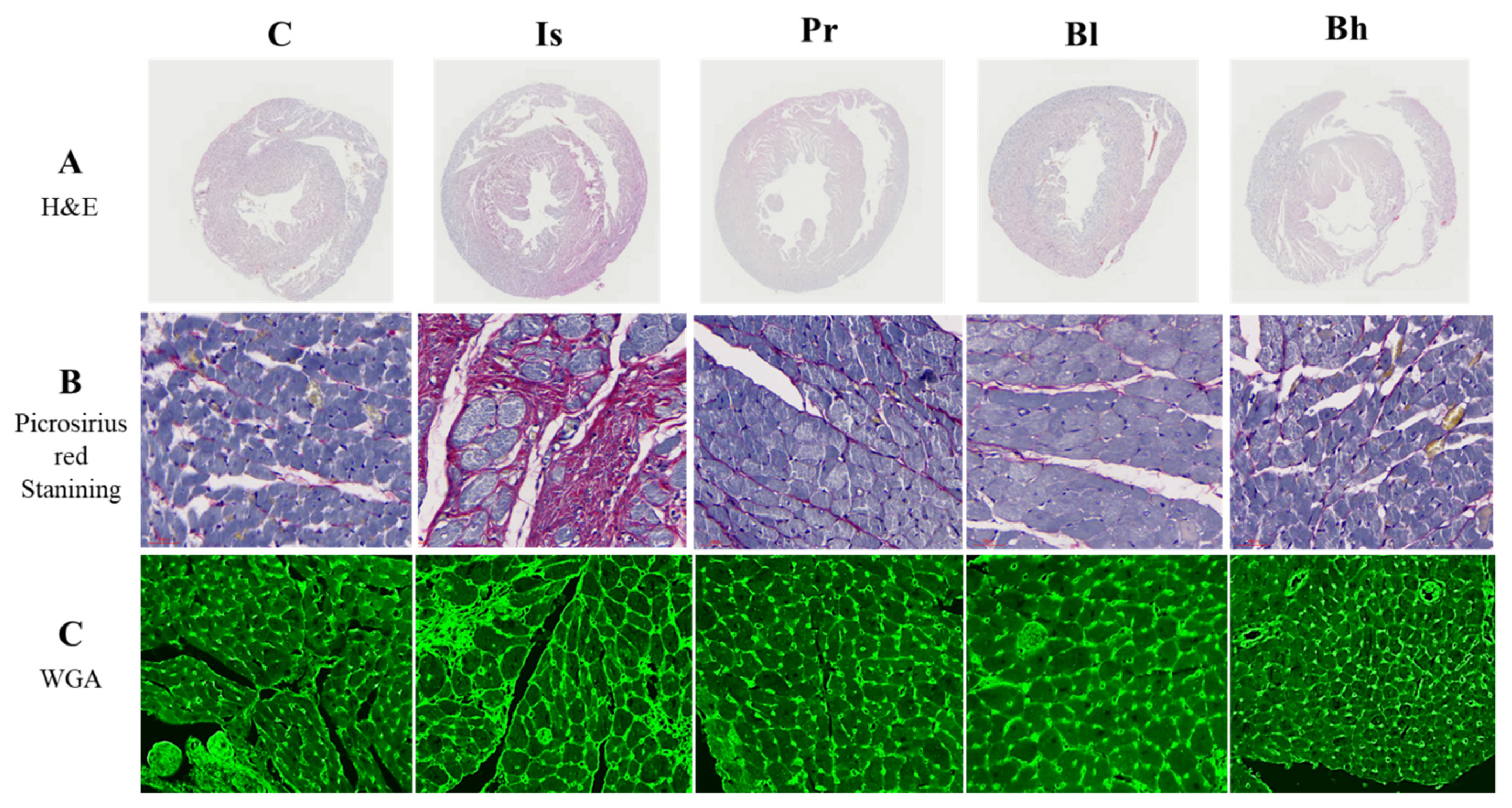
2.4. Histological Analysis
2.5. Immunoblotting
2.6. Measurements of Biomarkers in Serum
2.7. Monitoring of Renal Function
2.8. Statistical Analysis
3. Results
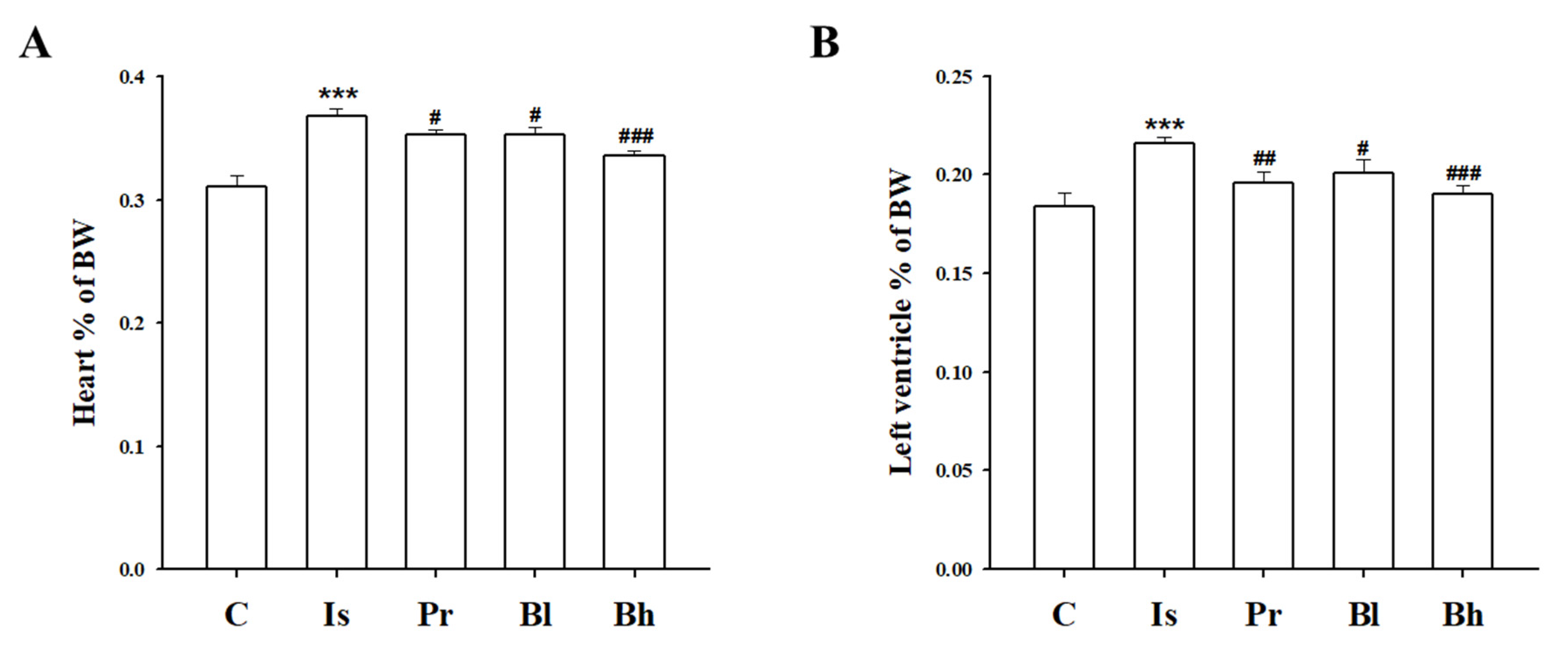
3.1. BA Ameliorated Heart Function in ISO-Induced LV Hypertrophy Rat
3.2. BA Changed Level of Serum Biomarkers in ISO-induced LV Hypertrophy Rat
3.3. BA Has Influenced Heart Morphology
3.4. BA Decreased Cardiac Hypertrophy Marker in ISO-induced LV Hypertrophy Rat
3.5. BA Attenuated Kidney Function in ISO-induced LV Hypertrophy Rat
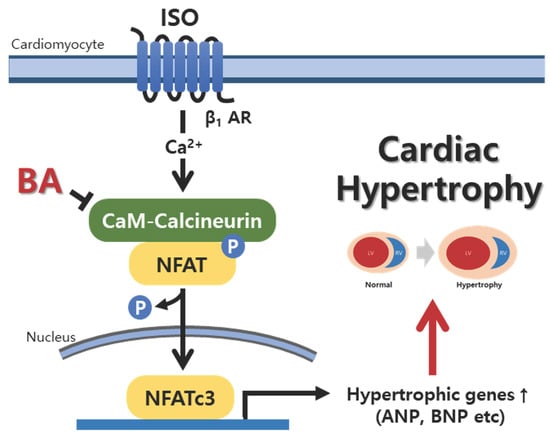
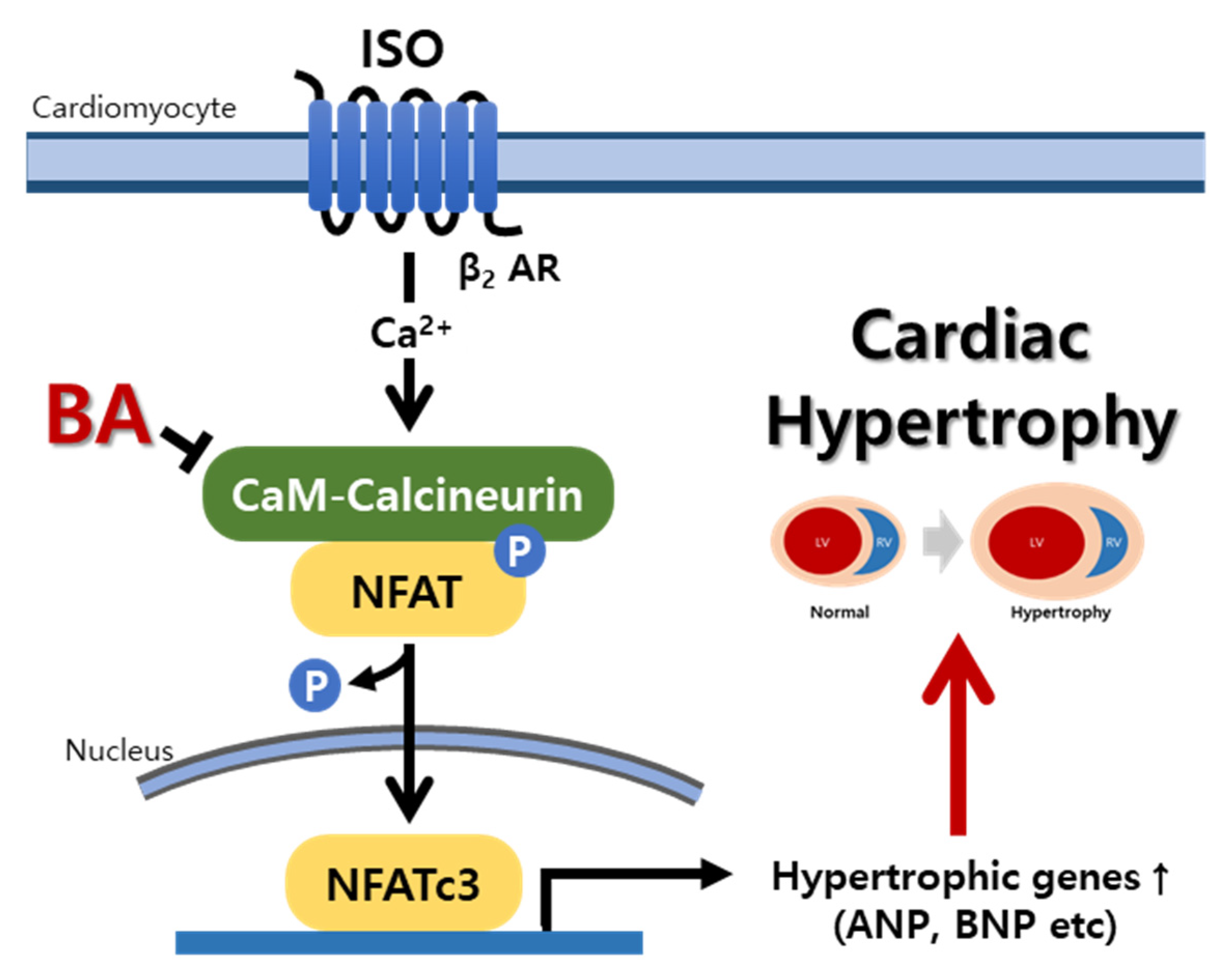
3.6. BA Inhibited Calcineurin/NFATc3 Signaling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donadio, C. Body Composition Analysis Allows the Prediction of Urinary Creatinine Excretion and of Renal Function in Chronic Kidney Disease Patients. Nutrients 2017, 9, 553. [Google Scholar] [CrossRef]
- Maron, B.J.; Rowin, E.J.; Maron, M.S. Global Burden of Hypertrophic Cardiomyopathy. JACC Heart Fail. 2018, 6, 376–378. [Google Scholar] [CrossRef]
- Burchfield, J.S.; Xie, M.; Hill, J.A. Pathological Ventricular Remodeling. Circulation 2013, 128, 388–400. [Google Scholar] [CrossRef]
- Lee, L.C.; Zhihong, Z.; Hinson, A.; Guccione, J.M. Reduction in Left Ventricular Wall Stress and Improvement in Function in Failing Hearts using Algisyl-LVR. J. Vis. Exp. 2013, e50096. [Google Scholar] [CrossRef]
- Imaeda, A.; Tanaka, S.; Tonegawa, K.; Fuchigami, S.; Obana, M.; Maeda, M.; Kihara, M.; Kiyonari, H.; Conway, S.J.; Fujio, Y.; et al. Myofibroblast β2 adrenergic signaling amplifies cardiac hypertrophy in mice. Biochem. Biophys. Res. Commun. 2019, 510, 149–155. [Google Scholar] [CrossRef]
- Schirmer, I.; Bualeong, T.; Budde, H.; Cimiotti, D.; Appukuttan, A.; Klein, N.; Steinwascher, P.; Reusch, P.; Mugge, A.; Meyer, R.; et al. Soluble adenylyl cyclase: A novel player in cardiac hypertrophy induced by isoprenaline or pressure overload. PLoS ONE 2018, 13, e0192322. [Google Scholar] [CrossRef]
- Khalilimeybodi, A.; Daneshmehr, A.; Sharif-Kashani, B. Ca2+-dependent calcineurin/NFAT signaling in β-adrenergic-induced cardiac hypertrophy. Gen. Physiol. Biophys. 2018, 37, 41–56. [Google Scholar] [CrossRef]
- Al-Majed, A.A.; Bakheit, A.H.; Aziz, H.A.A.; Alajmi, F.M.; AlRabiah, H. Propranolol. Profiles Drug Subst Excip Relat Methodol 2017, 42, 287–338. [Google Scholar] [CrossRef]
- Freitas, F.; Estato, V.; Lessa, M.A.; Tibiriçá, E. Cardiac microvascular rarefaction in hyperthyroid rats is reversed by losartan, diltiazem, and propranolol. Fundam. Clin. Pharmacol. 2014, 29, 31–40. [Google Scholar] [CrossRef]
- Appiah, B.; Amponsah, I.K.; Poudyal, A.; Mensah, M.L.K. Identifying strengths and weaknesses of the integration of biomedical and herbal medicine units in Ghana using the WHO Health Systems Framework: A qualitative study. BMC Complement. Altern. Med. 2018, 18, 286. [Google Scholar] [CrossRef]
- Prados, M.E.; García-Martín, A.; Unciti-Broceta, J.D.; Palomares, B.; Collado, J.A.; Minassi, A.; Calzado, M.A.; Appendino, G.; Muñoz, E. Betulinic acid hydroxamate prevents colonic inflammation and fibrosis in murine models of inflammatory bowel disease. Acta Pharmacol. Sin. 2020, 42, 1124–1138. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, F.-X.; Zang, S.-T.; Yang, Q.-F. Betulinic acid prevents high glucose-induced expression of extracellular matrix protein in cardiac fibroblasts by inhibiting the TGF-β1/Smad signaling pathway. Mol. Med. Rep. 2017, 16, 6320–6325. [Google Scholar] [CrossRef]
- Zhang, X.; Szeto, C.; Gao, E.; Tang, M.; Jin, J.; Fu, Q.; Makarewich, C.; Ai, X.; Li, Y.; Tang, A.; et al. Cardiotoxic and Cardioprotective Features of Chronic β-adrenergic Signaling. Circ. Res. 2013, 112, 498–509. [Google Scholar] [CrossRef]
- Chang, S.C.; Ren, S.; Rau, C.D.; Wang, J.J. Isoproterenol-Induced Heart Failure Mouse Model Using Osmotic Pump Implantation. Methods Mol Biol 2018, 1816, 207–220. [Google Scholar] [CrossRef]
- Akila, P.; Vennila, L. Chlorogenic acid a dietary polyphenol attenuates isoproterenol induced myocardial oxidative stress in rat myocardium: An in vivo study. Biomed. Pharmacother. 2016, 84, 208–214. [Google Scholar] [CrossRef]
- Yu, B.; Wang, W. Cardioprotective Effects of Morroniside in Rats Following Acute Myocardial Infarction. Inflammation 2018, 41, 432–436. [Google Scholar] [CrossRef]
- Houser, S.R.; Molkentin, J.D. Does Contractile Ca2+ Control Calcineurin-NFAT Signaling and Pathological Hypertrophy in Cardiac Myocytes? Sci. Signal. 2008, 1, pe31. [Google Scholar] [CrossRef]
- Parra, V.; Rothermel, B.A. Calcineurin signaling in the heart: The importance of time and place. J. Mol. Cell. Cardiol. 2016, 103, 121–136. [Google Scholar] [CrossRef]
- Chaklader, M.; Rothermel, B.A. Calcineurin in the heart: New horizons for an old friend. Cell. Signal. 2021, 87, 110134. [Google Scholar] [CrossRef]
- Olson, E.N.; Molkentin, J. Prevention of Cardiac Hypertrophy by Calcineurin Inhibition. Circ. Res. 1999, 84, 623–632. [Google Scholar] [CrossRef]
- Zhang, Q.; Qi, H.; Cao, Y.; Shi, P.; Song, C.; Ba, L.; Chen, Y.; Gao, J.; Li, S.; Li, B.; et al. Activation of transient receptor potential vanilloid 3 channel (TRPV3) aggravated pathological cardiac hypertrophy via calcineurin/NFATc3 pathway in rats. J. Cell. Mol. Med. 2018, 22, 6055–6067. [Google Scholar] [CrossRef]
- Lin, P.-P.; Tsai, C.-C.; Hsieh, Y.-M.; Kuo, W.-W.; Lin, C.-C.; Tsai, F.-J.; Tsai, C.-H.; Huang, C.-Y. Inhibition of cardiac hypertrophy by probiotic-fermented purple sweet potato yogurt in spontaneously hypertensive rat hearts. Int. J. Mol. Med. 2012, 30, 1365–1375. [Google Scholar] [CrossRef][Green Version]
- Molkentin, J.; Lu, J.; Antos, C.L.; Markham, B.; Richardson, J.; Robbins, J.; Grant, S.R.; Olson, E.N. A Calcineurin-Dependent Transcriptional Pathway for Cardiac Hypertrophy. Cell 1998, 93, 215–228. [Google Scholar] [CrossRef]
- Li, X.; Lan, Y.; Wang, Y.; Nie, M.; Lu, Y.; Zhao, E. Telmisartan suppresses cardiac hypertrophy by inhibiting cardiomyocyte apoptosis via the NFAT/ANP/BNP signaling pathway. Mol. Med. Rep. 2017, 15, 2574–2582. [Google Scholar] [CrossRef]
- Dickhout, J.G.; Carlisle, R.E.; Austin, R.C. Interrelationship Between Cardiac Hypertrophy, Heart Failure, and Chronic Kidney Disease. Circ. Res. 2011, 108, 629–642. [Google Scholar] [CrossRef]
- Melenovsky, V.; Cervenka, L.; Viklicky, O.; Franekova, J.; Havlenova, T.; Behounek, M.; Chmel, M.; Petrak, J. Kidney Response to Heart Failure: Proteomic Analysis of Cardiorenal Syndrome. Kidney Blood Press. Res. 2018, 43, 1437–1450. [Google Scholar] [CrossRef]
- Lee, M.J.; Chang, T.I.; Lee, J.; Kim, Y.H.; Oh, K.-H.; Lee, S.W.; Kim, S.W.; Park, J.T.; Yoo, T.-H.; Kang, S.-W.; et al. Urine Osmolality and Renal Outcome in Patients with Chronic Kidney Disease: Results from the KNOW-CKD. Kidney Blood Press. Res. 2019, 44, 1089–1100. [Google Scholar] [CrossRef]
- Metra, M.; Cotter, G.; Gheorghiade, M.; Cas, L.D.; Voors, A.A. The role of the kidney in heart failure. Eur. Heart J. 2012, 33, 2135–2142. [Google Scholar] [CrossRef]
- Al-Naher, A.; Wright, D.; Devonald, M.A.J.; Pirmohamed, M. Renal function monitoring in heart failure—What is the optimal frequency? A narrative review. Br. J. Clin. Pharmacol. 2017, 84, 5–17. [Google Scholar] [CrossRef]
- Donadio, C.; Lucchesi, A.; Tramonti, G.; Bianchi, C. Creatinine clearance predicted from body cell mass is a good indicator of renal function. Kidney Int. Suppl. 1997, 63, S166–S168. [Google Scholar]



| C | Is | Pr | Bl | Bh | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LVEDV (mL) | 0.755 | ± | 0.0166 | 0.89 | ± | 0.066 | 1.09 | ± | 0.099 | 0.836 | ± | 0.031 | 0.80 | ± | 0.086 |
| LVESV (mL) | 0.046 | ± | 0.011 | 0.111 | ± | 0.029 | 0.184 | ± | 0.031 | 0.104 | ± | 0.037 | 0.12 | ± | 0.016 |
| SV (mL) | 0.782 | ± | 0.099 | 0.635 | ± | 0.045 * | 0.82 | ± | 0.047 # | 0.712 | ± | 0.026 # | 0.711 | ± | 0.062 # |
| LVd Mass | 1.28 | ± | 0.071 | 1.077 | ± | 0.034 * | 1.234 | ± | 0.088 # | 1.135 | ± | 0.212 | 1.307 | ± | 0.065 ## |
| %IVS | 71.9 | ± | 4.85 | 71.7 | ± | 5.03 | 68.7 | ± | 7.45 | 90.47 | ± | 7.06 | 55.15 | ± | 2.88 |
| IVSd | 1.446 | ± | 0.249 | 0.868 | ± | 1.99 * | 1.808 | ± | 0.150 ### | 1.723 | ± | 0.154 ### | 2.068 | ± | 0.087 ### |
| IVSs | 3.71 | ± | 0.077 | 3.29 | ± | 0.14 | 3.13 | ± | 0.157 | 3.21 | ± | 0.20 | 3.35 | ± | 0.104 |
| %LVPW | 3.877 | ± | 0.168 | 2.317 | ± | 0.259 * | 3.514 | ± | 0.235 ## | 4.228 | ± | 0.378 # | 4.057 | ± | 0.192 |
| LVPWd | 2.23 | ± | 0.093 | 1.52 | ± | 0.141 ** | 2.02 | ± | 0.220 # | 2.35 | ± | 0.336 # | 2.71 | ± | 0.185 ### |
| LVPWs | 3.877 | ± | 0.168 | 2.317 | ± | 0.259 ** | 3.514 | ± | 0.235 ## | 4.228 | ± | 0.378 ## | 4.057 | ± | 0.192 ### |
| LVIDd | 7.22 | ± | 0.392 | 7.21 | ± | 0.164 | 7.72 | ± | 0.258 | 7.07 | ± | 0.115 | 7.16 | ± | 0.245 |
| LVIDs | 2.64 | ± | 0.172 | 2.88 | ± | 0.273 | 3.79 | ± | 0.304 | 3.07 | ± | 0.275 | 3.43 | ± | 0.318 |
| C | Is | Pr | Bl | Bh | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LDH (U/I) | 699.5 | ± | 37.11 | 1162.5 | ± | 74.16 *** | 801.5 | ± | 27.15 ## | 823.6 | ± | 144.4 # | 745.6 | ± | 68.08 ## |
| CK-MB (U/I) | 547.2 | ± | 65.9 | 872.5 | ± | 18.6 ** | 663.5 | ± | 88.3 # | 658.5 | ± | 76.2 # | 652.6 | ± | 34.1 ## |
| ALT (U/I) | 54.7 | ± | 2.75 | 72.2 | ± | 6.0 * | 54.2 | ± | 0.6 # | 53.5 | ± | 3.3 # | 56.8 | ± | 2.6 # |
| AST (U/I) | 164.2 | ± | 7.3 | 186.2 | ± | 9.6 | 169 | ± | 10.2 | 168.2 | ± | 17.6 | 175.5 | ± | 1.4 |
| Albumin (g/dL) | 4.07 | ± | 0.19 | 3.65 | ± | 0.11 * | 3.72 | ± | 0.1 | 3.8 | ± | 0.04 | 3.8 | ± | 0.13 # |
| Creatinine (mg/dL) | 0.27 | ± | 0.16 | 0.305 | ± | 0.02 | 0.295 | ± | 0.0095 | 0.315 | ± | 0.0025 | 0.294 | ± | 0.0097 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, M.-H.; Na, S.-W.; Jang, Y.-J.; Yoon, J.-J.; Lee, Y.-J.; Lee, H.-S.; Kim, H.-Y.; Kang, D.-G. Betulinic Acid Improves Cardiac-Renal Dysfunction Caused by Hypertrophy through Calcineurin-NFATc3 Signaling. Nutrients 2021, 13, 3484. https://doi.org/10.3390/nu13103484
Hong M-H, Na S-W, Jang Y-J, Yoon J-J, Lee Y-J, Lee H-S, Kim H-Y, Kang D-G. Betulinic Acid Improves Cardiac-Renal Dysfunction Caused by Hypertrophy through Calcineurin-NFATc3 Signaling. Nutrients. 2021; 13(10):3484. https://doi.org/10.3390/nu13103484
Chicago/Turabian StyleHong, Mi-Hyeon, Se-Won Na, Youn-Jae Jang, Jung-Joo Yoon, Yun-Jung Lee, Ho-Sub Lee, Hye-Yoom Kim, and Dae-Gill Kang. 2021. "Betulinic Acid Improves Cardiac-Renal Dysfunction Caused by Hypertrophy through Calcineurin-NFATc3 Signaling" Nutrients 13, no. 10: 3484. https://doi.org/10.3390/nu13103484
APA StyleHong, M.-H., Na, S.-W., Jang, Y.-J., Yoon, J.-J., Lee, Y.-J., Lee, H.-S., Kim, H.-Y., & Kang, D.-G. (2021). Betulinic Acid Improves Cardiac-Renal Dysfunction Caused by Hypertrophy through Calcineurin-NFATc3 Signaling. Nutrients, 13(10), 3484. https://doi.org/10.3390/nu13103484