Cornelian Cherry (Cornus mas L.) Iridoid and Anthocyanin Extract Enhances PPAR-α, PPAR-γ Expression and Reduces I/M Ratio in Aorta, Increases LXR-α Expression and Alters Adipokines and Triglycerides Levels in Cholesterol-Rich Diet Rabbit Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Chemicals and Materials
2.3. Plant Materials and Preparation of Extract
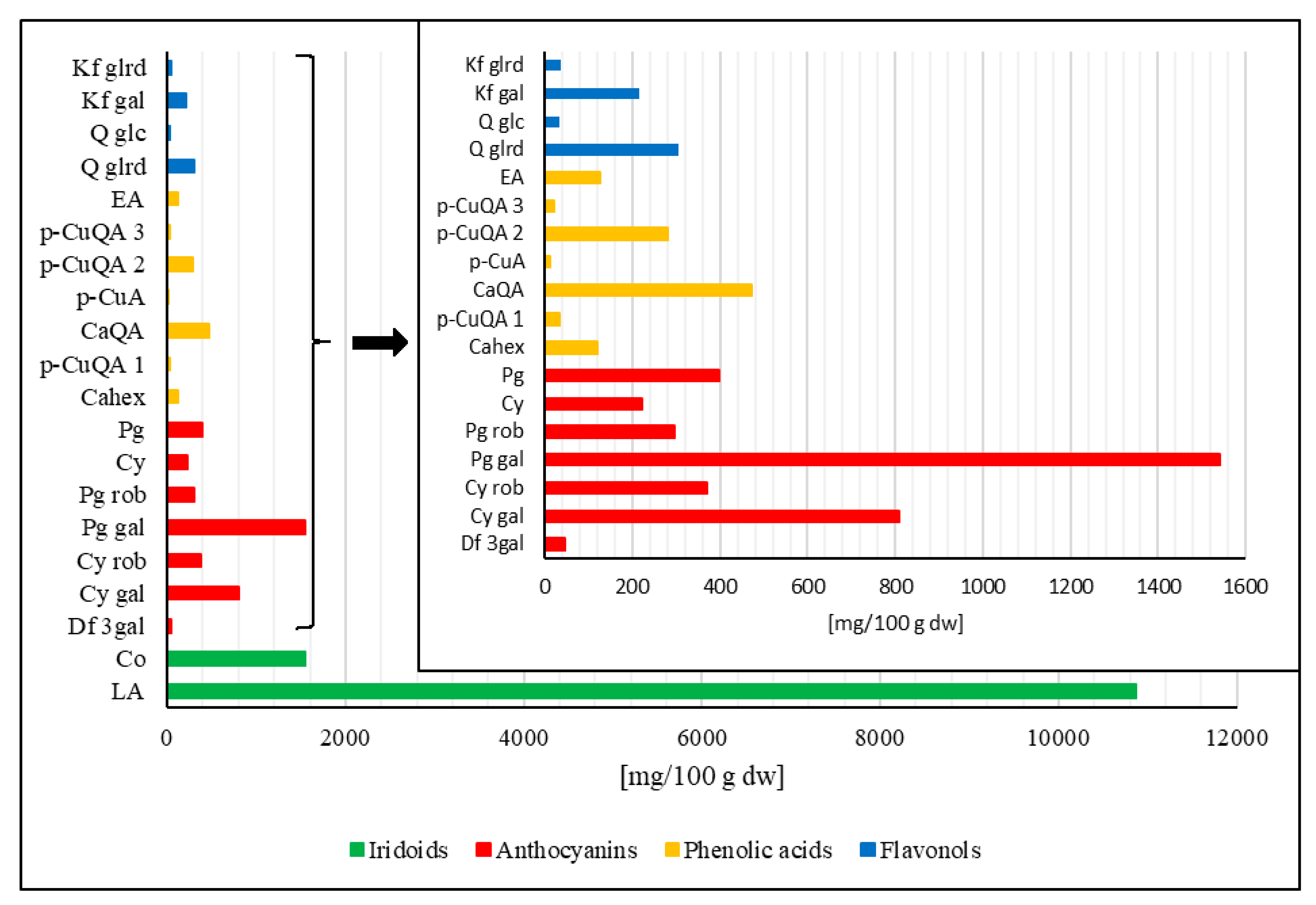
2.4. Quantification of Compounds by HPLC-PDA
2.5. Measurement of Physical and Biochemical Parameters
2.6. Quantification of Adiponectin, Leptin, Resistin, Apolipoproteins (A1, B100, E), and Insulin by Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Histopathological Assessment of the Thoracic and Abdominal Aorta
2.8. RNA Isolation, Reverse Transcription and Real-Time PCR
2.9. Western Blotting of LXR-α Expression
2.10. Statistical Analysis
3. Results
3.1. Body Weight and Serum Lipid Levels
3.2. Expression of PPAR-α and PPAR-γ in the Aorta and LXR-α in the Liver
3.3. Adipokines Serum Levels
3.4. Apolipoproteins, Glucose and Insulin Levels
3.5. Intima, Media and I/M Ratio in the Thoracic and the Abdominal Aorta
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghisalberti, E.L. Biological and pharmacological activity of naturally occurring iridoids and secoiridoids. Phytomedicine 1998, 5, 147–163. [Google Scholar] [CrossRef]
- Dinda, B.; Kyriakopoulos, A.M.; Dinda, S.; Zoumpourlis, V.; Thomaidis, N.S.; Velegraki, A.; Markopoulos, C.; Dinda, M. Cornus mas L. (cornelian cherry), an important European and Asian traditional food and medicine: Ethnomedicine, phytochemistry and pharmacology for its commercial utilization in drug industry. J. Ethnopharmacol. 2016, 193, 670–690. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Xia, M.; Ma, J.; Hao, Y.; Liu, J.; Mou, H.; Cao, L.; Ling, W. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am. J. Clin. Nutr. 2009, 90, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Sozański, T.; Kucharska, A.Z.; Rapak, A.; Szumny, D.; Trocha, M.; Merwid-Ląd, A.; Dzimira, S.; Piasecki, T.; Piórecki, N.; Magdalan, J.; et al. Iridoid-loganic acid versus anthocyanins from the Cornus mas fruits (cornelian cherry): Common and different effects on diet-induced atherosclerosis, PPARs expression and inflammation. Atherosclerosis 2016, 254, 151–160. [Google Scholar] [CrossRef]
- Park, M.; Sharma, A.; Lee, H.J. Anti-adipogenic effects of delphinidin-3-O-β-glucoside in 3T3-L1 preadipocytes and primary white adipocytes. Molecules 2019, 24, 1848. [Google Scholar] [CrossRef]
- Szandruk-Bender, M.; Rutkowska, M.; Merwid-Ląd, A.; Wiatrak, B.; Szeląg, A.; Dzimira, S.; Sobieszczańska, B.; Krzystek-Korpacka, M.; Kucharska, A.Z.; Matuszewska, A.; et al. Cornelian cherry iridoid-polyphenolic extract improves mucosal epithelial barrier integrity in rat experimental colitis and exerts antimicrobial and antiadhesive activities in vitro. Oxid. Med. Cell. Longev. 2020, 2020, 7697851. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Pogoń, P. Profile of japanese quince and cornelian cherry fruit. Zywnosc Nauka Technol. Jakosc 2010, 6, 100–108. [Google Scholar] [CrossRef]
- Czerwińska, M.E.; Melzig, M.F. Cornus mas and Cornus officinalis—analogies and differences of two medicinal plants traditionally used. Front. Pharmacol. 2018, 9, 894. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Wasilewski, T.; Bujak, T.; Gaweł-Bęben, K.; Osika, P.; Czerwonka, D. Cornus mas L. extract as a multifunctional material for manufacturing cosmetic emulsions. Chin. J. Nat. Med. 2018, 16, 284–292. [Google Scholar] [CrossRef]
- Przybylska, D.; Kucharska, A.Z.; Cybulska, I.; Sozański, T.; Piórecki, N.; Fecka, I. Cornus mas L. Stones: A valuable by-product as an ellagitannin source with high antioxidant potential. Molecules 2020, 25, 4646. [Google Scholar] [CrossRef]
- Hosseinpour-Jaghdani, F.; Shomali, T.; Gholipour-Shahraki, S.; Rahimi-Madiseh, M.; Rafieian-Kopaei, M. Cornus mas: A review on traditional uses and pharmacological properties. J. Complement. Integr. Med. 2017, 14, 20160137. [Google Scholar] [CrossRef]
- Kazimierski, M.; Regula, J.; Molska, M. Cornelian cherry (Cornus mas L.)—Characteristics, nutritional and pro-health properties. Acta Sci. Pol. Technol. Aliment. 2019, 18, 5–12. [Google Scholar]
- Siervo, M.; Lara, J.; Chowdhury, S.; Ashor, A.; Oggioni, C.; Mathers, J.C. Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: A systematic review and meta-analysis. Br. J. Nutr. 2015, 113, 1–15. [Google Scholar] [CrossRef]
- Karr, S. Epidemiology and management of hyperlipidemia. Am. J. Manag. Care 2017, 23 (Suppl. 9), S139–S148. [Google Scholar] [PubMed]
- Yao, Y.S.; Li, T.D.; Zeng, Z.H. Mechanisms underlying direct actions of hyperlipidemia on myocardium: An updated review. Lipids Health Dis. 2020, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Huynh, K. Non-HDL cholesterol levels linked with long-term risk of CVD. Nat. Rev. Cardiol. 2020, 17, 132–133. [Google Scholar] [CrossRef] [PubMed]
- Ramanath, V.S.; Oh, J.K.; Sundt, T.M., 3rd; Eagle, K.A. Acute aortic syndromes and thoracic aortic aneurysm. Mayo Clin. Proc. 2009, 84, 465–481. [Google Scholar] [CrossRef]
- Wang, S.; Miller, B.; Matthan, N.R.; Goktas, Z.; Wu, D.; Reed, D.B.; Yin, X.; Grammas, P.; Moustaid-Moussa, N.; Shen, C.L.; et al. Aortic cholesterol accumulation correlates with systemic inflammation but not hepatic and gonadal adipose tissue inflammation in low-density lipoprotein receptor null mice. Nutr. Res. 2013, 33, 1072–1082. [Google Scholar] [CrossRef]
- Zhao, S.P.; Yang, J.; Li, J.; Dong, S.Z.; Wu, Z.H. Effect of niacin on LXRalpha and PPARgamma expression and HDL-induced cholesterol efflux in adipocytes of hypercholesterolemic rabbits. Int. J. Cardiol. 2008, 124, 172–178. [Google Scholar] [CrossRef]
- Zou, Y.; Du, H.; Yin, M.; Zhang, L.; Mao, L.; Xiao, N.; Ren, G.; Zhang, C.; Pan, J. Effects of high dietary fat and cholesterol on expression of PPAR alpha, LXR alpha, and their responsive genes in the liver of apoE and LDLR double deficient mice. Mol. Cell. Biochem. 2009, 323, 195–205. [Google Scholar] [CrossRef]
- Su, X.; Peng, D. Adipokines as novel biomarkers of cardio-metabolic disorders. Clin. Chim. Acta 2020, 507, 31–38. [Google Scholar] [CrossRef]
- Sozański, T.; Kucharska, A.Z.; Szumny, A.; Magdalan, J.; Bielska, K.; Merwid-Ląd, A.; Woźniak, A.; Dzimira, S.; Piórecki, N.; Trocha, M. The protective effect of the Cornus mas fruits (cornelian cherry) on hypertriglyceridemia and atherosclerosis through PPARα activation in hypercholesterolemic rabbits. Phytomedicine 2014, 21, 1774–1784. [Google Scholar] [CrossRef]
- Sozański, T.; Kucharska, A.Z.; Dzimira, S.; Magdalan, J.; Szumny, D.; Matuszewska, A.; Nowak, B.; Piórecki, N.; Szeląg, A.; Trocha, M. Loganic acid and anthocyanins from cornelian cherry (Cornus mas L.) fruits modulate diet-induced atherosclerosis and redox status in rabbits. Adv. Clin. Exp. Med. 2018, 27, 1505–1513. [Google Scholar] [CrossRef]
- Sozański, T.; Kucharska, A.Z.; Wiśniewski, J.; Fleszar, M.G.; Rapak, A.; Gomułkiewicz, A.; Dzięgiel, P.; Magdalan, J.; Nowak, B.; Szumny, D.; et al. The iridoid loganic acid and anthocyanins from the cornelian cherry (Cornus mas L.) fruit increase the plasma l-arginine/ADMA ratio and decrease levels of ADMA in rabbits fed a high-cholesterol diet. Phytomedicine 2019, 52, 1–11. [Google Scholar] [CrossRef]
- Nowak, B.; Matuszewska, A.; Tomanik, M.; Filipiak, J.; Kucharska, A.Z.; Piórecki, N.; Jędrzejuk, D.; Zduniak, K.; Trocha, M.; Bolanowski, M.; et al. Cornelian cherry extract ameliorates osteoporosis associated with hypercholesterolemia in New Zealand rabbits. Adv. Clin. Exp. Med. 2020, 29, 1389–1397. [Google Scholar] [CrossRef]
- Kucharska, A.Z.; Sokół-Łętowska, A.; Oszmiański, J.; Piórecki, N.; Fecka, I. Iridoids, phenolic compounds and antioxidant activity of edible honeysuckle berries (Lonicera caerulea var. kamtschatica Sevast.). Molecules 2017, 22, 405. [Google Scholar] [CrossRef]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 2014, 129 (Suppl. S2), S102–S138. [Google Scholar] [CrossRef] [PubMed]
- Gadde, K.M.; Martin, C.K.; Berthoud, H.R.; Heymsfield, S.B. Obesity: Pathophysiology and management. J. Am. Coll. Cardiol. 2018, 71, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xu, K.; Zhang, X.; Cao, J.; Jia, Z.; Yang, R.; Ma, C.; Chen, C.; Zhang, T.; Yan, Z. Studies on the regulation of lipid metabolism and its mechanism of the iridoids rich fraction in Valeriana jatamansi Jones. Biomed. Pharmacother. 2016, 84, 1891–1898. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Chen, K.; Feng, M.; Shao, W.; Wu, J.; Chen, K.; Liang, T.; Liu, C. Genipin alleviates high-fat diet-induced hyperlipidemia and hepatic lipid accumulation in mice via miR-142a-5p/SREBP-1c axis. FEBS J. 2018, 285, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Ru, Q.; Xiong, Q.; Wen, R.; Chen, Y. Catalpol attenuates hepatic steatosis by regulating lipid metabolism via AMP-activated protein kinase activation. Biomed. Res. Int. 2020, 2020, 6708061. [Google Scholar] [CrossRef]
- Ishaq, M.; Tran, D.; Wu, Y.; Nowak, K.; Deans, B.J.; Xin, J.T.Z.; Loh, H.L.; Ng, W.Y.; Yee, C.W.; Southam, B.; et al. Asperuloside enhances taste perception and prevents weight gain in high-fat fed mice. Front. Endocrinol. 2021, 12, 615446. [Google Scholar] [CrossRef] [PubMed]
- Alarcon-Aguilar, F.J.; Zamilpa, A.; Perez-Garcia, M.D.; Almanza-Perez, J.C.; Romero-Nuñez, E.; Campos-Sepulveda, E.A.; Vazquez-Carrillo, L.I.; Roman-Ramos, R. Effect of Hibiscus sabdariffa on obesity in MSG mice. J. Ethnopharmacol. 2007, 114, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.P.; Choi, J.H.; Han, E.H.; Kim, H.G.; Wee, J.H.; Jung, K.O.; Jung, K.H.; Kwon, K.I.; Jeong, T.C.; Chung, Y.C.; et al. Purple sweet potato anthocyanins attenuate hepatic lipid accumulation through activating adenosine monophosphate-activated protein kinase in human HepG2 cells and obese mice. Nutr. Res. 2011, 31, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.; Damsud, T.; Wilson, M.; Grace, M.H.; Strauch, R.; Li, X.; Lila, M.A.; Komarnytsky, S. Black currant anthocyanins attenuate weight gain and improve glucose metabolism in diet-induced obese mice with intact, but not disrupted, gut microbiome. J. Agric. Food Chem. 2015, 63, 6172–6180. [Google Scholar] [CrossRef]
- Wu, T.; Jiang, Z.; Yin, J.; Long, H.; Zheng, X. Anti-obesity effects of artificial planting blueberry (Vaccinium ashei) anthocyanin in high-fat diet-treated mice. Int. J. Food Sci. Nutr. 2016, 67, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Kim, J.; Yeo, S.; Kim, G.; Ko, E.H.; Lee, S.W.; Li, W.Y.; Choi, C.W.; Jeong, S.Y. Antiadipogenic effects of loganic acid in 3T3-L1 preadipocytes and ovariectomized mice. Molecules 2018, 23, 1663. [Google Scholar] [CrossRef]
- Park, S.; Kang, S.; Jeong, D.Y.; Jeong, S.Y.; Park, J.J.; Yun, H.S. Cyanidin and malvidin in aqueous extracts of black carrots fermented with Aspergillus oryzae prevent the impairment of energy, lipid and glucose metabolism in estrogen-deficient rats by AMPK activation. Genes Nutr. 2015, 10, 455. [Google Scholar] [CrossRef]
- Liu, S.; Chang, X.; Yu, J.; Xu, W. Cerasus humilis cherry polyphenol reduces high-fat diet-induced obesity in C57BL/6 mice by mitigating fat deposition, inflammation, and oxidation. J. Agric. Food Chem. 2020, 68, 4424–4436. [Google Scholar] [CrossRef]
- Berger, J.; Moller, D.E. The mechanisms of action of PPARs. Annu. Rev. Med. 2002, 53, 409–435. [Google Scholar] [CrossRef]
- Yessoufou, A.; Wahli, W. Multifaceted roles of peroxisome proliferator-activated receptors (PPARs) at the cellular and whole organism levels. Swiss Med. Wkly. 2010, 140, w13071. [Google Scholar] [CrossRef]
- Yao, S.L.; Xu, Y.; Zhang, Y.Y.; Lu, Y.H. Black rice and anthocyanins induce inhibition of cholesterol absorption in vitro. Food Funct. 2013, 4, 1602–1608. [Google Scholar] [CrossRef]
- Liu, S.; Sui, Q.; Zhao, Y.; Chang, X. Lonicera caerulea berry polyphenols activate SIRT1, enhancing inhibition of RAW264.7 macrophage foam cell formation and promoting cholesterol efflux. J. Agric. Food Chem. 2019, 67, 7157–7166. [Google Scholar] [CrossRef]
- Shen, D.; Zhao, D.; Yang, X.; Zhang, J.; He, H.; Yu, C. Geniposide against atherosclerosis by inhibiting the formation of foam cell and lowering reverse lipid transport via p38/MAPK signaling pathways. Eur. J. Pharmacol. 2019, 864, 172728. [Google Scholar] [CrossRef]
- Chamnansilpa, N.; Aksornchu, P.; Adisakwattana, S.; Thilavech, T.; Mäkynen, K.; Dahlan, W.; Ngamukote, S. Anthocyanin-rich fraction from Thai berries interferes with the key steps of lipid digestion and cholesterol absorption. Heliyon 2020, 6, e05408. [Google Scholar] [CrossRef]
- Fki, I.; Sayadi, S.; Mahmoudi, A.; Daoued, I.; Marrekchi, R.; Ghorbel, H. Comparative study on beneficial effects of hydroxytyrosol- and oleuropein-rich olive leaf extracts on high-fat diet-induced lipid metabolism disturbance and liver injury in rats. Biomed. Res. Int. 2020, 2020, 1315202. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, Y.; Sun, C.; Liu, S.; Yan, Y.; Pan, H.; Fan, M.; Xue, L.; Nie, C.; Zhang, H.; et al. Geniposide reduces cholesterol accumulation and increases its excretion by regulating the FXR-mediated liver-gut crosstalk of bile acids. Pharmacol. Res. 2020, 152, 104631. [Google Scholar] [CrossRef] [PubMed]
- Danielewski, M.; Matuszewska, A.; Szeląg, A.; Sozański, T. The impact of anthocyanins and iridoids on transcription factors crucial for lipid and cholesterol homeostasis. Int. J. Mol. Sci. 2021, 22, 6074. [Google Scholar] [CrossRef] [PubMed]
- Wahli, W. Peroxisome proliferator-activated receptors (PPARs): From metabolic control to epidermal wound healing. Swiss Med. Wkly. 2002, 132, 83–91. [Google Scholar] [PubMed]
- Sokołowska, M.; Kowalski, M.L.; Pawliczak, R. Peroxisome proliferator-activated receptors-g (PPAR-g) and their role in immunoregulation and inflammation control. Postepy Hig. I Med. Dosw. 2005, 59, 472–484. [Google Scholar]
- Ronis, M.J. Effects of soy containing diet and isoflavones on cytochrome P450 enzyme expression and activity. Drug Metab. Rev. 2016, 48, 331–341. [Google Scholar] [CrossRef]
- Shuang, R.; Rui, X.; Wenfang, L. Phytosterols and dementia. Plant Foods Hum. Nutr. 2016, 71, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhong, H.; Leng, L.; Jiang, Z. Effects of soy isoflavone on hepatic steatosis in high fat-induced rats. J. Clin. Biochem. Nutr. 2017, 61, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Schoonjans, K.; Staels, B.; Auwerx, J. The peroxisome proliferator activated receptors (PPARS) and their effects on lipid metabolism and adipocyte differentiation. Biochim. Biophys. Acta 1996, 1302, 93–109. [Google Scholar] [CrossRef]
- Tontonoz, P.; Hu, E.; Devine, J.; Beale, E.G.; Spiegelman, B.M. PPAR gamma 2 regulates adipose expression of the phosphoenolpyruvate carboxykinase gene. Mol. Cell. Biol. 1995, 15, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Schoonjans, K.; Peinado-Onsurbe, J.; Lefebvre, A.M.; Heyman, R.A.; Briggs, M.; Deeb, S.; Staels, B.; Auwerx, J. PPARalpha and PPARgamma activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J. 1996, 15, 5336–5348. [Google Scholar] [CrossRef] [PubMed]
- Iida, K.T.; Kawakami, Y.; Suzuki, H.; Sone, H.; Shimano, H.; Toyoshima, H.; Okuda, Y.; Yamada, N. PPAR gamma ligands, troglitazone and pioglitazone, up-regulate expression of HMG-CoA synthase and HMG-CoA reductase gene in THP-1 macrophages. FEBS Lett. 2002, 520, 177–181. [Google Scholar] [CrossRef]
- Motojima, K.; Passilly, P.; Peters, J.M.; Gonzalez, F.J.; Latruffe, N. Expression of putative fatty acid transporter genes are regulated by peroxisome proliferator-activated receptor alpha and gamma activators in a tissue- and inducer-specific manner. J. Biol. Chem. 1998, 273, 16710–16714. [Google Scholar] [CrossRef]
- Gupta, R.A.; Brockman, J.A.; Sarraf, P.; Willson, T.M.; DuBois, R.N. Target genes of peroxisome proliferator-activated receptor gamma in colorectal cancer cells. J. Biol. Chem. 2001, 276, 29681–29687. [Google Scholar] [CrossRef]
- Schoonjans, K.; Watanabe, M.; Suzuki, H.; Mahfoudi, A.; Krey, G.; Wahli, W.; Grimaldi, P.; Staels, B.; Yamamoto, T.; Auwerx, J. Induction of the acyl-coenzyme A synthetase gene by fibrates and fatty acids is mediated by a peroxisome proliferator response element in the C promoter. J. Biol. Chem. 1995, 270, 19269–19276. [Google Scholar] [CrossRef]
- Welch, J.S.; Ricote, M.; Akiyama, T.E.; Gonzalez, F.J.; Glass, C.K. PPARgamma and PPARdelta negatively regulate specific subsets of lipopolysaccharide and IFN-gamma target genes in macrophages. Proc. Natl. Acad. Sci. USA 2003, 100, 6712–6717. [Google Scholar] [CrossRef]
- Moore, K.J.; Fitzgerald, M.L.; Freeman, M.W. Peroxisome proliferator-activated receptors in macrophage biology: Friend or foe? Curr. Opin. Lipidol. 2001, 12, 519–527. [Google Scholar] [CrossRef]
- Cao, G.; Liang, Y.; Broderick, C.L.; Oldham, B.A.; Beyer, T.P.; Schmidt, R.J.; Zhang, Y.; Stayrook, K.R.; Suen, C.; Otto, K.A.; et al. Antidiabetic action of a liver x receptor agonist mediated by inhibition of hepatic gluconeogenesis. J. Biol. Chem. 2003, 278, 1131–1136. [Google Scholar] [CrossRef]
- Danielewski, M.; Matuszewska, A.; Nowak, B.; Kucharska, A.Z.; Sozański, T. The effects of natural iridoids and anthocyanins on selected parameters of liver and cardiovascular system functions. Oxid. Med. Cell. Longev. 2020, 2020, 2735790. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Graf, D.; Seifert, S.; Jaudszus, A.; Bub, A.; Watzl, B. Anthocyanin-rich juice lowers serum cholesterol, leptin, and resistin and improves plasma fatty acid composition in Fischer rats. PLoS ONE 2013, 8, e66690. [Google Scholar] [CrossRef]
- Izquierdo, A.G.; Crujeiras, A.B.; Casanueva, F.F.; Carreira, M.C. Leptin, obesity, and leptin resistance: Where are we 25 years later? Nutrients 2019, 11, 2704. [Google Scholar] [CrossRef] [PubMed]
- Münzberg, H.; Morrison, C.D. Structure, production and signaling of leptin. Metabolism 2015, 64, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Yu, Z.; Tang, Q.; Song, H.; Gao, Z.; Chen, W.; Zheng, X. Honeysuckle anthocyanin supplementation prevents diet-induced obesity in C57BL/6 mice. Food Funct. 2013, 4, 1654–1661. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, J.H.; Lee, E.B.; Hur, W.; Kwon, O.J.; Park, H.J.; Yoon, S.K. Aronia melanocarpa extract ameliorates hepatic lipid metabolism through PPARγ2 downregulation. PLoS ONE 2017, 12, e0169685. [Google Scholar] [CrossRef]
- Nemes, A.; Homoki, J.R.; Kiss, R.; Hegedűs, C.; Kovács, D.; Peitl, B.; Gál, F.; Stündl, L.; Szilvássy, Z.; Remenyik, J. Effect of anthocyanin-rich tart cherry extract on inflammatory mediators and adipokines involved in type 2 diabetes in a high fat diet induced obesity mouse model. Nutrients 2019, 11, 1966. [Google Scholar] [CrossRef] [PubMed]
- Colorado, D.; Fernandez, M.; Orozco, J.; Lopera, Y.; Muñoz, D.L.; Acín, S.; Balcazar, N. Metabolic activity of anthocyanin extracts loaded into non-ionic niosomes in diet-induced obese mice. Pharm. Res. 2020, 37, 152. [Google Scholar] [CrossRef]
- Tian, L.; Ning, H.; Shao, W.; Song, Z.; Badakhshi, Y.; Ling, W.; Yang, B.B.; Brubaker, P.L.; Jin, T. Dietary cyanidin-3-glucoside attenuates high-fat-diet-induced body-weight gain and impairment of glucose tolerance in mice via effects on the hepatic hormone FGF21. J. Nutr. 2020, 150, 2101–2111. [Google Scholar] [CrossRef]
- Park, C.H.; Tanaka, T.; Kim, J.H.; Cho, E.J.; Park, J.C.; Shibahara, N.; Yokozawa, T. Hepato-protective effects of loganin, iridoid glycoside from Corni Fructus, against hyperglycemia-activated signaling pathway in liver of type 2 diabetic db/db mice. Toxicology 2011, 290, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Acquarone, E.; Monacelli, F.; Borghi, R.; Nencioni, A.; Odetti, P. Resistin: A reappraisal. Mech. Ageing Dev. 2019, 178, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.R.; Rangwala, S.M.; Shapiro, J.S.; Rich, A.S.; Rhoades, B.; Qi, Y.; Wang, J.; Rajala, M.W.; Pocai, A.; Scherer, P.E.; et al. Regulation of fasted blood glucose by resistin. Science 2004, 303, 1195–1198. [Google Scholar] [CrossRef]
- Qatanani, M.; Szwergold, N.R.; Greaves, D.R.; Ahima, R.S.; Lazar, M.A. Macrophage-derived human resistin exacerbates adipose tissue inflammation and insulin resistance in mice. J. Clin. Investig. 2009, 119, 531–539. [Google Scholar] [CrossRef]
- Park, H.K.; Kwak, M.K.; Kim, H.J.; Ahima, R.S. Linking resistin, inflammation, and cardiometabolic diseases. Korean J. Intern. Med. 2017, 32, 239–247. [Google Scholar] [CrossRef]
- Fujikawa, T.; Hirata, T.; Hosoo, S.; Nakajima, K.; Wada, A.; Yurugi, Y.; Soya, H.; Matsui, T.; Yamaguchi, A.; Ogata, M.; et al. Asperuloside stimulates metabolic function in rats across several organs under high-fat diet conditions, acting like the major ingredient of Eucommia leaves with anti-obesity activity. J. Nutr. Sci. 2012, 1, e10. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Zhang, Y.; Sun, R.; Xia, M. Anthocyanin increases adiponectin secretion and protects against diabetes-related endothelial dysfunction. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E975–E988. [Google Scholar] [CrossRef]
- Sangsefidi, Z.S.; Hosseinzadeh, M.; Ranjbar, A.M.; Akhondi-Meybodi, M.; Fallahzadeh, H.; Mozaffari-Khosravi, H. The effect of total anthocyanin-base standardized (Cornus mas L.) fruit extract on liver function, tumor necrosis factor α, malondealdehyde, and adiponectin in patients with non-alcoholic fatty liver: A study protocol for a double-blind randomized clinical trial. Nutr. J. 2019, 18, 39. [Google Scholar]
- Nguyen, T.M.D. Adiponectin: Role in physiology and pathophysiology. Int. J. Prev. Med. 2020, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Yoshida, H. Beneficial effects of adiponectin on glucose and lipid metabolism and atherosclerotic progression: Mechanisms and perspectives. Int. J. Mol. Sci. 2019, 20, 1190. [Google Scholar] [CrossRef] [PubMed]
- Sakakura, K.; Nakano, M.; Otsuka, F.; Ladich, E.; Kolodgie, F.D.; Virmani, R. Pathophysiology of atherosclerosis plaque progression. Heart Lung Circ. 2013, 22, 399–411. [Google Scholar] [CrossRef]
- Sozański, T.; Kucharska, A.Z.; Szumny, D.; Magdalan, J.; Merwid-Ląd, A.; Nowak, B.; Piórecki, N.; Dzimira, S.; Jodkowska, A.; Szeląg, A.; et al. Cornelian cherry consumption increases the L-arginine/ADMA ratio, lowers ADMA and SDMA levels in the plasma, and enhances the aorta glutathione level in rabbits fed a high cholesterol diet. J. Funct. Foods 2017, 34, 189–196. [Google Scholar] [CrossRef]
 p < 0.05 vs. CHOL.
p < 0.05 vs. CHOL.
 p < 0.05 vs. CHOL.
p < 0.05 vs. CHOL.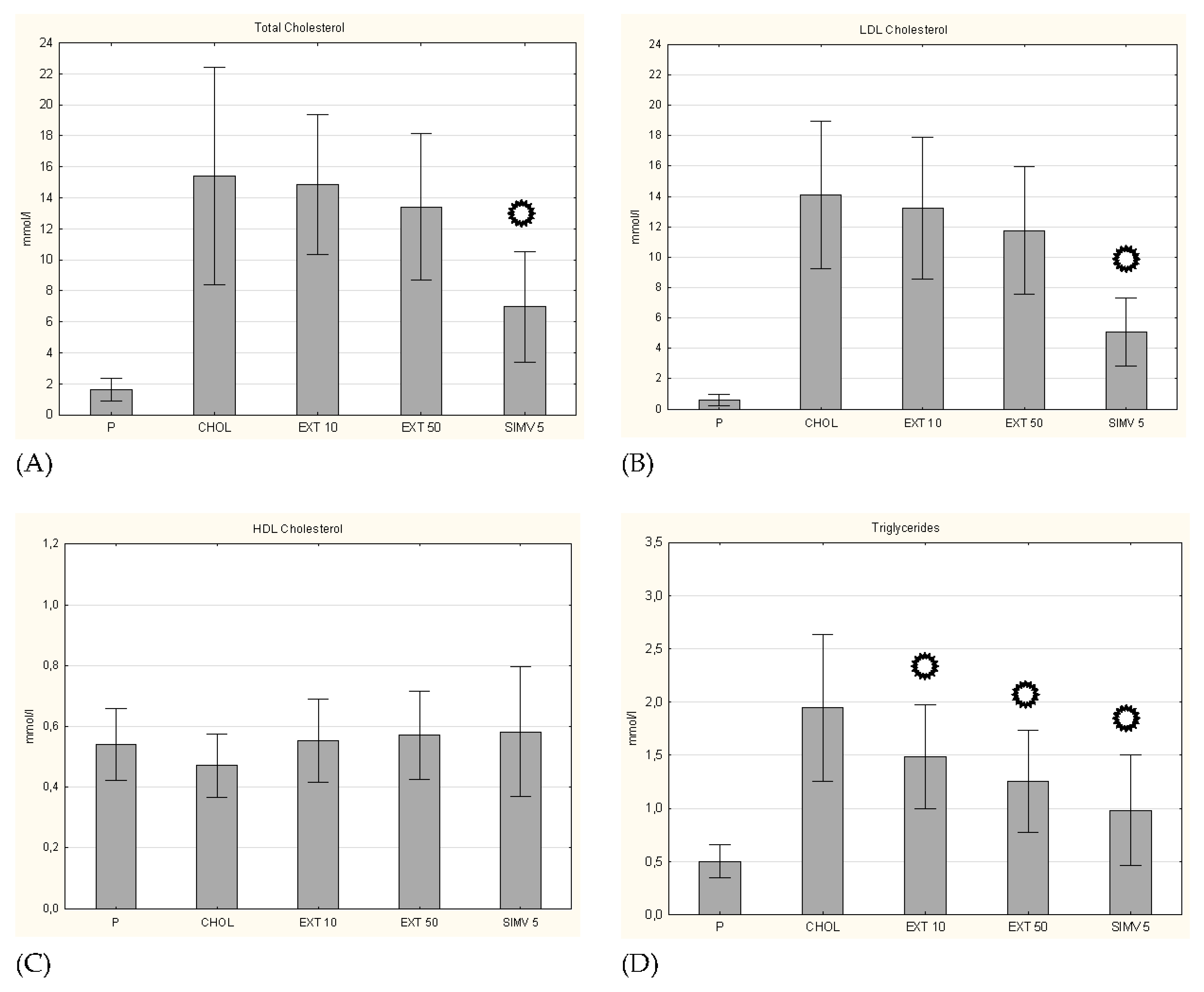
 p < 0.05 vs. CHOL.
p < 0.05 vs. CHOL.
 p < 0.05 vs. CHOL.
p < 0.05 vs. CHOL.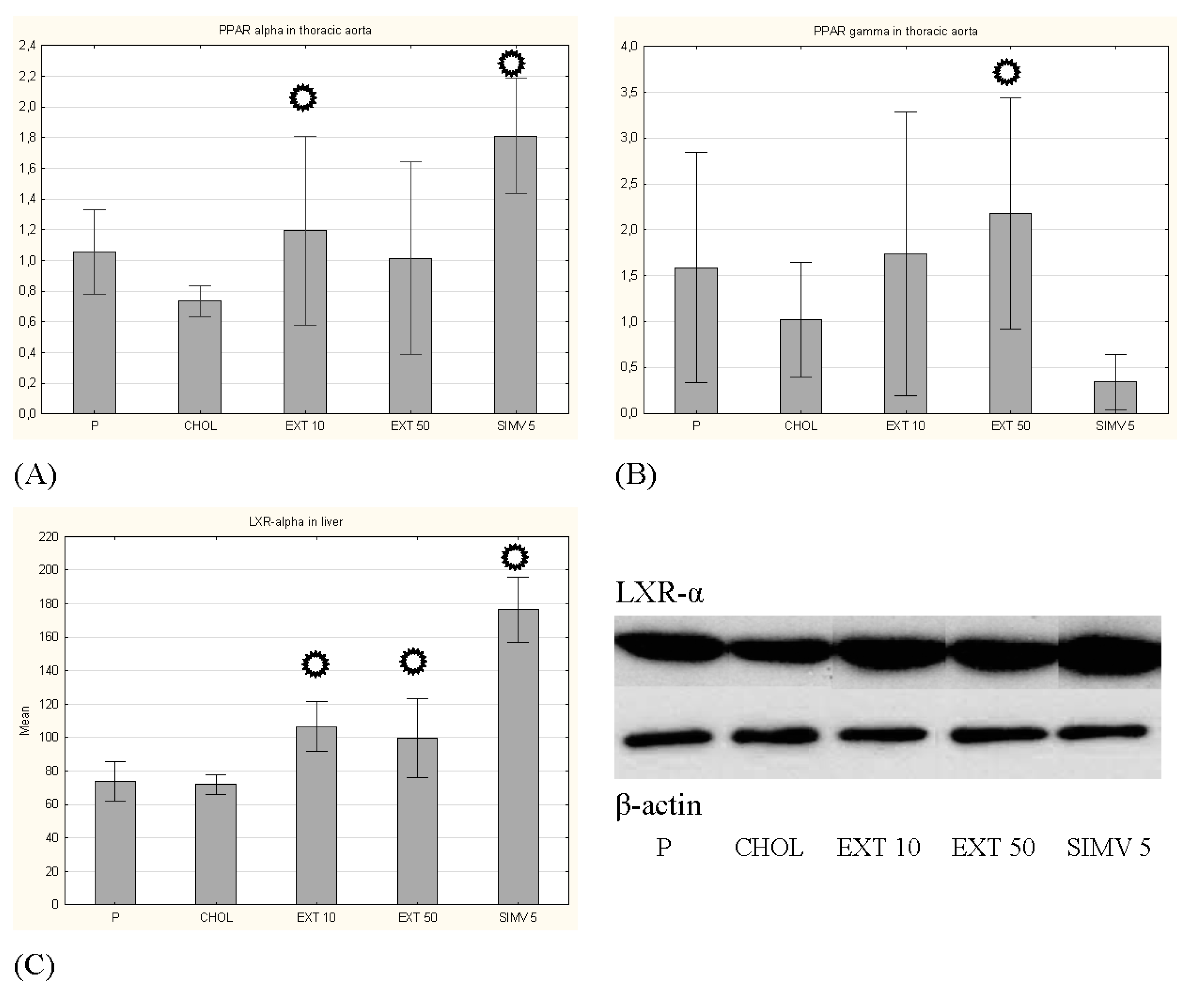
 p < 0.05 vs. CHOL.
p < 0.05 vs. CHOL.
 p < 0.05 vs. CHOL.
p < 0.05 vs. CHOL.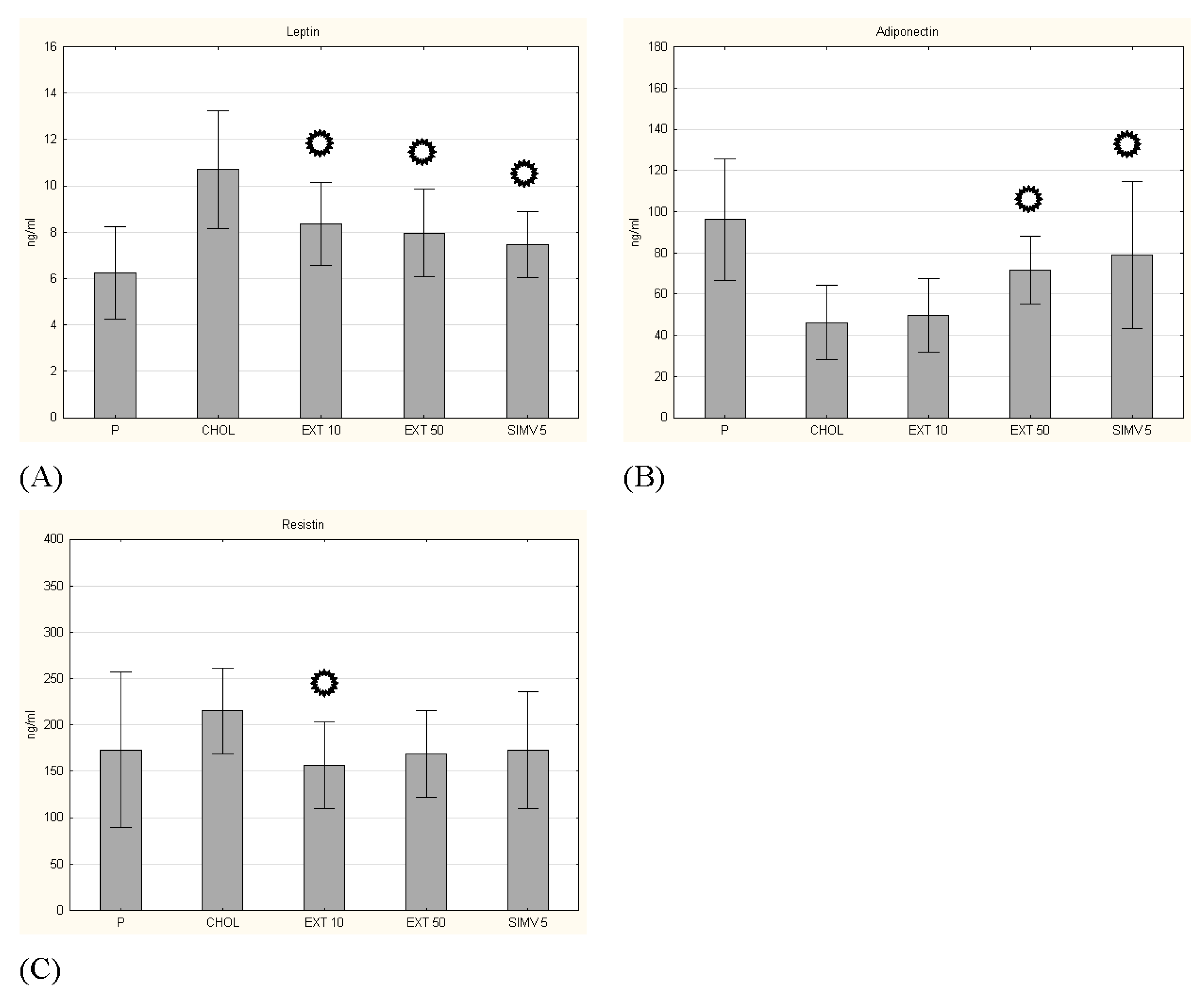
 p < 0.05 vs. CHOL.
p < 0.05 vs. CHOL.
 p < 0.05 vs. CHOL.
p < 0.05 vs. CHOL.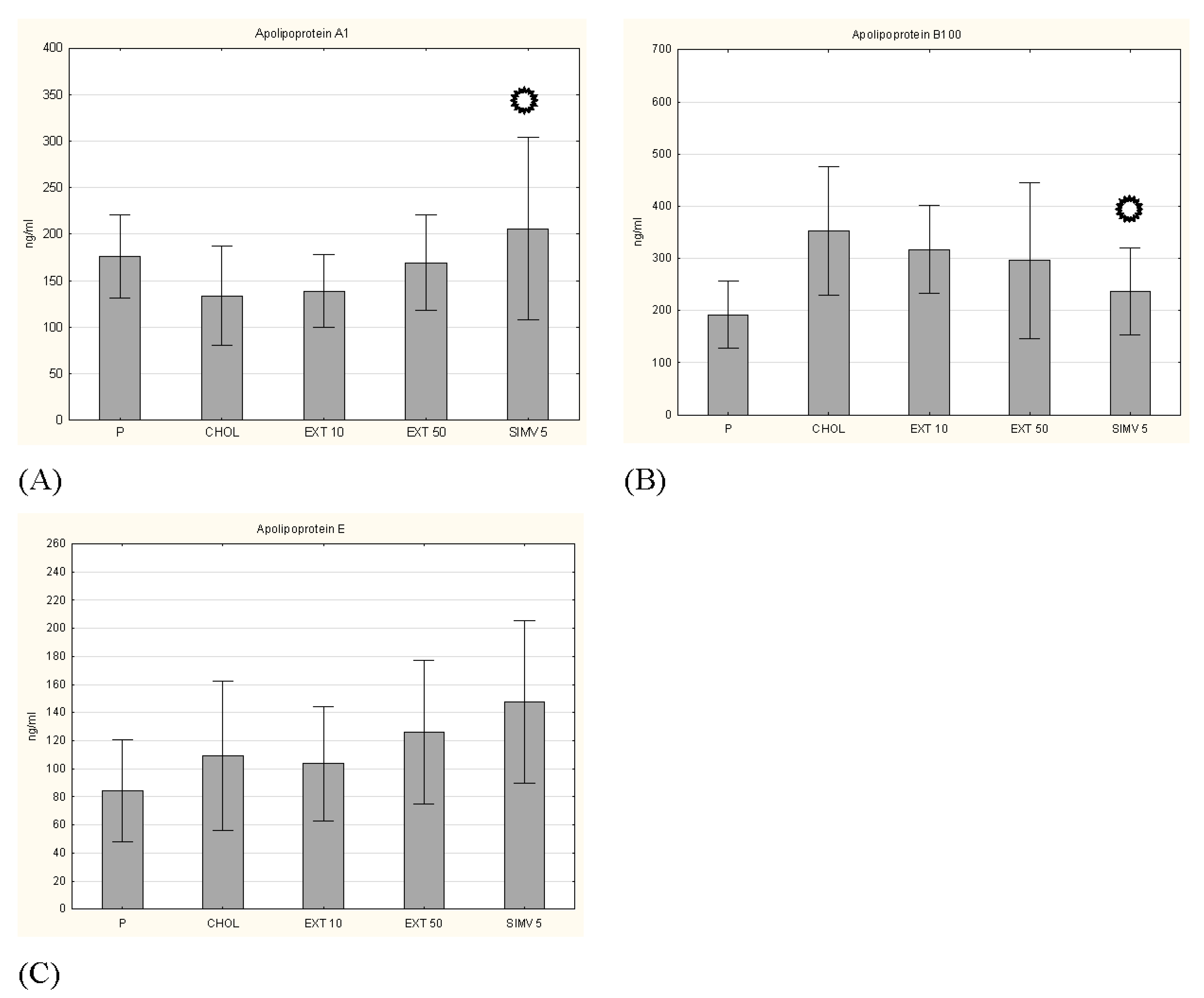
 p < 0.05 vs. CHOL.
p < 0.05 vs. CHOL.
 p < 0.05 vs. CHOL.
p < 0.05 vs. CHOL.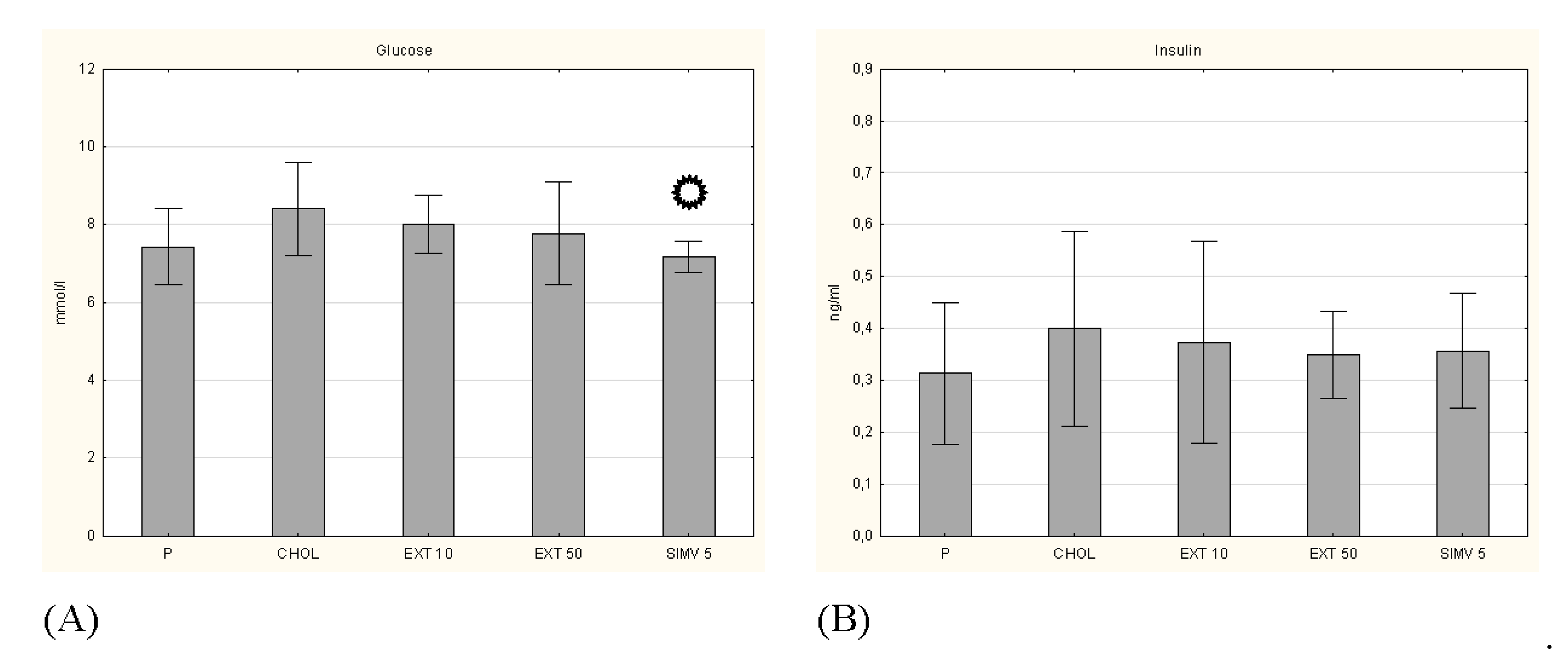
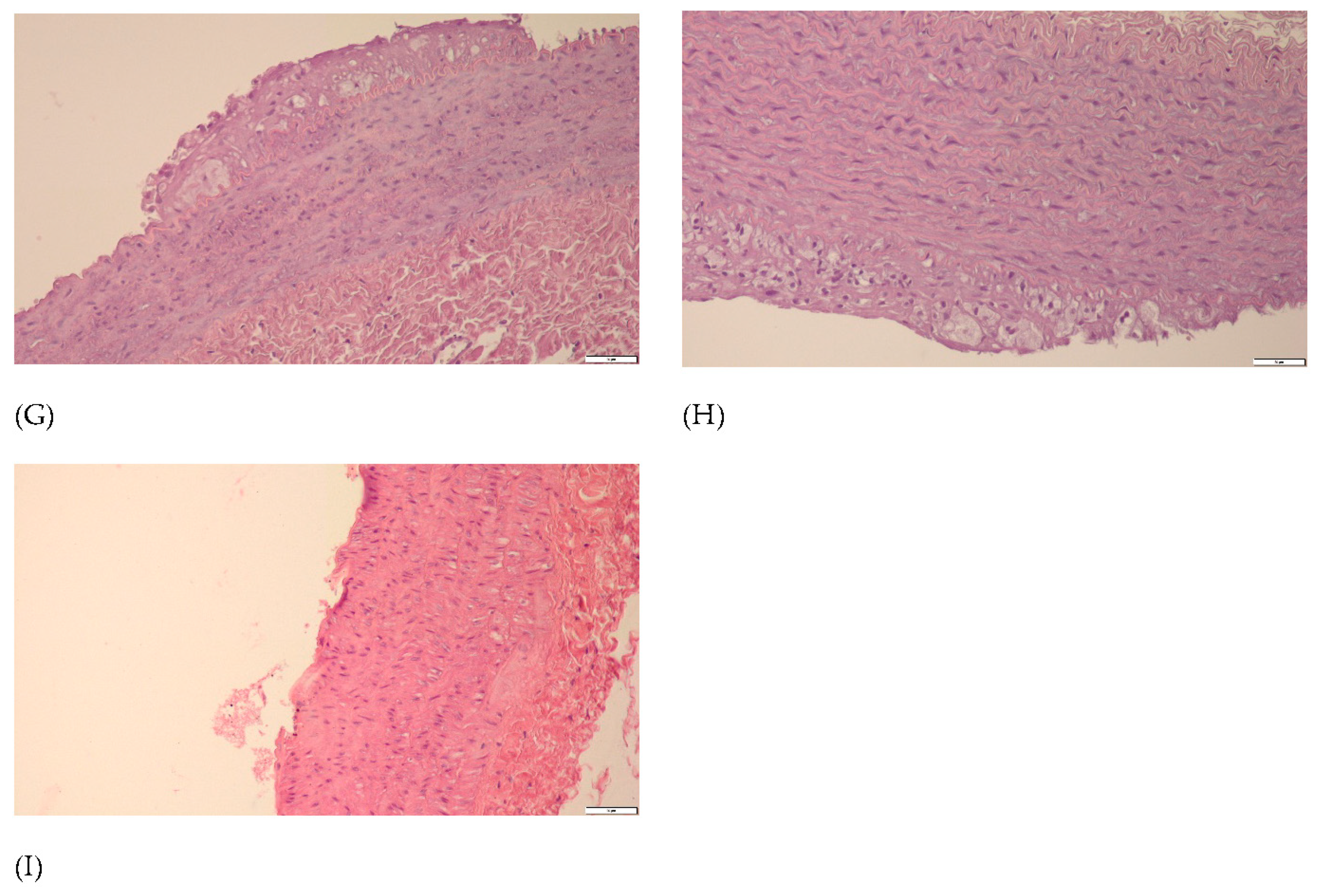
 p < 0.05 vs. CHOL. Representative cross-sections of the thoracic aorta segments, stained with hematoxylin-eosin in the (E) P group, (F) CHOL group, (G) EXT 10 group, (H) EXT 50 group and (I) SIMV 5 group.
p < 0.05 vs. CHOL. Representative cross-sections of the thoracic aorta segments, stained with hematoxylin-eosin in the (E) P group, (F) CHOL group, (G) EXT 10 group, (H) EXT 50 group and (I) SIMV 5 group.
 p < 0.05 vs. CHOL. Representative cross-sections of the thoracic aorta segments, stained with hematoxylin-eosin in the (E) P group, (F) CHOL group, (G) EXT 10 group, (H) EXT 50 group and (I) SIMV 5 group.
p < 0.05 vs. CHOL. Representative cross-sections of the thoracic aorta segments, stained with hematoxylin-eosin in the (E) P group, (F) CHOL group, (G) EXT 10 group, (H) EXT 50 group and (I) SIMV 5 group.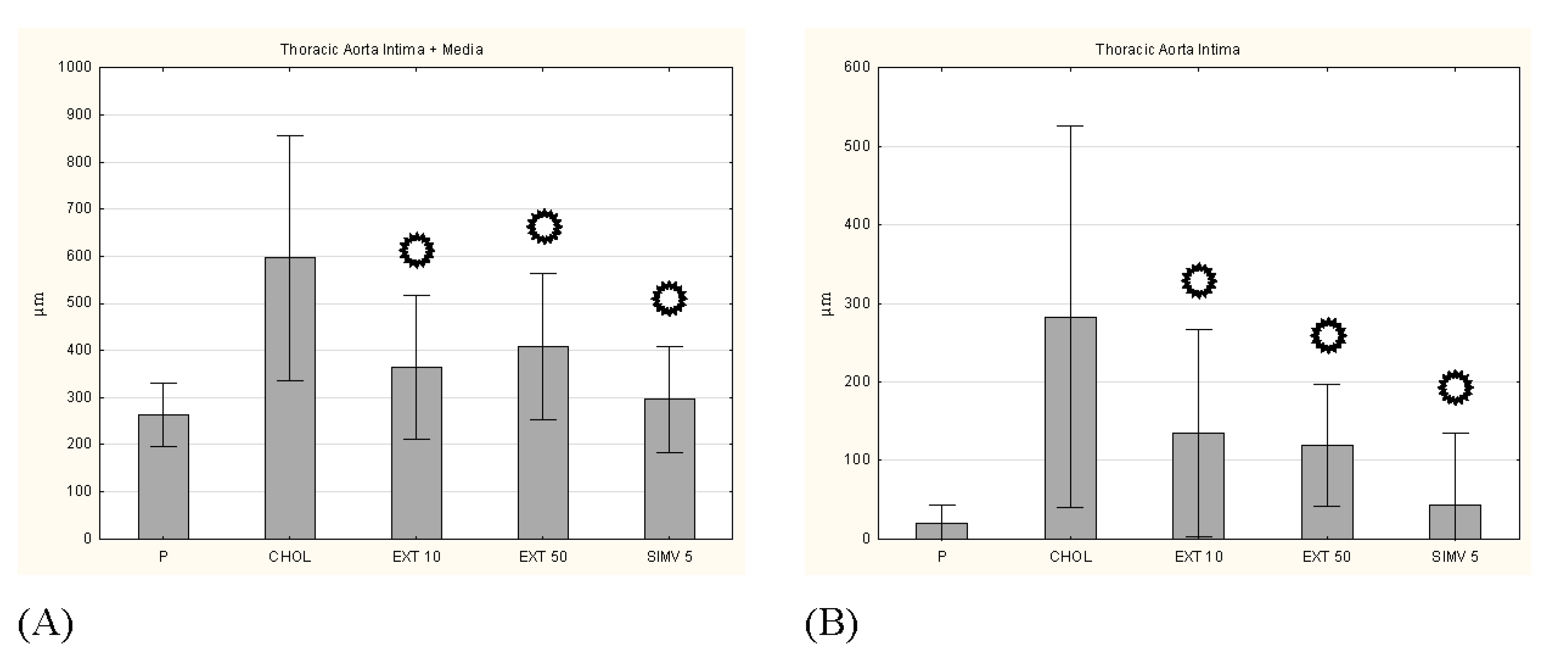

 p < 0.05 vs. CHOL. Representative cross-sections of the abdominal aorta segments, stained with hematoxylin-eosin in the (E) P group, (F) CHOL group, (G) EXT 10 group, (H) EXT 50 group and (I) SIMV 5 group.
p < 0.05 vs. CHOL. Representative cross-sections of the abdominal aorta segments, stained with hematoxylin-eosin in the (E) P group, (F) CHOL group, (G) EXT 10 group, (H) EXT 50 group and (I) SIMV 5 group.
 p < 0.05 vs. CHOL. Representative cross-sections of the abdominal aorta segments, stained with hematoxylin-eosin in the (E) P group, (F) CHOL group, (G) EXT 10 group, (H) EXT 50 group and (I) SIMV 5 group.
p < 0.05 vs. CHOL. Representative cross-sections of the abdominal aorta segments, stained with hematoxylin-eosin in the (E) P group, (F) CHOL group, (G) EXT 10 group, (H) EXT 50 group and (I) SIMV 5 group.
| Group | Chow | Tested Substance | Dose of Tested Substance |
|---|---|---|---|
| P | standard chow | none | none |
| CHOL | standard chow + 1% cholesterol | none | none |
| EXT 10 | standard chow + 1% cholesterol | cornelian cherry extract | 10 mg/kg b.w. |
| EXT 50 | standard chow + 1% cholesterol | cornelian cherry extract | 50 mg/kg b.w. |
| SIMV 5 | standard chow + 1% cholesterol | simvastatin | 5 mg/kg b.w. |
| Group | Average Weight (kg) [Mean ± SD] | Weight Change (kg) | Weight Change (%) | Change in Weight Gain in Comparison to P Group (%) | Change in Weight Gain in Comparison to CHOL Group (%) | |
|---|---|---|---|---|---|---|
| Day 0 | Day 60 | |||||
| P | 3.203 ± 0.338 | 3.380 ± 0.305 | 0.177 | 5.53 | 0 | −57.14 |
| CHOL | 3.117 ± 0.453 | 3.530 ± 0.377 | 0.413 | 13.25 | 133.33 | 0 |
| EXT 10 | 3.315 ± 0.320 | 3.696 ± 0.367 | 0.381 | 11.49 | 115.25 | −7.75 |
| EXT 50 | 3.161 ± 0.434 | 3.475 ± 0.422 | 0.314 | 9.93 | 77.40 | −23.97 |
| SIMV 5 | 2.975 ± 0.249 | 3.395 ± 0.274 | 0.420 | 14.12 | 137.29 | 1.69 |
| Group | P | CHOL | EXT 10 | EXT 50 | SIMV 5 |
|---|---|---|---|---|---|
| HOMA-IR | 2.48 | 3.58 | 3.19 | 2.90 | 2.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danielewski, M.; Kucharska, A.Z.; Matuszewska, A.; Rapak, A.; Gomułkiewicz, A.; Dzimira, S.; Dzięgiel, P.; Nowak, B.; Trocha, M.; Magdalan, J.; et al. Cornelian Cherry (Cornus mas L.) Iridoid and Anthocyanin Extract Enhances PPAR-α, PPAR-γ Expression and Reduces I/M Ratio in Aorta, Increases LXR-α Expression and Alters Adipokines and Triglycerides Levels in Cholesterol-Rich Diet Rabbit Model. Nutrients 2021, 13, 3621. https://doi.org/10.3390/nu13103621
Danielewski M, Kucharska AZ, Matuszewska A, Rapak A, Gomułkiewicz A, Dzimira S, Dzięgiel P, Nowak B, Trocha M, Magdalan J, et al. Cornelian Cherry (Cornus mas L.) Iridoid and Anthocyanin Extract Enhances PPAR-α, PPAR-γ Expression and Reduces I/M Ratio in Aorta, Increases LXR-α Expression and Alters Adipokines and Triglycerides Levels in Cholesterol-Rich Diet Rabbit Model. Nutrients. 2021; 13(10):3621. https://doi.org/10.3390/nu13103621
Chicago/Turabian StyleDanielewski, Maciej, Alicja Z. Kucharska, Agnieszka Matuszewska, Andrzej Rapak, Agnieszka Gomułkiewicz, Stanisław Dzimira, Piotr Dzięgiel, Beata Nowak, Małgorzata Trocha, Jan Magdalan, and et al. 2021. "Cornelian Cherry (Cornus mas L.) Iridoid and Anthocyanin Extract Enhances PPAR-α, PPAR-γ Expression and Reduces I/M Ratio in Aorta, Increases LXR-α Expression and Alters Adipokines and Triglycerides Levels in Cholesterol-Rich Diet Rabbit Model" Nutrients 13, no. 10: 3621. https://doi.org/10.3390/nu13103621
APA StyleDanielewski, M., Kucharska, A. Z., Matuszewska, A., Rapak, A., Gomułkiewicz, A., Dzimira, S., Dzięgiel, P., Nowak, B., Trocha, M., Magdalan, J., Piórecki, N., Szeląg, A., & Sozański, T. (2021). Cornelian Cherry (Cornus mas L.) Iridoid and Anthocyanin Extract Enhances PPAR-α, PPAR-γ Expression and Reduces I/M Ratio in Aorta, Increases LXR-α Expression and Alters Adipokines and Triglycerides Levels in Cholesterol-Rich Diet Rabbit Model. Nutrients, 13(10), 3621. https://doi.org/10.3390/nu13103621












