Dose- and Sex-Dependent Changes in Hemoglobin Oxygen Affinity by the Micronutrient 5-Hydroxymethylfurfural and α-Ketoglutaric Acid
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mairbaurl, H.; Weber, R.E. Oxygen transport by hemoglobin. Compr. Physiol. 2012, 2, 1463–1489. [Google Scholar]
- Imai, K. Allosteric Effects in Haemoglobin; Cambridge University Press: Cambridge, UK, 1982. [Google Scholar]
- Böning, D.; Littschwager, A.; Hütler, M.; Beneke, R.; Staab, D. Hemoglobin oxygen affinity in patients with cystic fibrosis. PLoS ONE 2014, 9, e97932. [Google Scholar] [CrossRef] [Green Version]
- Dempsey, J.A. With haemoglobin as with politics—Should we shift right or left? J. Physiol. 2020, 598, 1419–1420. [Google Scholar] [CrossRef]
- Srinivasan, A.J.; Morkane, C.; Martin, D.S.; Welsby, I.J. Should modulation of p50 be a therapeutic target in the critically ill? Expert Rev. Hematol. 2017, 10, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Woyke, S.; Rauch, S.; Strohle, M.; Gatterer, H. Modulation of Hb–O2 affinity to improve hypoxemia in COVID-19 patients. Clin. Nutr. 2021, 40, 38–39. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, O.; Cabrales, P. Increased hemoglobin O2 affinity protects during acute hypoxia. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H271–H281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulmalik, O.; Safo, M.K.; Chen, Q.; Yang, J.; Brugnara, C.; Ohene-Frempong, K.; Abraham, D.J.; Asakura, R. 5-hydroxymethyl-2-furfural modifies intracellular sickle haemoglobin and inhibits sickling of red blood cells. Br. J. Haematol. 2005, 128, 552–561. [Google Scholar] [CrossRef]
- Mahon, R.T.; Ciarlone, G.E.; Roney, N.G.; Swift, J.M. Cardiovascular Parameters in a Swine Model of Normobaric Hypoxia Treated With 5-Hydroxymethyl-2-Furfural (5-HMF). Front. Physiol. 2019, 10, 395. [Google Scholar] [CrossRef]
- Stern, W.; Matthews, D.; McKew, J.C.; Shen, X.; Kato, G.J. A phase 1, first-in-man, dose–response study of Aes-103 (5-HMF), an anti-sickling, allosteric modifier of hemoglobin oxygen affinity in healthy normal volunteers. Blood 2012, 120, 3210. [Google Scholar] [CrossRef]
- Hannemann, A.; Cytlak, U.M.; Rees, D.C.; Tewari, S.; Gibson, J.S. Effects of 5-hydroxymethyl-2-furfural on the volume and membrane permeability of red blood cells from patients with sickle cell disease. J. Physiol. 2014, 592, 4039–4049. [Google Scholar] [CrossRef] [Green Version]
- Kossler, F.; Mair, L.; Burtscher, M.; Gatterer, H. 5-Hydroxymethylfurfural and alpha-ketoglutaric acid supplementation increases oxygen saturation during prolonged exercise in normobaric hypoxia. Int. J. Vitam. Nutr. Res. 2019. [Google Scholar] [CrossRef]
- Parker, S.J.; Encarnación-Rosado, J.; Hollinshead, K.E.R.; Hollinshead, D.M.; Ash, L.J.; Rossi, J.A.K.; Lin, E.Y.; Sohn, A.S.W.; Philips, M.R.; Jones, D.R.; et al. Spontaneous hydrolysis and spurious metabolic properties of α-ketoglutarate esters. Nat. Commun. 2021, 12, 4905. [Google Scholar] [CrossRef] [PubMed]
- Whillier, S.; Garcia, B.; Chapman, B.E.; Kuchel, P.W.; Raftos, J.E. Glutamine and α-ketoglutarate as glutamate sources for glutathione synthesis in human erythrocytes. FEBS J. 2011, 278, 3152–3163. [Google Scholar] [CrossRef] [PubMed]
- Matzi, V.; Lindenmann, J.; Muench, A.; Greilberger, J.; Juan, H.; Wintersteiger, R.; Maier, A.; Smolle-Juettner, F.M. The impact of preoperative micronutrient supplementation in lung surgery. A prospective randomized trial of oral supplementation of combined alpha-ketoglutaric acid and 5-hydroxymethylfurfural. Eur. J. Cardiothorac. Surg. 2007, 32, 776–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Formenti, F.; Vogel, D.J.; Camporota, L. Nutritional interventions to modulate haemoglobin-oxygen affinity in COVID-19 patients. Clin. Nutr. 2020, 39, 3843–3844. [Google Scholar] [CrossRef]
- Woyke, S.; Gatterer, H.; Rauch, S.; Ströhle, M. Reply—Letter to the editor—Nutritional interventions to modulate haemoglobin-oxygen affinity in COVID-19 patients. Clin. Nutr. 2020, 39, 3842. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, S. Physiology, Blood Volume; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- National Toxicology Program. NTP toxicology and carcinogenesis studies of 5-(Hydroxymethyl)-2-furfural (CAS No. 67-47-0) in F344/N rats and B6C3F1 mice (gavage studies). Natl. Toxicol. Program Tech. Rep. Ser. 2010, 554, 7–13, 15–19, 21–31. [Google Scholar]
- Zaitsev, A.N.; Simonian, T.A.; Pozdniakov, A.L. Hygienic standards for hydroxymethylfurfural in food products. Vopr. Pitan. 1975, 1, 52–55. [Google Scholar]
- Woyke, S.; Ströhle, M.; Brugger, H.; Strapazzon, G.; Gatterer, H.; Mair, N.; Haller, T. High-throughput determination of oxygen dissociation curves in a microplate reader-A novel, quantitative approach. Physiol. Rep. 2021, 9, e14995. [Google Scholar] [CrossRef]
- Goutelle, S.; Maurin, M.; Rougier, F.; Barbaut, X.; Bourguignon, L.; Ducher, M.; Maire, P. The Hill equation: A review of its capabilities in pharmacological modelling. Fundam. Clin. Pharmacol. 2008, 22, 633–648. [Google Scholar] [CrossRef]
- Nooren, I.M.; Thornton, J.M. Diversity of protein-protein interactions. EMBO J. 2003, 22, 3486–3492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böning, D.; Draude, W.; Trost, F.; Meier, U. Interrelation between Bohr and temperature effects on the oxygen dissociation curve in men and women. Respir. Physiol. 1978, 34, 195–207. [Google Scholar] [CrossRef]
- Humpeler, E.; Vogel, S.; Schobersberger, W.; Mairbäurl, H. Red cell oxygen transport in man in relation to gender and age. Mech. Ageing Dev. 1989, 47, 229–239. [Google Scholar] [CrossRef]
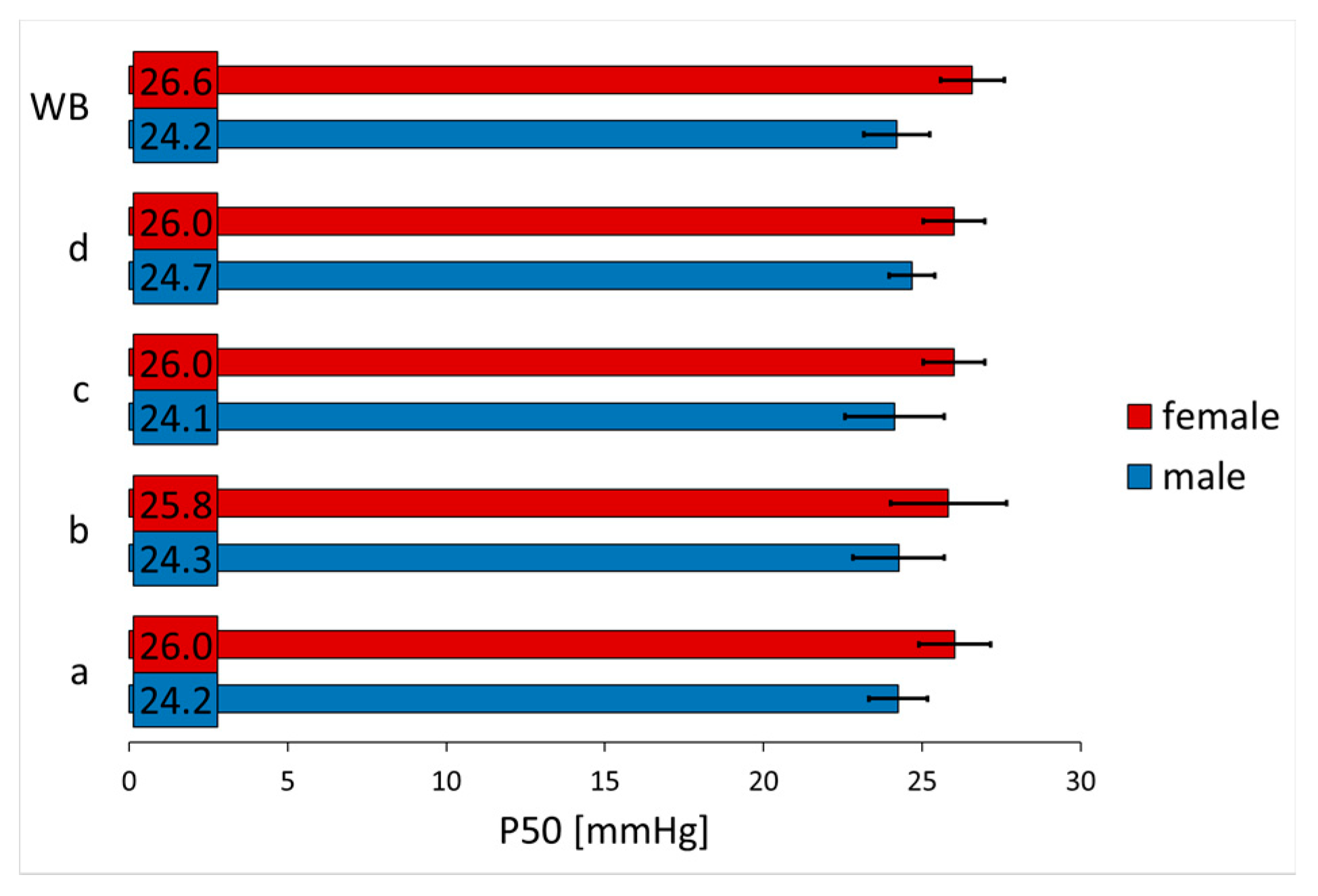
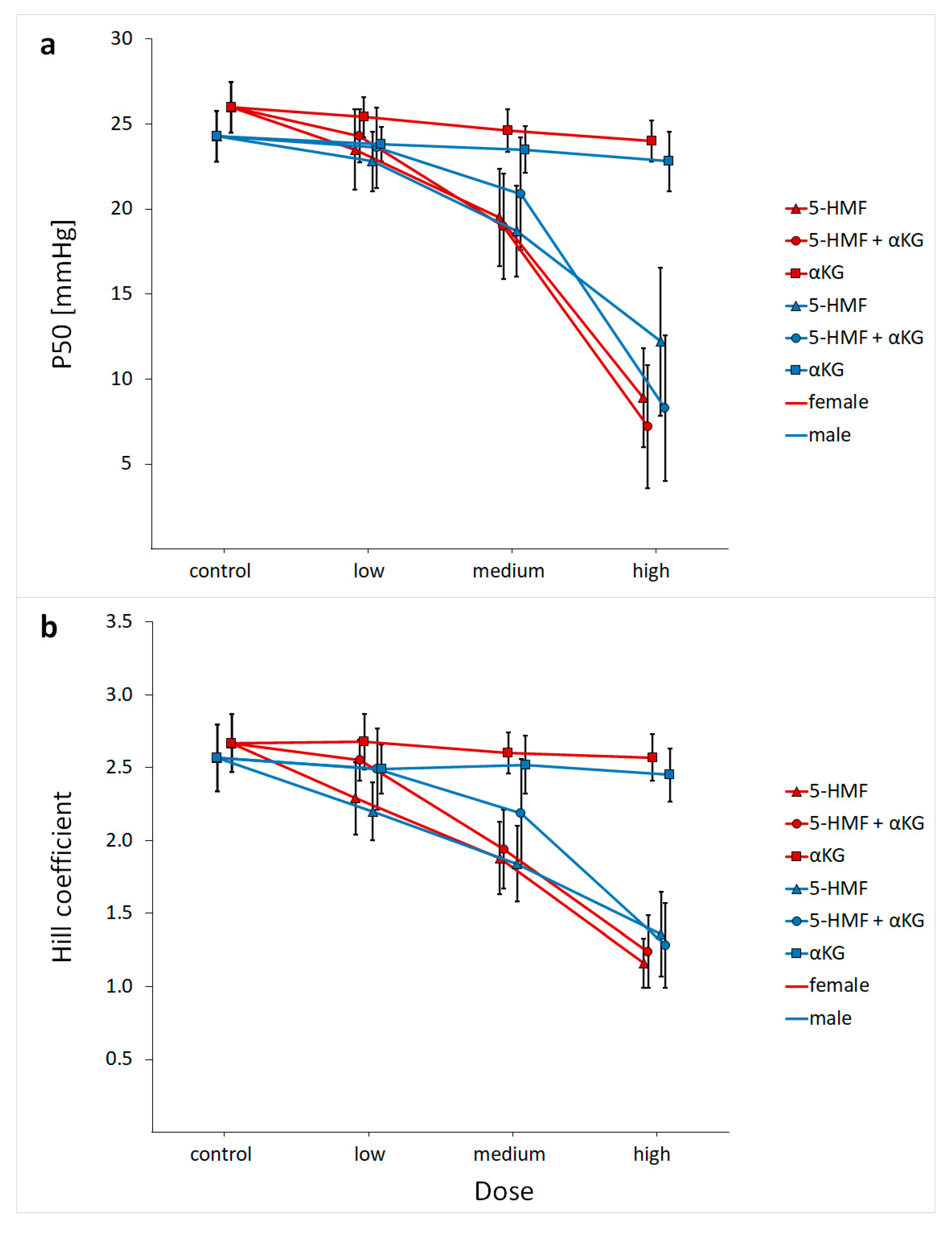

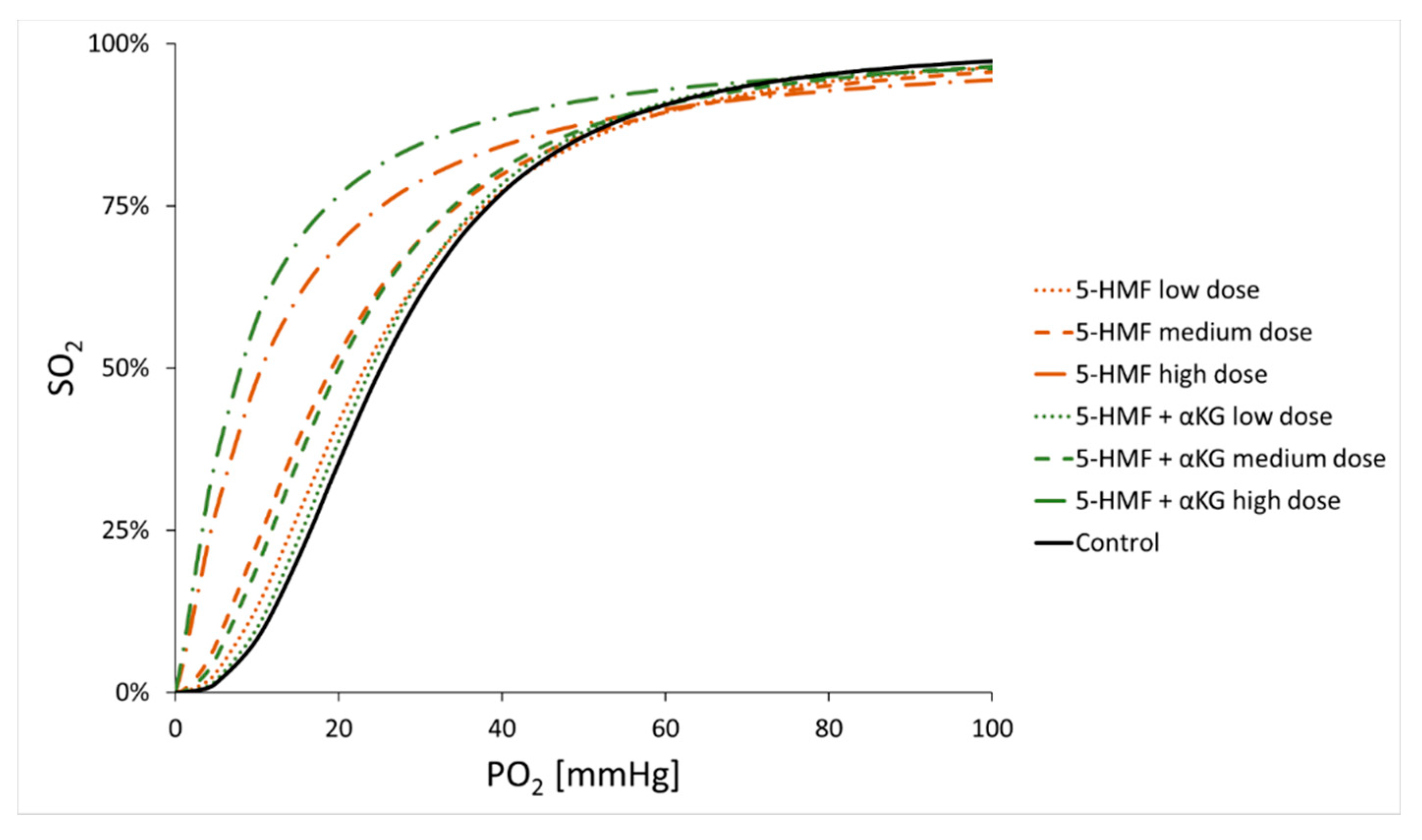
| Age (Years) | Hb (g/dL) | Hct (%) | pH | PCO2 (mmHg) | |
|---|---|---|---|---|---|
| male | 30.3 ± 4.0 | 15.5 ± 0.9 | 47.6 ± 2.6 | 7.35 ± 0.03 | 51.0 ± 6.7 |
| female | 28.9 ± 1.4 | 13.4 ± 0.7 | 41.3 ± 2.1 | 7.35 ± 0.04 | 45.8 ± 7.4 |
| p | 0.31 | <0.001 | <0.001 | 0.76 | 0.12 |
| P50 (mmHg) | HC | ||||
|---|---|---|---|---|---|
| Females | Males | Females | Males | ||
| control | 26.0 ± 1.5 | 24.3 ± 1.5 | 2.7 ± 0.2 | 2.6 ± 0.2 | |
| 5-HMF | low dose | 23.5 ± 2.3 | 22.8 ± 1.7 | 2.3 ± 0.3 | 2.2 ± 0.2 |
| medium dose | 19.5 ± 2.9 | 18.7 ± 2.7 | 1.9 ± 0.3 | 1.8 ± 0.3 | |
| high dose | 8.9 ± 2.9 | 12.2 ± 4.3 | 1.2 ± 0.2 | 1.4 ± 0.3 | |
| low dose | 24.3 ± 1.6 | 23.6 ± 2.4 | 2.6 ± 0.1 | 2.5 ± 0.3 | |
| 5-HMF + αKG | medium dose | 19.0 ± 3.1 | 20.9 ± 3.3 | 1.9 ± 0.3 | 2.2 ± 0.4 |
| high dose | 7.2 ± 3.6 | 8.3 ± 4.3 | 1.2 ± 0.3 | 1.3 ± 0.3 | |
| low dose | 25.4 ± 1.2 | 23.8 ± 1.0 | 2.7 ± 0.2 | 2.5 ± 0.2 | |
| αKG | medium dose | 24.6 ± 1.3 | 23.5 ± 1.4 | 2.6 ± 0.1 | 2.5 ± 0.2 |
| high dose | 24.0 ± 1.2 | 22.8 ± 1.7 | 2.6 ± 0.2 | 2.4 ± 0.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woyke, S.; Mair, N.; Ortner, A.; Haller, T.; Ronzani, M.; Rugg, C.; Ströhle, M.; Wintersteiger, R.; Gatterer, H. Dose- and Sex-Dependent Changes in Hemoglobin Oxygen Affinity by the Micronutrient 5-Hydroxymethylfurfural and α-Ketoglutaric Acid. Nutrients 2021, 13, 3448. https://doi.org/10.3390/nu13103448
Woyke S, Mair N, Ortner A, Haller T, Ronzani M, Rugg C, Ströhle M, Wintersteiger R, Gatterer H. Dose- and Sex-Dependent Changes in Hemoglobin Oxygen Affinity by the Micronutrient 5-Hydroxymethylfurfural and α-Ketoglutaric Acid. Nutrients. 2021; 13(10):3448. https://doi.org/10.3390/nu13103448
Chicago/Turabian StyleWoyke, Simon, Norbert Mair, Astrid Ortner, Thomas Haller, Marco Ronzani, Christopher Rugg, Mathias Ströhle, Reinhold Wintersteiger, and Hannes Gatterer. 2021. "Dose- and Sex-Dependent Changes in Hemoglobin Oxygen Affinity by the Micronutrient 5-Hydroxymethylfurfural and α-Ketoglutaric Acid" Nutrients 13, no. 10: 3448. https://doi.org/10.3390/nu13103448
APA StyleWoyke, S., Mair, N., Ortner, A., Haller, T., Ronzani, M., Rugg, C., Ströhle, M., Wintersteiger, R., & Gatterer, H. (2021). Dose- and Sex-Dependent Changes in Hemoglobin Oxygen Affinity by the Micronutrient 5-Hydroxymethylfurfural and α-Ketoglutaric Acid. Nutrients, 13(10), 3448. https://doi.org/10.3390/nu13103448






