Effects of Delayed-Release Olive Oil and Hydrolyzed Pine Nut Oil on Glucose Tolerance, Incretin Secretion and Appetite in Humans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Subjective Appetite and Gastrointestinal Tolerability
2.4. Indices of Insulin Sensitivity and Beta-Cell Function
2.5. Analysis of Blood Biomarkers
2.6. Intervention Oils
2.7. Outcomes and Statistics
3. Results
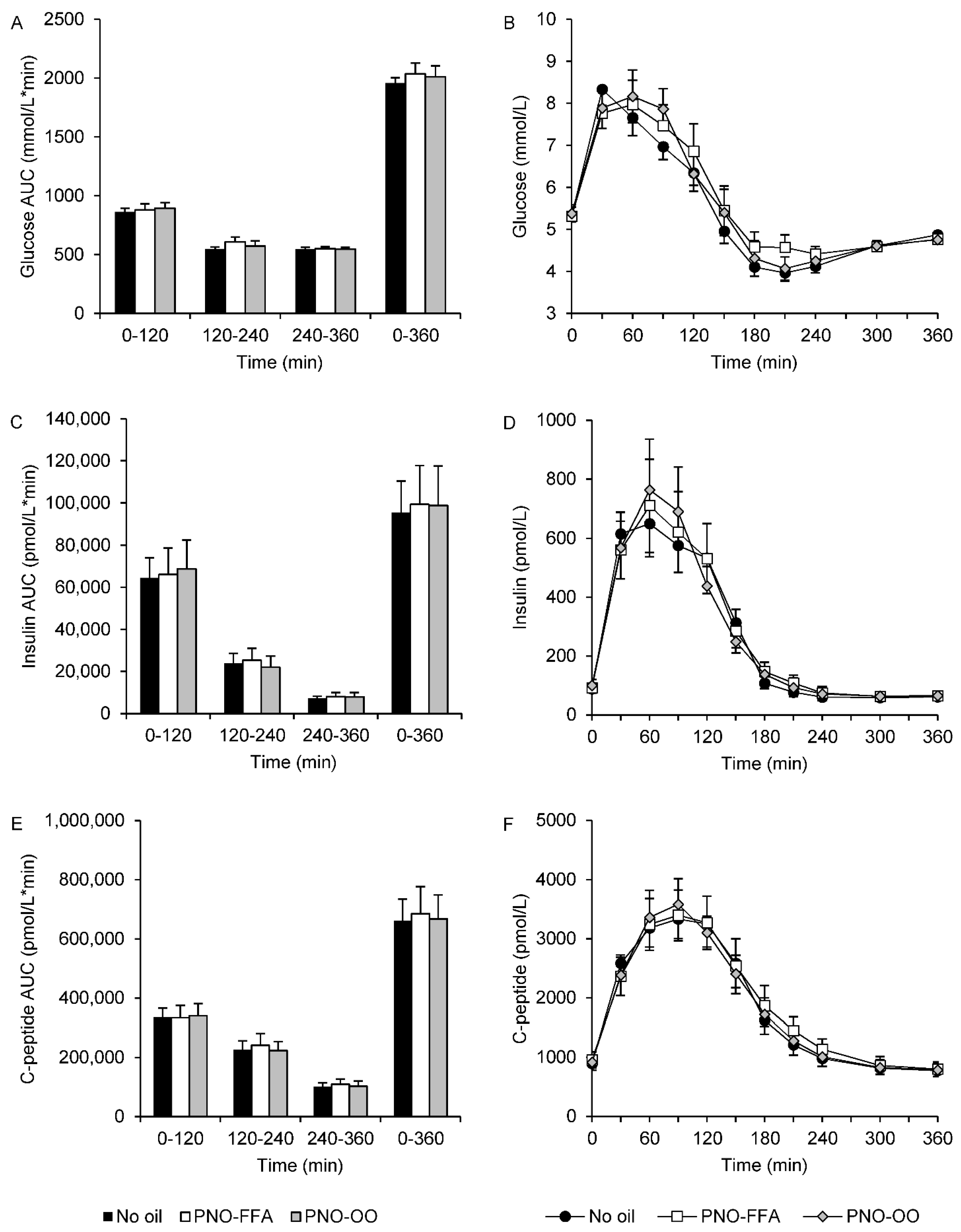
3.1. Glucose, Insulin and C-Peptide
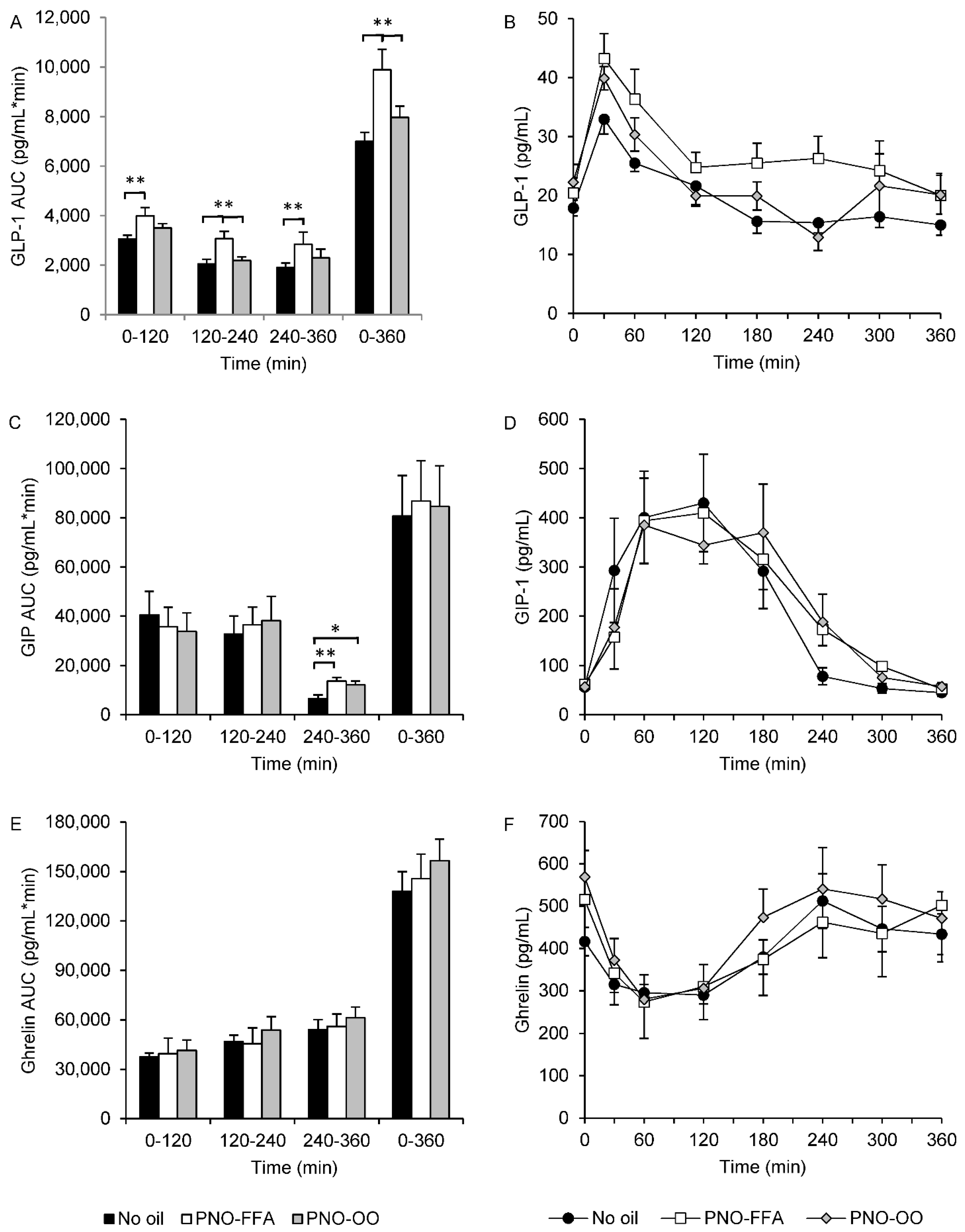
3.2. Hormone Secretion of GLP-1, GIP and Ghrelin
3.3. FFA
3.4. Insulin Sensitivity and Beta-Cell Function
3.5. Subjective Appetite
3.6. Gastrointestinal Tolerability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christiansen, E.; Watterson, K.R.; Stocker, C.J.; Sokol, E.; Jenkins, L.; Simon, K.; Grundmann, M.; Petersen, R.K.; Wargent, E.; Hudson, B.; et al. Activity of dietary fatty acids on FFA1 and FFA4 and characterisation of pinolenic acid as a dual FFA1/FFA4 agonist with potential effect against metabolic diseases. Br. J. Nutr. 2015, 113, 1677–1688. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.W.; Kim, H.S.; Choi, Y.; Kim, Y.; Kang, K.W. Therapeutic application ofGPR119ligands in metabolic disorders. Diabetes Obes. Metab. 2017, 20, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.S.; Rosenkilde, M.M.; Holst, J.J.; Schwartz, T.W. GPR119 as a fat sensor. Trends Pharmacol. Sci. 2012, 33, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Edfalk, S.; Steneberg, P.; Edlund, H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes 2008, 57, 2280–2287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirasawa, A.; Tsumaya, K.; Awaji, T.; Katsuma, S.; Adachi, T.; Yamada, M.; Sugimoto, Y.; Miyazaki, S.; Tsujimoto, G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 2004, 11, 90–94. [Google Scholar] [CrossRef]
- Iwasaki, K.; Harada, N.; Sasaki, K.; Yamane, S.; Iida, K.; Suzuki, K.; Hamasaki, A.; Nasteska, D.; Shibue, K.; Joo, E.; et al. Free Fatty Acid Receptor GPR120 Is Highly Expressed in Enteroendocrine K Cells of the Upper Small Intestine and Has a Critical Role in GIP Secretion After Fat Ingestion. Endocrinology 2015, 156, 837–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekberg, J.H.; Hauge, M.; Kristensen, L.V.; Madsen, A.N.; Engelstoft, M.S.; Husted, A.S.; Sichlau, R.; Egerod, K.L.; Timshel, P.; Kowalski, T.J.; et al. GPR119, a Major Enteroendocrine Sensor of Dietary Triglyceride Metabolites Coacting in Synergy with FFA1 (GPR40). Endocrinology 2016, 157, 4561–4569. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20 (Suppl. 1), 5–21. [Google Scholar] [CrossRef]
- Andersen, A.; Lund, A.; Knop, F.K.; Vilsbøll, T. Glucagon-like peptide 1 in health and disease. Nat. Rev. Endocrinol. 2018, 14, 390–403. [Google Scholar] [CrossRef]
- Itoh, Y.; Kawamata, Y.; Harada, M.; Kobayashi, M.; Fujii, R.; Fukusumi, S.; Ogi, K.; Hosoya, M.; Tanaka, Y.; Uejima, H.; et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature 2003, 422, 173–176. [Google Scholar] [CrossRef]
- Chu, Z.L.; Jones, R.M.; He, H.; Carroll, C.; Gutierrez, V.; Lucman, A.; Moloney, M.; Gao, H.; Mondala, H.; Bagnol, D.; et al. A role for beta-cell-expressed G protein-coupled receptor 119 in glycemic control by enhancing glucose-dependent insulin release. Endocrinology 2007, 148, 2601–2609. [Google Scholar] [CrossRef]
- Kaku, K.; Enya, K.; Nakaya, R.; Ohira, T.; Matsuno, R. Efficacy and safety of fasiglifam (TAK-875), a G protein-coupled receptor 40 agonist, in Japanese patients with type 2 diabetes inadequately controlled by diet and exercise: A randomized, double-blind, placebo-controlled, phase III trial. Diabetes Obes. Metab. 2015, 17, 675–681. [Google Scholar] [CrossRef] [Green Version]
- Engelstoft, M.S.; Park, W.-M.; Sakata, I.; Kristensen, L.V.; Husted, A.S.; Osborne-Lawrence, S.; Piper, P.K.; Walker, A.K.; Pedersen, M.H.; Nøhr, M.K.; et al. Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol. Metab. 2013, 2, 376–392. [Google Scholar] [CrossRef]
- Mihalache, L.; Gherasim, A.; Niță, O.; Ungureanu, M.C.; Pădureanu, S.S.; Gavril, R.S.; Arhire, L.I. Effects of ghrelin in energy balance and body weight homeostasis. Hormones 2016, 15, 186–196. [Google Scholar] [CrossRef] [Green Version]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 Is an Omega-3 Fatty Acid Receptor Mediating Potent Anti-inflammatory and Insulin-Sensitizing Effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, D.Y.; Walenta, E.; Akiyama, T.E.; Lagakos, W.S.; Lackey, D.; Pessentheiner, A.; Sasik, R.; Hah, N.; Chi, T.J.; Cox, J.M.; et al. A Gpr120-selective agonist improves insulin resistance and chronic inflammation in obese mice. Nat. Med. 2014, 20, 942–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sørensen, K.V.; Korfitzen, S.S.; Kaspersen, M.H.; Ulven, E.R.; Ekberg, J.H.; Bauer-Brandl, A.; Ulven, T.; Højlund, K. Acute effects of delayed-release hydrolyzed pine nut oil on glucose tolerance, incretins, ghrelin and appetite in healthy humans. Clin. Nutr. 2021, 40, 2169–2179. [Google Scholar] [CrossRef] [PubMed]
- Mandøe, M.J.; Hansen, K.B.; Windeløv, J.A.; Knop, F.K.; Rehfeld, J.F.; Rosenkilde, M.M.; Holst, J.J.; Hansen, H.S. Comparing olive oil and C4-dietary oil, a prodrug for the GPR119 agonist, 2-oleoyl glycerol, less energy intake of the latter is needed to stimulate incretin hormone secretion in overweight subjects with type 2 diabetes. Nutr. Diabetes 2018, 8, 2. [Google Scholar] [CrossRef]
- Hansen, K.B.; Rosenkilde, M.M.; Knop, F.K.; Wellner, N.; Diep, T.A.; Rehfeld, J.F.; Andersen, U.B.; Holst, J.J.; Hansen, H.S. 2-Oleoyl Glycerol Is a GPR119 Agonist and Signals GLP-1 Release in Humans. J. Clin. Endocrinol. Metab. 2011, 96, E1409–E1417. [Google Scholar] [CrossRef] [Green Version]
- Mandoe, M.J.; Hansen, K.B.; Hartmann, B.; Rehfeld, J.F.; Holst, J.J.; Hansen, H.S. The 2-monoacylglycerol moiety of dietary fat appears to be responsible for the fat-induced release of GLP-1 in humans. Am. J. Clin. Nutr. 2015, 102, 548–555. [Google Scholar] [CrossRef]
- Flint, A.; Raben, A.; Blundell, J.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. 2000, 24, 38–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bengtsson, M.; Persson, J.; Sjölund, K.; Ohlsson, B. Further Validation of the Visual Analogue Scale for Irritable Bowel Syndrome after Use in Clinical Practice. Gastroenterol. Nurs. 2013, 36, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.; Drivsholm, T.; Urhammer, S.A.; Palacios, R.T.; Vølund, A.; Borch-Johnsen, K.; Pedersen, O. The BIGTT test: A novel test for simultaneous measurement of pancreatic beta-cell function, insulin sensitivity, and glucose tolerance. Diabetes Care 2007, 30, 257–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, W.J.; Erkelens, D.W.; Reitsma, W.D.; Doorenbos, H. Glucose tolerance and insulin release, a mathematical approach I. Assay of the beta-cell response after oral glucose loading. Diabetes 1976, 25, 241–244. [Google Scholar] [CrossRef]
- ProductDescription_DRcaps™. A Matter of Timing. Ensure Optimal Delivery for Acid-Sensitive Products. 2016. Available online: https://s3.amazonaws.com/cpsl-web/kc/library/c1a-32029_DRCaps-A4_FIN.PDF (accessed on 22 January 2019).
- Hughes, G.M.; Boyland, E.J.; Williams, N.J.; Mennen, L.; Scott, C.; Kirkham, T.C.; Harrold, J.A.; Keizer, H.G.; Halford, J.C. The effect of Korean pine nut oil (PinnoThin) on food intake, feeding behaviour and appetite: A double-blind placebo-controlled trial. Lipids Health Dis. 2008, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Pasman, W.J.; Heimerikx, J.; Rubingh, C.M.; Berg, R.V.D.; O’Shea, M.; Gambelli, L.; Hendriks, H.F.; Einerhand, A.W.; Scott, C.; Keizer, H.G.; et al. The effect of Korean pine nut oil on in vitro CCK release, on appetite sensations and on gut hormones in post-menopausal overweight women. Lipids Health Dis. 2008, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Curry, S.H.; Whelpton, R. Appendix 1. Mathematical Consepts and the Trapezoidal Method. In Introduction to Drug Disposition and Pharmacokinetics; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 293–299. [Google Scholar]
- Carr, R.D.; Larsen, M.O.; Winzell, M.S.; Jelic, K.; Lindgren, O.; Deacon, C.F.; Ahren, B. Incretin and islet hormonal responses to fat and protein ingestion in healthy men. Am. J. Physiol. Metab. 2008, 295, E779–E784. [Google Scholar] [CrossRef]
- Gentilcore, D.; Chaikomin, R.; Jones, K.; Russo, A.; Feinle-Bisset, C.; Wishart, J.M.; Rayner, C.K.; Horowitz, M. Effects of Fat on Gastric Emptying of and the Glycemic, Insulin, and Incretin Responses to a Carbohydrate Meal in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2006, 91, 2062–2067. [Google Scholar] [CrossRef]
- Engelstoft, M.; Schwartz, T. Opposite Regulation of Ghrelin and Glucagon-like Peptide-1 by Metabolite G-Protein-Coupled Receptors. Trends Endocrinol. Metab. 2016, 27, 665–675. [Google Scholar] [CrossRef]
| Age (Years) | 54 ± 8 |
| Sex, m/f (n) | 4/5 |
| Height (cm) | 174 ± 9 |
| Weight (kg) | 105 ± 21 |
| BMI (kg/m2) | 34.4 ± 4.2 |
| Fat mass (kg) | 42 ± 10 |
| Fat free mass (kg) | 61 ± 17 |
| Fat % | 41 ± 8 |
| Systolic blood pressure (mmHg) | 135 ± 13 |
| Diastolic blood pressure (mmHg) | 85 ± 5 |
| Plasma glucose (mmol/L) | 5.3 ± 0.4 |
| Creatinine (umol/L) | 77 ± 10 |
| Triglycerides (mmol/L) | 1.2 ± 0.3 |
| HDL-cholesterol (mmol/L) | 1.5 ± 0.4 |
| LDL-cholesterol (mmol/L) | 3.1 ± 0.5 |
| Total cholesterol (mmol/L) | 5.1 ± 0.6 |
| HOMA-IR * | 3.0 ± 1.4 |
| No Oil | PNO-FFA | PNO-OO | |
|---|---|---|---|
| Insulin sensitivity | |||
| BIGTTSi | 3.7 ± 0.9 | 4.0 ± 1.1 | 4.0 ± 0.8 |
| MATSUDA | 3.7 ± 0.8 | 4.0 ± 1.0 | 3.5 ± 0.7 |
| Beta-cell function | |||
| BIGTTAIR | 4362 ± 641 | 4807 ± 1023 | 4847 ± 1109 |
| CIR30 min | 1791 ± 235 | 2026 ± 336 | 1760 ± 286 |
| No Oil | PNO-FFA | PNO-OO | |
|---|---|---|---|
| Satiety | |||
| AUC−30–120 min | 5261 ± 303 1 | 6183 ± 449 | 5917 ± 622 |
| AUC120–240 min | 2733 ± 359 | 3220 ± 285 | 3785 ± 511 |
| AUC240–360 min | 1527 ± 318 | 2297 ± 358 | 2260 ± 449 |
| AUCtotal | 9364 ± 789 1 | 11,700 ± 822 * | 11,962 ± 1390 * |
| Hunger | |||
| AUC−30–120 min | 9087 ± 137 1 | 7448 ± 509 * | 8586 ± 495 |
| AUC120–240 min | 8202 ± 529 | 8113 ± 442 | 8052 ± 429 |
| AUC240–360 min | 9690 ± 474 | 9123 ± 435 * | 9157 ± 477 * |
| AUCtotal | 27,380 ± 878 1 | 24,684 ± 1027 * | 25,794 ± 1123 * |
| Fullness | |||
| AUC−30–120 min | 4784 ± 654 1 | 5487 ± 787 | 5343 ± 874 |
| AUC120–240 min | 2268 ± 363 | 2782 ± 459 | 3245 ± 687 * |
| AUC240–360 min | 1317 ± 279 | 1920 ± 376 * | 1930 ± 521 * |
| AUCtotal | 8499 ± 1380 1 | 10,188 ± 1451 | 10,518 ± 1965 * |
| Prospective food consumption | |||
| AUC−30–120 min | 9815 ± 560 1 | 8082 ± 759 * | 8819 ± 753 * |
| AUC120–240 min | 9098 ± 436 | 8348 ± 501 * | 8387 ± 603 * |
| AUC240–360 min | 9797 ± 496 | 8993 ± 630 * | 9227 ± 687 * |
| AUCtotal | 28,850 ± 1442 1 | 25,423 ± 1658 * | 26,433 ± 1847 * |
| Overall well-being | |||
| AUC−30–120 min | 12,111 ± 877 1 | 10,256 ± 1187 | 11,184 ± 1073 |
| AUC120–240 min | 9150 ± 676 | 8095 ± 953 * | 9022 ± 769 |
| AUC240–360 min | 8486 ± 793 | 8070 ± 918 | 9000 ± 713 |
| AUCtotal | 30,004 ± 1993 1 | 26,421 ± 2981 | 29,206 ± 2496 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sørensen, K.V.; Kaspersen, M.H.; Ekberg, J.H.; Bauer-Brandl, A.; Ulven, T.; Højlund, K. Effects of Delayed-Release Olive Oil and Hydrolyzed Pine Nut Oil on Glucose Tolerance, Incretin Secretion and Appetite in Humans. Nutrients 2021, 13, 3407. https://doi.org/10.3390/nu13103407
Sørensen KV, Kaspersen MH, Ekberg JH, Bauer-Brandl A, Ulven T, Højlund K. Effects of Delayed-Release Olive Oil and Hydrolyzed Pine Nut Oil on Glucose Tolerance, Incretin Secretion and Appetite in Humans. Nutrients. 2021; 13(10):3407. https://doi.org/10.3390/nu13103407
Chicago/Turabian StyleSørensen, Karina V., Mads H. Kaspersen, Jeppe H. Ekberg, Annette Bauer-Brandl, Trond Ulven, and Kurt Højlund. 2021. "Effects of Delayed-Release Olive Oil and Hydrolyzed Pine Nut Oil on Glucose Tolerance, Incretin Secretion and Appetite in Humans" Nutrients 13, no. 10: 3407. https://doi.org/10.3390/nu13103407
APA StyleSørensen, K. V., Kaspersen, M. H., Ekberg, J. H., Bauer-Brandl, A., Ulven, T., & Højlund, K. (2021). Effects of Delayed-Release Olive Oil and Hydrolyzed Pine Nut Oil on Glucose Tolerance, Incretin Secretion and Appetite in Humans. Nutrients, 13(10), 3407. https://doi.org/10.3390/nu13103407





