Abstract
Insulin resistance is a risk of sarcopenia, and the presence of sarcopenia is high in patients with type 2 diabetes (T2DM). It has been reported that habitual miso soup consumption was associated with lower insulin resistance. However, the association between habitual miso consumption and the presence of sarcopenia in patients with T2DM, especially sex difference, was unclear. In this cross-sectional study, 192 men and 159 women with T2DM were included. Habitual miso consumption was defined as consuming miso soup regularly. Having both low skeletal muscle mass index (<28.64% for men, <24.12% for women) and low adjusted hand grip strength (<51.26% for men, <35.38% for women) was defined as sarcopenia. The proportions of sarcopenia were 8.7% in men and 22.6% in women. The proportions of habitual miso consumption were 88.0% in men and 83.6% in women. Among women, the presence of sarcopenia was lower in the group with habitual miso consumption (18.8% versus 42.3%, p = 0.018); however, there was no association between habitual miso consumption and the presence of sarcopenia in men. Habitual miso consumption was negatively associated with the presence of sarcopenia in women (adjusted odds ratio (OR), 0.20 (95% confidence interval (CI): 0.06–0.62), p = 0.005) but not in men. This study indicated that habitual miso consumption was associated with the presence of sarcopenia in women but not in men.
1. Introduction
The number of older patients with type 2 diabetes (T2DM) in Japan is increasing, since the aging of the adult population is increasing [1]. These patients often complicate with sarcopenia, which is characterized as loss of muscle mass, power, and function [2]. In the last decade, sarcopenia has been reported as a risk factor for fatty liver, cardiovascular diseases, and death [3,4,5]. In insulin-resistant conditions including T2DM, insulin-stimulated glucose disposal is severely impaired in the skeletal muscle [6]. Insulin resistance and oxidative stress are reported to be a cause of sarcopenia [7,8,9], and they are related to vascular modification [10], chronic inflammation [11], and lipid inflation in muscles [12,13]. Moreover, low skeletal muscle mass, defined as the weight-adjusted absolute muscle mass, has been described as a risk factor for T2DM [14] and to be associated with insulin sensitivity [15]. Furthermore, low handgrip strength, defined as weight-adjusted handgrip strength, has been described as a risk factor for T2DM [16]. Therefore, it is useful to determine the percentage of muscle mass or handgrip strength per body weight in patients with diabetes.
Miso soup is a traditional Japanese dish, made from a fermented soybean food called miso, and is consumed widely in Japan. It includes vitamins, minerals, vegetable proteins, microorganisms, salts, carbohydrates, and fat [17]. A previous study has revealed that miso can suppress the development of gastric, colon, liver, and lung tumors and abnormal crypt foci in mice and rats [18]. Despite its high salt content, miso was reported to prevent the induction of hypertension in Dahl salt-sensitive hypertensive rats [17]. Previous studies have revealed that miso intake has a protective effect against hypertension in Japanese individuals without hypertension [19,20]. In addition, habitual miso soup consumption is associated with lower insulin resistance [21,22]. Moreover, a recent study from Japan revealed that the intake of fermented soy products including miso, rather than soy products, is related to a lower risk of total mortality [23]. Furthermore, miso intake suppresses visceral fat accumulation and hepatic lipid deposits in mice [24]. Soybeans, which are the main ingredient of miso, contain bioactive substances such as isoflavones and dietary fiber, and are useful in the prevention of various diseases [25,26,27]. In addition, fermented soybean products contain more bioactive ingredients and nutrients than nonfermented soybean products [28,29]. Therefore, nutrients and bioactive components in fermented soybean products may have multifaceted benefits for health, including survival. However, no previous studies investigated the association between habitual miso consumption and sarcopenia in patients with T2DM. Furthermore, the effect of sex of the patient on this association is also an important component that has not been clarified yet. Therefore, this cross-sectional study researched the relationship between habitual miso consumption and sarcopenia in men and women with T2DM, separately.
2. Materials and Methods
2.1. Study Participants
The KAMOGAWA-DM cohort study, a prospective cohort study, started in 2014 and is currently ongoing [30]. In this cross-sectional study, patients with data of questionnaires from January 2016 to December 2018 were included. Exclusion criteria were as follows: no data of multifrequency impedance body composition analyzer, no data of hand grip strength, incomplete questionnaires, and patients without T2DM. This study was approved by the local research ethics committee (No. RBMR-E-466-6) and was carried out in accordance with the Declaration of Helsinki. All patients gave written informed consent.
2.2. Data Collection
Duration of diabetes, family history of diabetes, smoking status, and exercise habit were asked by a standardized questionnaire. The patients were divided into smokers and nonsmokers. We defined regular exercisers as patients who regularly played any type of sport >1×/week. Further, venous blood was collected from the participants who had fasted overnight, and the levels of triglycerides, high-density lipoprotein cholesterol, creatinine (Cr), uric acid, and fasting plasma glucose were measured. Estimated glomerular filtration rate (eGFR) was calculated using the equation of the Japanese Society of Nephrology, that is, eGFR = 194 × Cr-1.094 × age-0.287 (mL/min/1.73 m2) (×0.739, if woman) [31]. Hemoglobin A1c (HbA1c) levels were estimated using high-performance liquid chromatography and were expressed as National Glycohemoglobin Standardization Program (NGSP) units. After the participants had 5 min of rest, blood pressure was measured by a HEM-906 device (OMRON, Kyoto, Japan) in a quiet space, automatically. Additionally, the data on medications, including those for diabetes and hypertension (renin–angiotensin–aldosterone inhibitor) were obtained from the patients’ medical records.
2.3. Definition of Sarcopenia
Using InBody 720 (InBody, Japan, Tokyo, Japan), a multifrequency impedance body composition analyzer, the data of body weight (kg), appendicular muscle mass (kg), and body fat mass (kg) were gathered [32]. Then, body mass index (BMI, kg/m2), dividing body weight (kg) by the square of height (m) and ideal body weight (IBW), 22 multiplied by the square of the patient’s height in meters squared [33], were calculated. Percent body fat mass (%), dividing body fat mass (kg) by body weight (kg) × 100 and skeletal muscle mass index (SMI, %), appendicular muscle mass divided by body weight × 100 [34] for muscle mass were also calculated. Hand grip strength (HGS) was measured twice with each hand on a handgrip dynamometer (Smedley; Takei Scientific Instruments, Niigata, Japan), and the highest value was recorded. Adjusted grip strength (AGS) was calculated as HGS value divided by body weight × 100 [35] for muscle strength. The cutoff points for low muscle mass and low muscle strength were 28.64% and 51.26% in men and 24.12% and 35.38% in women [36]. Having both low muscle mass and low muscle strength was defined as sarcopenia [36].
2.4. Data of Habitual Diet Intake, Including Habitual Miso Consumption
Using a brief-type self-administered diet history questionnaire (BDHQ), habitual food and nutrient intake during the preceding 1 month period were evaluated [37]. The details and validity of BDHQ have been presented previously [38]. Patient data on the intakes of energy, fat, carbohydrate, protein, including animal and vegetable proteins, fiber, miso soup consumption, and alcohol consumption were obtained by BDHQ. Energy intake (kcal/kg IBW/day), protein intake (g/kg IBW/day), animal protein intake (g/kg IBW/day), vegetable protein intake (g/kg IBW/day), fat intake (g/kg IBW/day), and carbohydrate intake (g/kg IBW/day) were calculated. The ratio of carbohydrate to fiber intake was defined as carbohydrate intake divided by fiber intake [39]. In addition, the data on the intake frequency of miso soup were also collected and habitual miso consumption was defined as consuming miso soup regularly.
2.5. Statistical Analysis
The data are shown as means (standard deviation (SD)) or frequencies of potential confounding variables. Because the characteristics and dietary intakes differed between men and women, the data was analyzed by sex.
Patients were divided into two groups according to habitual miso consumption. We performed a Shapiro–Wilk test to investigate the distribution of variables. The differences in the continuous variables and categorical variables were evaluated by Student’s t-test or the Mann–Whitney U test and the chi-square test, respectively. The correlation was analyzed by Pearson’s correlation coefficient.
Further, to examine the effects of habitual miso consumption on the prevalence of sarcopenia, a logistic regression analysis was performed. The independent variables were sex, age, insulin treatment, habit of exercise, habit of smoking, duration of diabetes, HbA1c, energy intake, protein intake, and alcohol consumption. In addition, we performed a logistic regression analysis to examine the effect of miso soup intake, as a continuous variable, on the prevalence of sarcopenia. The independent variables were sex, age, insulin treatment, habit of exercise, habit of smoking, duration of diabetes, HbA1c, energy intake, protein intake, and alcohol consumption. Because miso soup intake was a skewed variable, logarithmic transformation was done before performing a logistic regression analysis, which were performed to evaluate the association of sarcopenia with log (miso soup intake + 1).
We performed subanalyses to examine the effects of habitual miso soup consumption on the prevalence of sarcopenia adjusted by age, according to the presence or absence of habit of smoking.
We used EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [40] for statistical analyses. Differences with p values < 0.05 were set as statistically significant.
3. Results
In the present study, 523 patients (276 men and 247 women) were included. Among them, 109 patients (50 men and 59 women) who did not undergo the bioelectrical impedance analysis (BIA) test, 24 patients with no date of BDHQ (15 men and 9 women), a patient who did not undergo the hand grip strength test, and 38 patients without T2DM (19 men and 19 women) were excluded from the study. Therefore, 351 patients (192 men and 159 women) were the study participants (Figure 1).
Figure 1.
Inclusion and exclusion flow. BDHQ, Brief-type self-administered diet history questionnaire; T2DM, type 2 diabetes mellitus.
Table 1 shows the clinical characteristics of the study participants. Mean age was 66.6 ± 10.6 years in all subjects. Mean BMI was 23.9 ± 3.7 kg/m2 in men and 25.1 ± 5.1 kg/m2 in women. Mean SMI and AGS were 31.7 ± 3.2% and 50.7 ± 10.1% in men and 25.7 ± 2.9% and 36.1 ± 9.0% in women, respectively. The proportions of men and women with sarcopenia were 8.7% (n = 22/192) and 22.6% (n = 36/159), respectively, and the proportion of women with sarcopenia was significantly higher than that of men with sarcopenia (p = 0.008).

Table 1.
Clinical characteristics of study participants.
Table 2 shows the results of the data of dietary intake. The percentages of habitual miso consumption were 88.0% (n = 169/192) and 83.6% (n = 133/159) in men and women, respectively.

Table 2.
Habitual diet intake of study participants.
Table 3 shows the clinical characteristics of patients according to habitual miso consumption. Both in men (24.4 ± 7.2% vs. 27.4 ± 5.6%, p = 0.056) and women (33.8 ± 8.0% vs. 39.3 ± 7.3%, p = 0.001), patients with habitual miso consumption had a lower percentage of body fat than those without it. Among women, patients with habitual miso consumption had lower BMI (24.7 ± 5.0 kg/m2 vs. 27.3 ± 5.0 kg/m2, p = 0.022) and lower HbA1c levels (7.2 ± 1.0% vs. 8.1 ± 1.9%, p = 0.006) than those without it. In addition, in women with habitual miso consumption, the proportions of patients with low skeletal muscle mass (35/133 (26.3%) vs. 13/26 (50.0%), p = 0.024), low muscle strength (60/133 (45.1%) vs. 19/26 (73.1%), p = 0.009), and sarcopenia (25/133 (18.8%) vs. 11/26 (42.3%), p = 0.018) were lower than those in women without habitual miso consumption.

Table 3.
Clinical characteristics according to habitual miso consumption.
Table 4 shows the habitual diet intakes according to the presence of habitual miso consumption. In women, the patients with habitual miso consumption had higher intakes of energy (30.8 ± 11.2 kg/IBW kg/day vs. 23.5 ± 6.3 kg/IBW kg/day, p < 0.001), protein (1.4 ± 0.6 g/IBW kg/day vs. 1.0 ± 0.3 g/IBW kg/day, p < 0.001), animal protein (0.9 ± 0.5 g/IBW kg/day vs. 0.6 ± 0.3 g/IBW kg/day, p = 0.006), vegetable protein (0.5 ± 0.2 g/IBW kg/day vs. 0.4 ± 0.1 g/IBW kg/day, p < 0.001), fat (1.0 ± 0.5 g/IBW kg/day vs. 0.8 ± 0.3 g/IBW kg/day, p = 0.006), carbohydrate (3.9 ± 1.4 g/IBW kg, day vs. 3.0 ± 0.8 g/IBW kg/day, p = 0.003), and dietary fiber (12.4 ± 4.6 g/day vs. 8.1 ± 2.9 g/day, p < 0.001) and lower carbohydrate-to-fiber ratios (17.0 ± 5.6 vs. 20.1 ± 6.6, p = 0.028) in their diets than those without habitual miso consumption.

Table 4.
Habitual diet intake according to habitual miso consumption.
Figure 2 shows the correlation between the log (miso soup intake + 1) and skeletal muscle mass or muscle strength. In women, the log (miso soup intake + 1) was correlated with skeletal muscle mass index (r = 0.25, p = 0.003) and adjusted grip strength (r = 0.17, p = 0.030). However, the log (miso soup intake + 1) was not correlated with skeletal muscle mass index (r = 0.14, p = 0.079) and adjusted grip strength (r = 0.14, p = 0.061) in men.
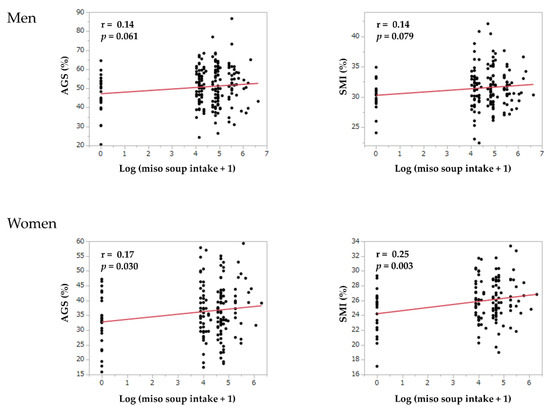
Figure 2.
The correlation between miso soup intake and skeletal muscle mass or muscle strength. AGS, adjusted grip strength; log, logarithm; SMI, skeletal muscle mass index.
Furthermore, habitual miso consumption was negatively related to the presence of sarcopenia (adjusted OR, 0.20 (95% CI: 0.06–0.62), p = 0.005) in women, whereas this association was absent in men (adjusted OR, 1.11 (95% CI: 0.27–4.57), p = 0.882) (Table 5).

Table 5.
Odds ratio of habitual miso consumption on the presence of sarcopenia.
The log (miso soup intake + 1) was negatively related to the presence of sarcopenia (adjusted OR, 0.67 (95% CI: 0.52–0.86), p = 0.002) in women, whereas this association was absent in men (adjusted OR, 1.01 (95% CI: 0.76–1.34), p = 0.952) (Table 6).

Table 6.
Odds ratio of the quantity of miso soup intake on the presence of sarcopenia.
In men, the association between habitual miso consumption and the presence of sarcopenia was absent in men both with (adjusted OR, 0.68 (95% CI: 0.02–29.90), p = 0.839), and without a habit of smoking (adjusted OR, 1.26 (95% CI: 0.27–5.98), p = 0.768). On the other hand, in women without a habit of smoking, habitual miso consumption was negatively related to the presence of sarcopenia (adjusted OR, 0.36 (95% CI: 0.14–0.94), p = 0.036), whereas habitual miso consumption was not associated with the presence of sarcopenia in women with a habit of smoking (adjusted OR, 0.07 (95% CI: 0.00–3.70), p = 0.187).
4. Discussion
We firstly investigated the relationship between habitual miso consumption and the presence of sarcopenia in patients with T2DM in this study. We revealed that habitual miso consumption was associated with a low prevalence of sarcopenia in women but not in men. Additionally, habitual miso consumption was associated with lower percentage of body fat mass in women.
The possible explanation for the association between habitual miso consumption and the low prevalence of sarcopenia is as follows. A previous study has shown that miso consumption suppresses fat accumulation in fat mass and showed anti-obesity effects [24]. Furthermore, there is a close association between increased visceral fat and muscle atrophy [41,42]. Secretion of proinflammatory cytokines such as interleukin-6 and tumor necrosis factor-α from obesity-induced hypertrophic fat cells causes muscle atrophy [43]. The consumption of miso soup every day is related to lower insulin resistance in women [21]. In fact, the percent body fat mass was lower in women with habitual miso consumption than in those without it, in this study. Furthermore, miso is a product made of fermented soybeans. Soybeans are rich in soybean-specific proteins (glycinin and conglycinin), vitamin E, isoflavones (daidzein, daidzin, genistein, and glycitein), lipids rich in polyunsaturated fatty acids, lecithin, and saponin [17,44,45]. Miso also contains pyroglutamyl leucine [46], which is spontaneously generated from peptides with a glutaminyl residue at the amino terminal, during storage and processing [47]. Pyroglutamyl leucine was found to improve gut dysbiosis and colitis in mice [46,48,49] and, in fact, suppresses the excess proliferation of phylum Firmicutes, which is associated with obesity [49]. The main ingredient of miso is soybeans. Soybeans contain high amounts of omega-3 and omega-6 fatty acids [50]. There is a negative correlation between omega-3 fatty acids intake and the presence of sarcopenia in elderly patients with T2DM [51]. In addition, both omega-3 and omega-6 intake is known to be important for muscle maintenance [52]. Moreover, soybean proteins and isoflavones are known to reduce accumulation of visceral fat mass in animal models [53,54]. Isoflavones have a structure similar to that of estradiol and have a high binding ability to the primary estrogen receptor in the vascular wall [55]. Therefore, isoflavones in miso may inhibit the accumulation of visceral fat and the loss of muscle mass and strength in women.
Another possible mechanism is the association between habitual miso consumption and diet quality. In this study, women without habitual miso consumption had lower energy intakes than those with habitual miso consumption. Moreover, compared to those with habitual miso consumption, the diets of women without habitual miso consumption showed higher carbohydrate-to-fiber ratios, which has a reported association with metabolic syndrome [39] and visceral fat accumulation [56]. Thus, women without habitual miso consumption may have had poor diet qualities, which may have contributed to obesity and sarcopenia. Adherence to a dietary pattern rich in foods characteristic of the Japanese diet is associated with a lower prevalence of sarcopenia [57]. Miso soup is well-known as a traditional Japanese food. Therefore, the consumption of miso soup contributes to the traditional Japanese diet, suggesting that women with habitual miso consumption have a lower prevalence of sarcopenia. On the other hand, habitual miso consumption was not associated with the presence of sarcopenia in men. However, it is unclear why there is no association between miso consumption and sarcopenia in men. It is probably because there was no association between habitual miso consumption and diet quality in men. In fact, a previous study revealed an association between habitual miso soup consumption and insulin resistance in women but not in men [21].
In women without a habit of smoking, habitual miso consumption was associated with the presence of sarcopenia, but in women with a habit of smoking, habitual miso consumption was not associated with the presence of sarcopenia. This is due to the high oxidative stress caused by smoking [58]. In fact, the habit of smoking is known to be a risk factor of sarcopenia [59].
However, our study had certain limitations. First, the data regarding the frequency of miso soup consumption were self-reported, and there were some concerns regarding the accuracy of the data. Second, because of a cross-sectional study, it was not possible to show the causal relationship. Third, the sample size of this study was relatively small. Fourth, women without habitual miso consumption might have poor quality of diet, thus, we cannot deny the possibility that diet quality contributed to this result. Finally, all study participants were Japanese patients only; hence, it is unclear whether the results can be generalized to other ethnic groups.
In conclusion, this study firstly revealed that habitual miso consumption was associated with a low presence of sarcopenia in women but not in men. Although further research is needed, miso soup is a part of the traditional Japanese diet, and it has been suggested that consuming miso soup may help prevent sarcopenia.
Author Contributions
Conception: Y.H., A.K., R.S. and M.F.; design of the work: F.T., Y.H., A.K., R.S. and M.H. data acquisition: Y.H., A.K., R.S., Y.K., T.O., E.U., N.K., S.M., T.S., H.O., N.N., M.H., M.A., M.Y., and M.F.; data analysis: F.T, and Y.H.; interpretation of data: F.T, and Y.H.; writing the manuscript: F.T.; revising the manuscript: Y.H. and M.F.; contributed discussion: A.K., R.S., Y.K., T.O., E.U., N.K., S.M., T.S., H.O., N.N., M.H., M.A., and M.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI, Grant-in-Aid for Young Scientists, Grant Number 19K20187.
Institutional Review Board Statement
The study was conducted to the guidelines of the Declaration of Helsinki, and approved by the local research ethics committee (No. RBMR-E-466-6).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to restrictions eg privacy or ethical.
Conflicts of Interest
Hashimoto reports grants from Asahi Kasei Pharma and personal fees from Sanofi K.K., Mitsubishi Tanabe Pharma Corp., Daiichi Sankyo Co. Ltd., and Novo Nordisk Pharma Ltd. outside the submitted work. Ushigome received grant support from the Japanese Study Group for Physiology and Management of Blood Pressure, and the Astellas Foundation for Research on Metabolic Disorders and donated fund Laboratory of Diabetes therapeutics is an endowment department, supported with an unrestricted grant from Ono Pharma. Co., Ltd., and received personal fees from Mitsubishi Tanabe Pharma Corp., MSD K.K., Daiichi Sankyo Co. Ltd., Sumitomo Dainippon Pharma Co. Ltd., Astellas Pharma Inc., AstraZeneca plc, Kyowa Kirin Co. Ltd., Novo Nordisk Pharma Ltd., Taisho Toyama Pharma Co., Ltd., Kowa Pharma Co. Ltd., Takeda Pharma Co., Ltd., and Nippon Boehringer Ingelheim Co. Ltd. outside the submitted work. Hamaguchi reports grants from Sanofi K.K., Takeda Pharma Co. Ltd., Novo Nordisk Pharma Ltd., Asahi Kasei Pharma, Mitsubishi Tanabe Pharma Corp., Kyowa Kirin Co. Ltd., Eli Lilly Japan K.K., Astellas Pharma Inc., Sumitomo Dainippon Pharma Co. Ltd., Nippon Boehringer Ingelheim Co. Ltd., and Daiichi Sankyo Co. Ltd. outside the submitted work. Asano reports personal fees from Novo Nordisk Pharma Ltd., Ono Pharma Co. Ltd., Abbott Japan Co. Ltd., AstraZeneca plc, Kowa Pharma Co. Ltd., Sumitomo Dainippon Pharma Co. Ltd., and Takeda Pharma Co. Ltd. outside the submitted work. Yamazaki reports personal fees from Kowa Pharma Co. Ltd., MSD K.K., Kyowa Kirin Co. Ltd., AstraZeneca plc, Takeda Pharma Co. Ltd., Daiichi Sankyo Co. Ltd., Kowa Pharma Co. Ltd., Ono Phama Co., Ltd. and Sumitomo Dainippon Pharma Co. Ltd., outside the submitted work. Fukui received grants from Taisho Pharma Co., Ltd., Ono Pharma Co. Ltd., Mitsubishi Tanabe Pharma Corp, Kissei Pharma Co. Ltd., Sumitomo Dainippon Pharma Co., Ltd., Daiichi Sankyo Co. Ltd., Nippon Boehringer Ingelheim Co. Ltd., Takeda Pharma Co. Ltd., Tejin Pharma Ltd., Nippon Chemiphar Co., Ltd., Eli Lilly Japan K.K., Johnson & Johnson k.k. MSD K.K., Sanwa Kagagu Kenkyusho Co., LtD., Sanofi K.K., Astellas Pharma Inc., Kowa Pharma Co. Ltd., Abbott japan Co. Ltd., Novo Nordisk Pharma Ltd.,Kyowa Kirin Co., Ltd., Medical Co., and Terumo Corp., and received honoraria from Nippon Boehringer Ingelheim Co., Ltd., Ono Pharma Co. Ltd., Taisho Pharma Co., Ltd., Sumitomo Dainippon Pharma Co. Ltd., Mitsubishi Tanabe Pharma Corp., Eli Lilly Japan K.K., Mochida Pharma Co. Ltd., Bayer Yakuhin, Ltd., Sanwa Kagaku Kenkyusho Co. Ltd., Astellas Pharma Inc., Sanofi K.K., AstraZeneca K.K., Novo Nordisk Pharma Ltd., Daiichi Sankyo Co. Ltd., Abbott japan Co. Ltd., Medtronic Japan Co. Ltd., Kowa Pharma Co. Ltd., Kissei Pharma Co., Ltd., Teijin Pharma Ltd., Nipro Corp. Takeda Pharma Co. Ltd., MSD K.K., Arkray Inc., and Kyowa Kirin Co. Ltd. outside the submitted work. The other authors have nothing to disclose.
References
- Charvat, H.; Goto, A.; Goto, M.; Inoue, M.; Heianza, Y.; Arase, Y.; Sone, H.; Nakagami, T.; Song, X.; Qiao, Q.; et al. Impact of population aging on trends in diabetes prevalence: A meta-regression analysis of 160,000 Japanese adults. J. Diabetes Investig. 2015, 6, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Umegaki, H. Sarcopenia and frailty in older patients with diabetes mellitus. Geriatr. Gerontol. Int. 2016, 16, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Osaka, T.; Fukuda, T.; Tanaka, M.; Yamazaki, M.; Fukui, M. The relationship between hepatic steatosis and skeletal muscle mass index in men with type 2 diabetes. Endocr. J. 2016, 63, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Kaji, A.; Sakai, R.; Hamaguchi, M.; Okada, H.; Ushigome, E.; Asano, M.; Yamazaki, M.; Fukui, M. Sarcopenia is associated with blood pressure variability in older patients with type 2 diabetes: A cross-sectional study of the KAMOGAWA-DM cohort study. Geriatr. Gerontol. Int. 2018, 18, 1345–1349. [Google Scholar] [CrossRef]
- Chin, S.O.; Rhee, S.Y.; Chon, S.; Hwang, Y.C.; Jeong, I.K.; Oh, S.; Ahn, K.J.; Chung, H.Y.; Woo, J.T.; Kim, S.W.; et al. Sarcopenia Is Independently Associated with Cardiovascular Disease in Older Korean Adults: The Korea National Health and Nutrition Examination Survey (KNHANES) from 2009. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; Defronzo, R.A. Pathogenesis of insulin resistance in skeletal muscle. J. Biomed. Biotechnol. 2010, 2010. [Google Scholar] [CrossRef]
- Khamseh, M.E.; Malek, M.; Aghili, R.; Emami, Z. Sarcopenia and diabetes: Pathogenesis and consequences. Br. J. Diabetes Vasc. Dis. 2011, 11, 230–234. [Google Scholar] [CrossRef]
- Morley, J.E.; Malmstrom, T.K.; Rodriguez-Mañas, L.; Sinclair, A.J. Frailty, Sarcopenia and Diabetes. J. Am. Med. Dir. Assoc. 2014, 15, 853–859. [Google Scholar]
- Kim, T.N.; Choi, K.M. Sarcopenia: Definition, Epidemiology, and Pathophysiology. J. Bone Metab. 2013, 20, 1–10. [Google Scholar]
- Abbatecola, A.M.; Olivieri, F.; Corsonello, A.; Strollo, F.; Fumagalli, A.; Lattanzio, F. Frailty and safety: The example of diabetes. Drug Saf. 2012, 35, 63–71. [Google Scholar]
- Pedersen, M.; Bruunsgaard, H.; Weis, N.; Hendel, H.W.; Andreassen, B.U.; Eldrup, E.; Dela, F.; Pedersen, B.K. Circulating levels of TNF-alpha and IL-6-relation to truncal fat mass and muscle mass in healthy elderly individuals and in patients with type-2 diabetes. Mech. Ageing Dev. 2003, 124, 495–502. [Google Scholar] [PubMed]
- Song, M.Y.; Ruts, E.; Kim, J.; Janumala, I.; Heymsfield, S.; Gallagher, D. Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am. J. Clin. Nutr. 2004, 79, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Delmonico, M.J.; Harris, T.B.; Visser, M.; Park, S.W.; Conroy, M.B.; Velasquez-Mieyer, P.; Boudreau, R.; Manini, T.M.; Nevitt, M.; Newman, A.B.; et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am. J. Clin. Nutr. 2009, 90, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Son, J.W.; Lee, S.S.; Kim, S.R.; Yoo, S.J.; Cha, B.Y.; Son, H.Y.; Cho, N.H. Low muscle mass and risk of type 2 diabetes in middle-aged and older adults: Findings from the KoGES. Diabetologia 2017, 60, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Takamura, T.; Kita, Y.; Nakagen, M.; Sakurai, M.; Isobe, Y.; Takeshita, Y.; Kawai, K.; Urabe, T.; Kaneko, S. Weight-adjusted lean body mass and calf circumference are protective against obesity-associated insulin resistance and metabolic abnormalities. Heliyon 2017, 3. [Google Scholar] [CrossRef]
- Brown, E.C.; Buchan, D.S.; Madi, S.A.; Gordon, B.N.; Drignei, D. Grip Strength Cut Points for Diabetes Risk Among Apparently Healthy U.S. Adults. Am. J. Prev. Med. 2020, 58, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Kashimoto, N.; Kajimura, J.; Kamiya, K. A miso (Japanese soybean paste) diet conferred greater protection against hypertension than a sodium chloride diet in Dahl salt-sensitive rats. Hypertens. Res. 2006, 29, 731–738. [Google Scholar] [CrossRef]
- Watanabe, H. Beneficial biological effects of miso with reference to radiation injury, cancer and hypertension. J. Toxicol. Pathol. 2013, 26, 91–103. [Google Scholar] [CrossRef][Green Version]
- Kanda, A.; Hoshiyama, Y.; Kawaguchi, T. Association of lifestyle parameters with the prevention of hypertension in elderly Japanese men and women: A Four-Year followup of normotensive subjects. Asia-Pac. J. Public Health 1999, 11, 77–81. [Google Scholar] [CrossRef]
- Manzoni, M.S.J.; Rossi, E.A.; Carlos, I.Z.; Vendramini, R.C.; Duarte, A.C.G.O.; Dâmaso, A.R. Fermented soy product supplemented with isoflavones affected fat depots in juvenile rats. Nutrition 2005, 21, 1018–1024. [Google Scholar] [CrossRef]
- Ikeda, K.; Sato, T.; Nakayama, T.; Tanaka, D.; Nagashima, K.; Mano, F.; Joo, E.; Fujimoto, S.; Takahashi, Y.; Kosugi, S.; et al. Dietary habits associated with reduced insulin resistance: The Nagahama study. Diabetes Res. Clin. Pract. 2018, 141, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, M.; Uemura, H.; Sakai, T.; Katsuura-Kamano, S.; Yamaguchi, M.; Hiyoshi, M.; Arisawa, K. Inverse association between soya food consumption and insulin resistance in Japanese adults. Public Health Nutr. 2015, 18, 2031–2040. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Katagiri, R.; Sawada, N.; Goto, A.; Yamaji, T.; Iwasaki, M.; Noda, M.; Iso, H.; Tsugane, S. Association of soy and fermented soy product intake with total and cause specific mortality: Prospective cohort study. BMJ 2020, 368, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Okouchi, R.; Sakanoi, Y.; Tsuduki, T. Miso (Fermented soybean paste) suppresses visceral fat accumulation in mice, especially in combination with exercise. Nutrients 2019, 11, 560. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Wada, K.; Tamura, T.; Konishi, K.; Goto, Y.; Koda, S.; Kawachi, T.; Tsuji, M.; Nakamura, K. Dietary soy and natto intake and cardiovascular disease mortality in Japanese adults: The Takayama study. Am. J. Clin. Nutr. 2017, 105, 426–431. [Google Scholar] [CrossRef]
- Taku, K.; Lin, N.; Cai, D.; Hu, J.; Zhao, X.; Zhang, Y.; Wang, P.; Melby, M.K.; Hooper, L.; Kurzer, M.S.; et al. Effects of soy isoflavone extract supplements on blood pressure in adult humans: Systematic review and meta-analysis of randomized placebo-controlled trials. J. Hypertens. 2010, 28, 1971–1982. [Google Scholar] [CrossRef]
- Taku, K.; Umegaki, K.; Sato, Y.; Taki, Y.; Endoh, K.; Watanabe, S. Soy isoflavones lower serum total and LDL cholesterol in humans: A meta-analysis of 11 randomized controlled trials. Am. J. Clin. Nutr. 2007, 85, 1148–1156. [Google Scholar] [CrossRef]
- Okamoto, A.; Sugi, E.; Koizumi, Y.; Yanagida, F.; Udaka, S. Polyamine content of ordinary foodstuffs and various fermented foods. Biosci. Biotechnol. Biochem. 1997, 61, 1582–1584. [Google Scholar] [CrossRef]
- Sumi, H.; Hamada, H.; Tsushima, H.; Mihara, H.; Muraki, H. A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese Natto; a typical and popular soybean food in the Japanese diet. Experientia 1987, 43, 1110–1111. [Google Scholar] [CrossRef]
- Sakai, R.; Hashimoto, Y.; Ushigome, E.; Miki, A.; Okamura, T.; Matsugasumi, M.; Fukuda, T.; Majima, S.; Matsumoto, S.; Senmaru, T.; et al. Late-night-dinner is associated with poor glycemic control in people with type 2 diabetes: The KAMOGAWA-DM cohort study. Endocr. J. 2018, 65, 395–402. [Google Scholar] [CrossRef]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised Equations for Estimated GFR From Serum Creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Kaji, A.; Hashimoto, Y.; Kobayashi, Y.; Sakai, R.; Okamura, T.; Miki, A.; Hamaguchi, M.; Kuwahata, M.; Yamazaki, M.; Fukui, M. Sarcopenia is associated with tongue pressure in older patients with type 2 diabetes: A cross-sectional study of the KAMOGAWA-DM cohort study. Geriatr. Gerontol. Int. 2019, 19, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, H.J.M.; Brodsky, J.B.; Bernstein, D.P. Estimating ideal body weight—A new formula. Obes. Surg. 2005, 15, 1082–1083. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef]
- Meng, G.; Wu, H.; Fang, L.; Li, C.; Yu, F.; Zhang, Q.; Liu, L.; Du, H.; Shi, H.; Xia, Y.; et al. Relationship between grip strength and newly diagnosed nonalcoholic fatty liver disease in a large-scale adult population. Sci. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef]
- Gan, D.; Wang, L.; Jia, M.; Ru, Y.; Ma, Y.; Zheng, W.; Zhao, X.; Yang, F.; Wang, T.; Mu, Y.; et al. Low muscle mass and low muscle strength associate with nonalcoholic fatty liver disease. Clin. Nutr. 2020, 39, 1124–1130. [Google Scholar] [CrossRef]
- Okamura, T.; Miki, A.; Hashimoto, Y.; Kaji, A.; Sakai, R.; Osaka, T.; Hamaguchi, M.; Yamazaki, M.; Fukui, M. Shortage of energy intake rather than protein intake is associated with sarcopenia in elderly patients with type 2 diabetes: A cross-sectional study of the KAMOGAWA-DM cohort. J. Diabetes 2019, 11, 477–483. [Google Scholar] [CrossRef]
- Kobayashi, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Comparison of relative validity of food group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 d dietary records in Japanese adults. Public Health Nutr. 2011, 14, 1200–1211. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Tanaka, M.; Miki, A.; Kobayashi, Y.; Wada, S.; Kuwahata, M.; Kido, Y.; Yamazaki, M.; Fukui, M. Intake of Carbohydrate to Fiber Ratio Is a Useful Marker for Metabolic Syndrome in Patients with Type 2 Diabetes: A Cross-Sectional Study. Ann. Nutr. Metab. 2018, 72, 329–335. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Zhu, S.; Tian, Z.; Torigoe, D.; Zhao, J.; Xie, P.; Sugizaki, T.; Sato, M.; Horiguchi, H.; Terada, K.; Kadomatsu, T.; et al. Aging- And obesity-related peri-muscular adipose tissue accelerates muscle atrophy. PLoS ONE 2019, 14, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.N.; Park, M.S.; Ryu, J.Y.; Choi, H.Y.; Hong, H.C.; Yoo, H.J.; Kang, H.J.; Song, W.; Park, S.W.; Baik, S.H.; et al. Impact of visceral fat on skeletal muscle mass and vice versa in a prospective cohort study: The Korean Sarcopenic Obesity Study (KSOS). PLoS ONE 2014, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Beyer, I.; Mets, T.; Bautmans, I. Chronic low-grade inflammation and age-related sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, M.; Toda, N.; Tamura, Y.; Terakado, S.; Ueno, M.; Otsuka, K.; Numabe, A.; Kawabata, Y.; Uehara, Y. Japanese traditional miso soup attenuates salt-induced hypertension and its organ damage in Dahl salt-sensitive rats. Nutrition 2012, 28, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Prevention by Long-Term Fermented Miso of Induction of Colonic Aberrant Crypt Foci by Azoxymethane in F344 Rats—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/11748458/ (accessed on 6 November 2020).
- Shirako, S.; Kojima, Y.; Tomari, N.; Nakamura, Y.; Matsumura, Y.; Ikeda, K.; Inagaki, N.; Sato, K. Pyroglutamyl leucine, a peptide in fermented foods, attenuates dysbiosis by increasing host antimicrobial peptide. Npj Sci. Food 2019, 3, 18. [Google Scholar] [CrossRef]
- Ejima, A.; Nakamura, M.; Suzuki, Y.A.; Sato, K. Identification of food-derived peptides in human blood after ingestion of corn and wheat gluten hydrolysates. J. Food Bioact. 2018, 2, 104–111. [Google Scholar] [CrossRef]
- Wada, S.; Sato, K.; Ohta, R.; Wada, E.; Bou, Y.; Fujiwara, M.; Kiyono, T.; Park, E.Y.; Aoi, W.; Takagi, T.; et al. Ingestion of low dose pyroglutamyl leucine improves dextran sulfate sodium-induced colitis and intestinal microbiota in mice. J. Agric. Food Chem. 2013, 61, 8807–8813. [Google Scholar] [CrossRef]
- Castaner, O.; Goday, A.; Park, Y.M.; Lee, S.H.; Magkos, F.; Toh Ee Shiow, S.A. The Gut Microbiome Profile in Obesity: A Systematic Review. Int. J. Endocrinol. 2018, 2018, 4095789. [Google Scholar] [CrossRef]
- Dhakal, K.H.; Jung, K.H.; Chae, J.H.; Shannon, J.G.; Lee, J.D. Variation of unsaturated fatty acids in soybean sprout of high oleic acid accessions. Food Chem. 2014, 164, 70–73. [Google Scholar] [CrossRef]
- Okamura, T.; Hashimoto, Y.; Miki, A.; Kaji, A.; Sakai, R.; Iwai, K.; Osaka, T.; Ushigome, E.; Hamaguchi, M.; Yamazaki, M.; et al. Reduced dietary omega-3 fatty acids intake is associated with sarcopenia in elderly patients with type 2 diabetes: A cross-sectional study of KAMOGAWA-DM cohort study. J. Clin. Biochem. Nutr. 2020, 66, 233–237. [Google Scholar] [CrossRef]
- Oh, S.L.; Lee, S.R.; Kim, J.S. Effects of conjugated linoleic acid/n-3 and resistance training on muscle quality and expression of atrophy-related ubiquitin ligases in middle-aged mice with high-fat diet-induced obesity. J. Exerc. Nutr. Biochem. 2017, 30, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Fukui, K.; Takamatsu, K.; Hashimoto, Y.; Yamamoto, T. Soy protein isolate and its hydrolysate reduce body fat of dietary obese rats and genetically obese mice (yellow KK). Nutrition 2000, 16, 349–354. [Google Scholar] [CrossRef]
- Davis, J.; Higginbotham, A.; O’Connor, T.; Moustaid-Moussa, N.; Tebbe, A.; Kim, Y.C.; Cho, K.W.; Shay, N.; Adler, S.; Peterson, R.; et al. Soy protein and isoflavones influence adiposity and development of metabolic syndrome in the obese male ZDF rat. Ann. Nutr. Metab. 2007, 51, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic activity, biological effect and bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Takase, H.; Sakane, N.; Morimoto, T.; Uchida, T.; Mori, K.; Katashima, M.; Katsuragi, Y.; Sallam, R.M. Development of a Dietary Factor Assessment Tool for Evaluating Associations between Visceral Fat Accumulation and Major Nutrients in Japanese Adults. J. Obes. 2019, 2019. [Google Scholar] [CrossRef]
- Suthuvoravut, U.; Takahashi, K.; Murayama, H.; Tanaka, T.; Akishita, M.; Iijima, K. Association between Traditional Japanese Diet Washoku and Sarcopenia in Community-Dwelling Older Adults: Findings from the Kashiwa Study. J. Nutr. Health Aging 2020, 24, 282–289. [Google Scholar] [CrossRef]
- Zuo, L.; He, F.; Sergakis, G.G.; Koozehchian, M.S.; Stimpfl, J.N.; Rong, Y.; Diaz, P.T.; Best, T.M. Interrelated role of cigarette smoking, oxidative stress, and immune response in COPD and corresponding treatments. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L205–L218. [Google Scholar] [CrossRef]
- Rom, O.; Kaisari, S.; Aizenbud, D.; Reznick, A.Z. Sarcopenia and smoking: A possible cellular model of cigarette smoke effects on muscle protein breakdown. Ann. N. Y. Acad. Sci. 2012, 1259, 47–53. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).