The Effect of Continuous Intake of Lactobacillus gasseri OLL2716 on Mild to Moderate Delayed Gastric Emptying: A Randomized Controlled Study
Abstract
1. Introduction
2. Materials and Methods
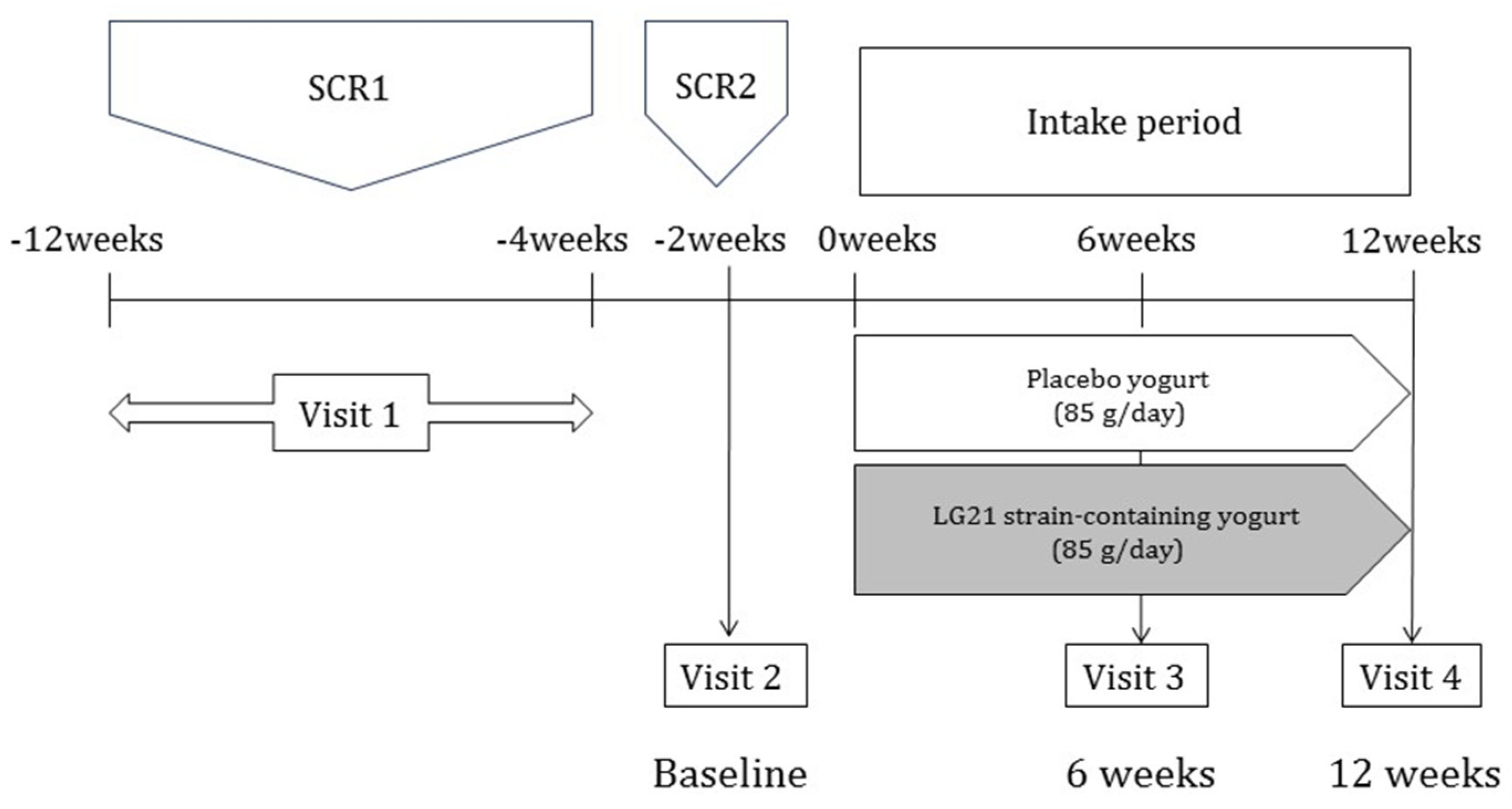
2.1. Study Design
2.2. Participants
2.3. Criteria for Tmax at Screening
2.4. Study Protocol
2.5. Assessments
2.5.1. 13C Gastric Emptying Breath Test
2.5.2. Salivary Amylase Test
2.5.3. Participant’s Global Assessment of Gastric Condition
2.5.4. Endpoints
- Primary endpoint: An intergroup comparison of the ΔTmax-area under the curve (AUC) during the intake period was performed. The ΔTmax-AUC of each participant during the intake period was obtained by plotting the origin, ΔTmax after 6 weeks of intake, and ΔTmax after 12 weeks of intake on a graph with intake time (weeks) on the x-axis and ΔTmax (minutes) on the y-axis, and connecting each plot with a straight line to calculate the AUC relative to baseline.
- Secondary endpoints: (1) Changes in salivary amylase concentration (Δsalivary amylase concentration) after 6 and 12 weeks of intake were compared to before intake. (2) A global assessment of gastric condition after intake was conducted.
2.5.5. Safety Assessment
2.6. Statistical Analysis
3. Results
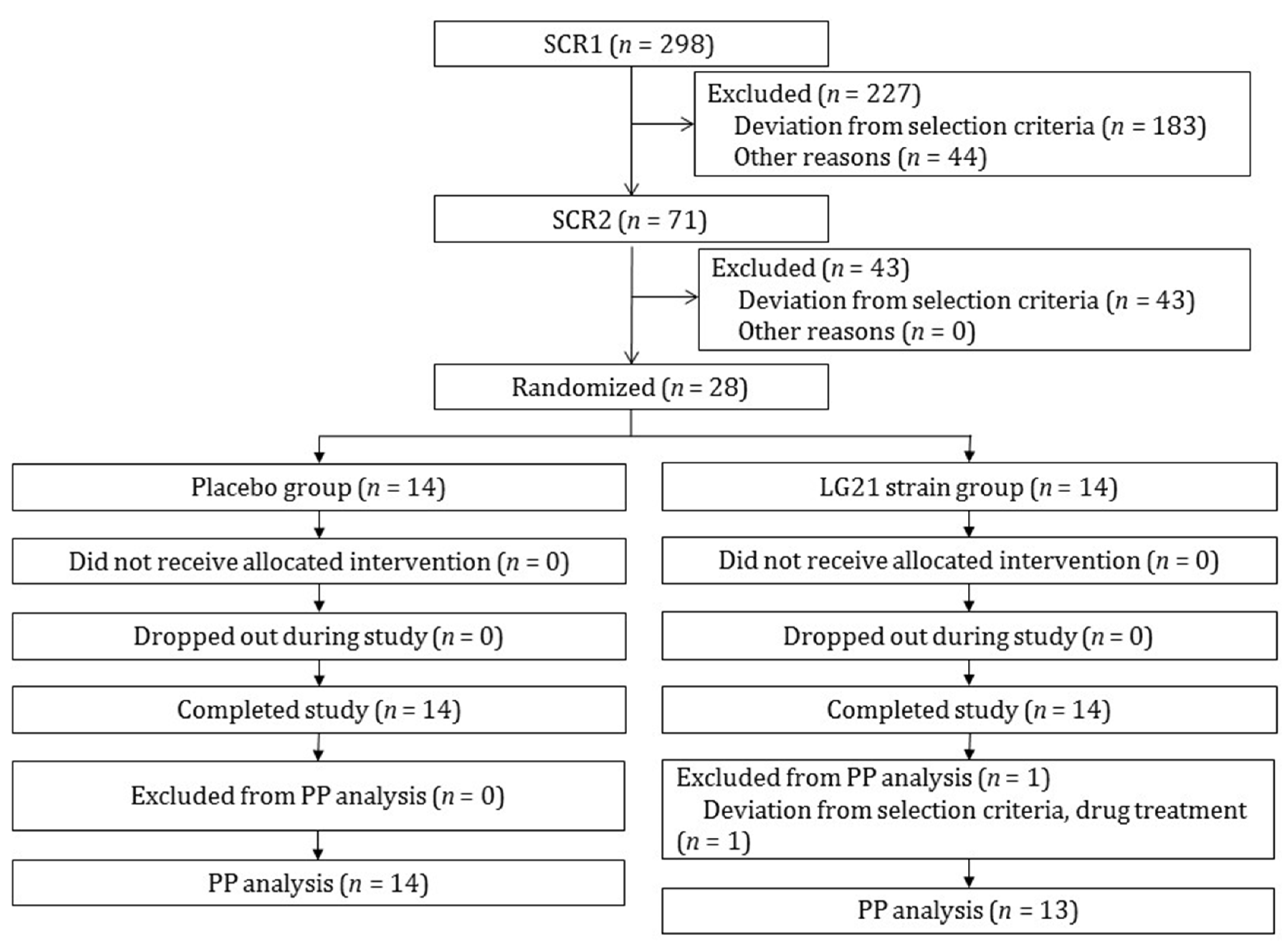
3.1. Participant Selection and Baseline Characteristics
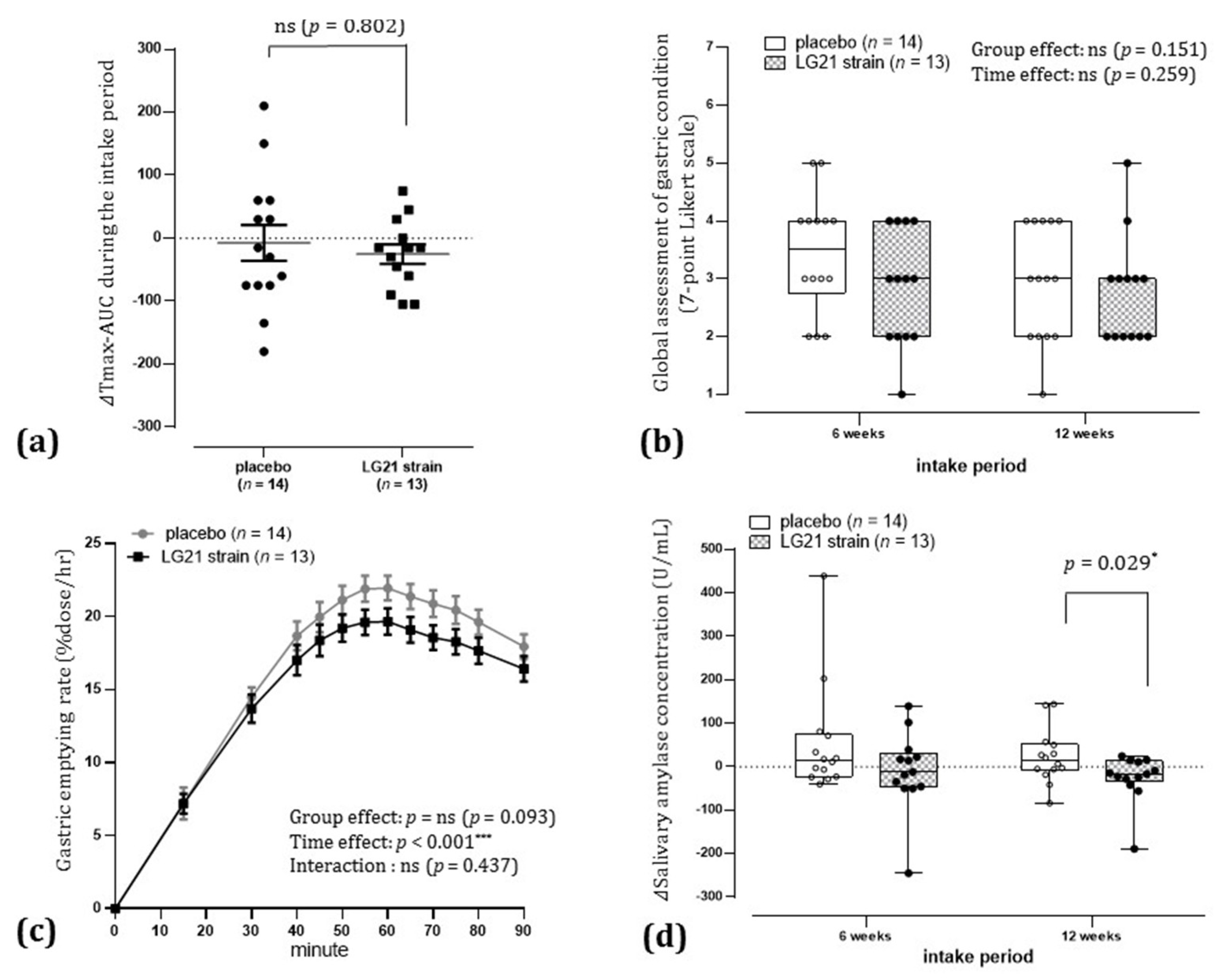
3.2. Primary Endpoint
3.3. Secondary Endpoints
3.3.1. Participant’s Global Assessment of Gastric Condition
3.3.2. Δ Salivary Amylase Concentration after 12 Weeks of Intake
3.3.3. Tmax after 6 and 12 Weeks of Intake Compared to Before Intake
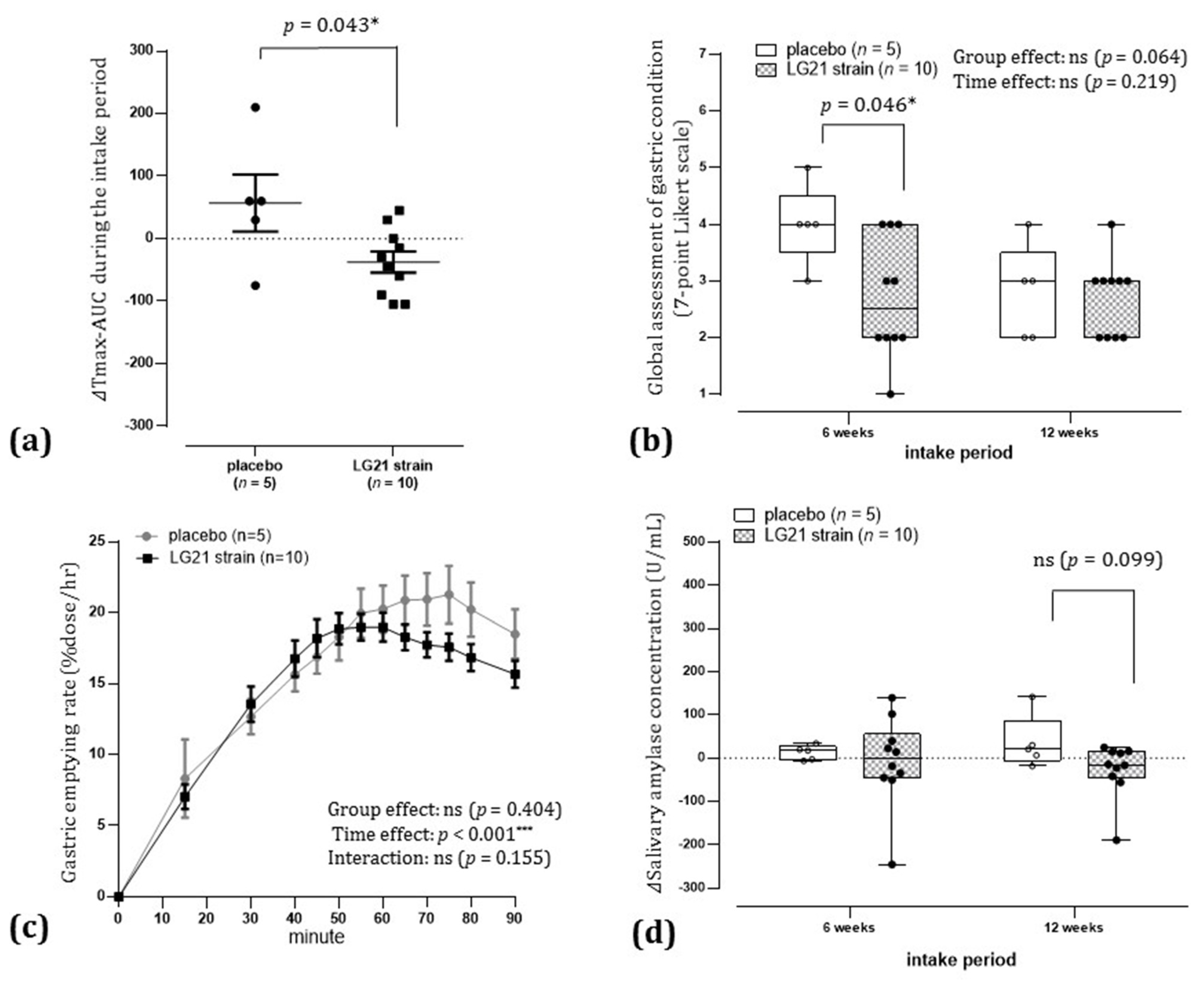
3.3.4. Odds Ratio for Improvement in Delayed Gastric Emptying after 12 Weeks of Intake
3.3.5. Gastric Emptying Rate following Ingestion of a Liquid Meal after 12 Weeks of Intake
3.3.6. Relationship between Changes in Salivary Amylase Concentration after 12 Weeks of Intake and Baseline Salivary Amylase Concentration
3.3.7. Effect of Gender on the Results of the Intervention in Delayed Gastric Emptying
3.4. Safety Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Camilleri, M.; Chedid, V.; Ford, A.C.; Haruma, K.; Horowitz, M.; Jones, K.L.; Low, P.A.; Park, S.-Y.; Parkman, H.P.; Stanghellini, V. Gastroparesis. Nat. Rev. Dis. Prim. 2018, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Grover, M.; Farrugia, G.; Stanghellini, V. Gastroparesis: A turning point in understanding and treatment. Gut 2019, 68, 2238–2250. [Google Scholar] [CrossRef] [PubMed]
- Travagli, R.A.; Anselmi, L. Vagal neurocircuitry and its influence on gastric motility. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 389–401. [Google Scholar] [CrossRef] [PubMed]
- McMenamin, C.A.; Travagli, R.A.; Browning, K.N. Inhibitory neurotransmission regulates vagal efferent activity and gastric motility. Exp. Biol. Med. 2016, 241, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, G.; Gibbons, S.J.; Kashyap, P.C.; Farrugia, G. Intrinsic Gastrointestinal Macrophages: Their Phenotype and Role in Gastrointestinal Motility. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 120–130.e1. [Google Scholar] [CrossRef] [PubMed]
- Parkman, H.P.; Yates, K.; Hasler, W.L.; Nguyen, L.; Pasricha, P.J.; Snape, W.J.; Farrugia, G.; Koch, K.L.; Calles, J.; Abell, T.L.; et al. Similarities and Differences Between Diabetic and Idiopathic Gastroparesis. Clin. Gastroenterol. Hepatol. 2011, 9, 1056–1064. [Google Scholar] [CrossRef]
- Lee, H.S.; An, Y.-S.; Kang, J.; Yoo, J.H.; Lee, K.J. The effect of acute auditory stress on gastric motor responses to a meal in healthy volunteers. J. Gastroenterol. Hepatol. 2013, 28, 1699–1704. [Google Scholar] [CrossRef]
- Motokubota, N.; Komai, N.; Suzuki, M.; Hayashi, I.; Moritani, T.; Nagai, N. Influence of Evening Preference on Daytime Variation of Autonomic Nervous System Activity, Gastric Motility, and Appetite Sensations in Young Women. Nippon. Eiyo Shokuryo Gakkaishi 2016, 69, 65–74. [Google Scholar] [CrossRef][Green Version]
- Ohtsu, T.; Takagi, A.; Uemura, N.; Inoue, K.; Sekino, H.; Kawashima, A.; Uchida, M.; Koga, Y. The Ameliorating Effect of Lactobacillus gasseri OLL2716 on Functional Dyspepsia in Helicobacter pylori-Uninfected Individuals: A Randomized Controlled Study. Digestion 2017, 96, 92–102. [Google Scholar] [CrossRef]
- Matsueda, K.; Hongo, M.; Tack, J.; Aoki, H.; Saito, Y.; Kato, H. Clinical trial: Dose-dependent therapeutic efficacy of acotiamide hydrochloride (Z-338) in patients with functional dyspepsia—100 mg t.i.d. is an optimal dosage. Neurogastroenterol. Motil. 2010, 22, 618-e173. [Google Scholar] [CrossRef]
- Otomi, K.; Ymaguchi, T.; Watanabe, S.; Kobayashi, A.; Kobayashi, H.; Hashiguchi, N. Effects of Yogurt Containing Lactobacillus gasseri OLL2716 on Autonomic Nerve Activities and Physiological Functions. Health 2015, 7, 397–405. [Google Scholar] [CrossRef]
- Nakata, K.; Aoyama, N.; Nakagawa, M.; Kawasaki, S.; Shirasaka, D.; Zai, H.; Urita, Y.; Kitagawa, Y.; Koyama, S.; Shishido, T.; et al. The present and the future in gastric emptying study assessed by 13C-acetate breath test-with special reference to the standardization of the method. J. Smooth Muscle Res. 2002, 6, 75–91. [Google Scholar] [CrossRef]
- Stanghellini, V.; Tack, J. Gastroparesis: Separate entity or just a part of dyspepsia? Gut 2014, 63, 1972–1978. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Bassotti, G.; Clarke, J.; Dinning, P.; Fox, M.; Grover, M.; Hellström, P.M.; Ke, M.; Layer, P.; Malagelada, C. Expert consensus document: Advances in the diagnosis and classification of gastric and intestinal motility disorders. Nat. Rev. Gastroenterol Hepatol. 2018, 15, 291–308. [Google Scholar] [CrossRef]
- Braden, B.; Adams, S.; Duan, L.-P.; Orth, K.-H.; Maul, F.-D.; Lembcke, B.; Hör, G.; Caspary, W.F. The [13C]acetate breath test accurately reflects gastric emptying of liquids in both liquid and semisolid test meals. Gastroenterology 1995, 108, 1048–1055. [Google Scholar] [CrossRef]
- Goetze, O.; Fox, M.; Kwiatek, M.A.; Treier, R.; Schwizer, W.; Thumshirn, M.; Fried, M.; Fruehauf, H. Effects of postgastric13C-acetate processing on measurement of gastric emptying: A systematic investigation in health. Neurogastroenterol. Motil. 2009, 21, 1047-e85. [Google Scholar] [CrossRef]
- Futagami, S.; Shimpuku, M.; Song, J.; Kodaka, Y.; Yamawaki, H.; Nagoya, H.; Shindo, T.; Kawagoe, T.; Horie, A.; Gudis, K.; et al. Nizatidine Improves Clinical Symptoms and Gastric Emptying in Patients with Functional Dyspepsia Accompanied by Impaired Gastric Emptying. Digestion 2012, 86, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Matsueda, K.; Hongo, M.; Tack, J.F.; Saito, Y.; Kato, H. A placebo-controlled trial of acotiamide for meal-related symptoms of functional dyspepsia. Gut 2011, 61, 821–828. [Google Scholar] [CrossRef]
- Ali, N.; Nater, U.M. Salivary Alpha-Amylase as a Biomarker of Stress in Behavioral Medicine. Int. J. Behav. Med. 2020, 27, 337–342. [Google Scholar] [CrossRef]
- Wan, C.; Couture-Lalande, M.È.; Narain, T.A.; Lebel, S.; Bielajew, C. Salivary alpha-amylase reactivity in breast cancer survivors. Int. J. Environ. Res. Public Health 2016, 13, 353. [Google Scholar] [CrossRef]
- Mann, S.D.; Debinski, H.S.; Kamm, M.A. Clinical characteristics of chronic idiopathic intestinal pseudo-obstruction in adults. Gut 1997, 41, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Cogliandro, R.F.; Rizzoli, G.; Bellacosa, L.; de Giorgio, R.; Cremon, C.; Barbara, G.; Stanghellini, V. Is gastroparesis a gastric disease? Neurogastroenterol. Motil. 2019, 31, e13562. [Google Scholar] [CrossRef]
- Vijayvargiya, P.; Jameie-Oskooei, S.; Camilleri, M.; Chedid, V.; Erwin, P.J.; Murad, M.H. Association between delayed gastric emptying and upper gastrointestinal symptoms: A systematic review and meta-analysis. Gut 2019, 68, 804–813. [Google Scholar] [CrossRef]
- Kusano, M.; Zai, H.; Shimoyama, Y.; Hosaka, H.; Kuribayashi, S.; Kawamura, O.; Mori, M. Rapid gastric emptying, rather than delayed gastric emptying, might provoke functional dyspepsia. J. Gastroenterol. Hepatol. 2011, 26, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-J.; Vos, R.; Janssens, J.; Tack, J. Influence of duodenal acidification on the sensorimotor function of the proximal stomach in humans. Am. J. Physiol. Liver Physiol. 2004, 286, G278–G284. [Google Scholar] [CrossRef][Green Version]
- Tack, J.; Arts, J.; Caenepeel, P.; De Wulf, D.; Bisschops, R. Pathophysiology, diagnosis and management of postoperative dumping syndrome. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Orthey, P.; Dadparvar, S.; Kamat, B.; Parkman, H.P.; Maurer, A.H. Using gastric emptying scintigraphy to evaluate antral contractions and duodenal bolus propagation. Am. J. Physiol. Liver Physiol. 2020, 318, G203–G209. [Google Scholar] [CrossRef]
- Vella, A.; Camilleri, M. The Gastrointestinal Tract as an Integrator of Mechanical and Hormonal Response to Nutrient Ingestion. Diabetes 2017, 66, 2729–2737. [Google Scholar] [CrossRef]
- Kimura, K.; Sakamoto, I.; Igarashi, M.; Takagi, A.; Miwa, T.; Aiba, Y.; Koga, Y. Development of Probiotics for Helicobacter pylori Infection. Biosci. Microflora 2003, 22, 1–4. [Google Scholar] [CrossRef][Green Version]
- Fujimura, S.; Kato, S.; Oda, M.; Miyahara, M.; Ito, Y.; Kimura, K.; Kawamura, T.; Ohnuma, M.; Tateno, H.; Watanabe, A. Detection ofLactobacillus gasseriOLL2716 strain administered with yogurt drink in gastric mucus layer in humans. Lett. Appl. Microbiol. 2006, 43, 578–581. [Google Scholar] [CrossRef]
- Sakamoto, I.; Igarashi, M.; Kimura, K.; Takagi, A.; Miwa, T.; Koga, Y. Suppressive effect of Lactobacillus gasseri OLL 2716 (LG21) on Helicobacter pylori infection in humans. J. Antimicrob. Chemother. 2001, 47, 709–710. [Google Scholar] [CrossRef]
- Deguchi, R.; Nakaminami, H.; Rimbara, E.; Noguchi, N.; Sasatsu, M.; Suzuki, T.; Matsushima, M.; Koike, J.; Igarashi, M.; Ozawa, H.; et al. Effect of pretreatment with Lactobacillus gasseri OLL2716 on first-line Helicobacter pylori eradication therapy. J. Gastroenterol. Hepatol. 2012, 27, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Fukudo, S.; Nomura, T.; Muranaka, M.; Taguchi, F. Brain-Gut Response to Stress and Cholinergic Stimulation in Irritable Bowel Syndrome. J. Clin. Gastroenterol. 1993, 17, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Rebollo, I.; Devauchelle, A.-D.; Béranger, B.; Tallon-Baudry, C. Stomach-brain synchrony reveals a novel, delayed-connectivity resting-state network in humans. eLife 2018, 7, e33321. [Google Scholar] [CrossRef] [PubMed]
- Nishino, R.; Mikami, K.; Takahashi, H.; Tomonaga, S.; Furuse, M.; Hiramoto, T.; Aiba, Y.; Koga, Y.; Sudo, N. Commensal microbiota modulate murine behaviors in a strictly contamination-free environment confirmed by culture-based methods. Neurogastroenterol. Motil. 2013, 25, 521-e371. [Google Scholar] [CrossRef] [PubMed]
- De Palma, G.; Blennerhassett, P.; Lu, J.; Deng, Y.; Park, A.J.; Green, W.; Denou, E.; Silva, M.A.; Santacruz, A.; Sanz, Y.; et al. Microbiota and host determinants of behavioural phenotype in maternally separated mice. Nat. Commun. 2015, 6, 7735. [Google Scholar] [CrossRef] [PubMed]
- Takada, M.; Nishida, K.; Kataoka-Kato, A.; Gondo, Y.; Ishikawa, H.; Suda, K.; Kawai, M.; Hoshi, R.; Watanabe, O.; Igarashi, T.; et al. Probiotic Lactobacillus casei strain Shirota relieves stress-associated symptoms by modulating the gut–brain interaction in human and animal models. Neurogastroenterol. Motil. 2016, 28, 1027–1036. [Google Scholar] [CrossRef]
- Wauters, L.; Talley, N.J.; Walker, M.M.; Tack, J.; Vanuytsel, T. Novel concepts in the pathophysiology and treatment of functional dyspepsia. Gut 2019, 69, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Tziatzios, G.; Gkolfakis, P.; Papanikolaou, I.S.; Mathur, R.; Pimentel, M.; Giamarellos-Bourboulis, E.J.; Triantafyllou, K. Gut Microbiota Dysbiosis in Functional Dyspepsia. Microorganisms 2020, 8, 691. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, H.M.; Wang, X.; Xie, J.; Li, X.; Ma, J.; Wang, F.; Tang, X. Efficacy of prebiotics and probiotics for functional dyspepsia: A systematic review and meta-analysis. Medicine 2020, 99, e19107. [Google Scholar] [CrossRef] [PubMed]
- Nakae, H.; Tsuda, A.; Matsuoka, T.; Mine, T.; Koga, Y. Gastric microbiota in the functional dyspepsia patients treated with probiotic yogurt. BMJ Open Gastroenterol. 2016, 3, e000109. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Nakae, H.; Matsuoka, T.; Takahashi, S.; Hisada, T.; Tomita, J.; Koga, Y. Alteration in the gastric microbiota and its restoration by probiotics in patients with functional dyspepsia. BMJ Open Gastroenterol. 2017, 4, e000144. [Google Scholar] [CrossRef] [PubMed]
- Koga, Y.; Ohtsu, T.; Kimura, K.; Asami, Y.; Probiotic, L. gasseri strain (LG21) for the upper gastrointestinal tract acting through improvement of indigenous microbiota. BMJ Open Gastroenterol. 2019, 6, e000314. [Google Scholar] [CrossRef] [PubMed]
- Uchida, M.; Kurakazu, K. Yogurt Containing Lactobacillus gasseri OLL2716 Exerts Gastroprotective Action Agaisnt Acute Gastric Lesion and Antral Ulcer in Rats. J. Pharmacol. Sci. 2004, 96, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Akama, F.; Nishino, R.; Makino, S.; Kobayashi, K.; Kamikaseda, K.; Nagano, J.; Koga, Y. The effect of probiotics on gastric mucosal permeability in humans administered with aspirin. Scand. J. Gastroenterol. 2011, 46, 831–836. [Google Scholar] [CrossRef]
- Suzuki, T.; Masui, A.; Nakamura, J.; Shiozawa, H.; Aoki, J.; Nakae, H.; Tsuda, S.; Imai, J.; Hideki, O.; Matsushima, M.; et al. Yogurt Containing Lactobacillus gasseri Mitigates Aspirin-Induced Small Bowel Injuries: A Prospective, Randomized, Double-Blind, Placebo-Controlled Trial. Digestion 2017, 95, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Satoh-Takayama, N.; Kato, T.; Motomura, Y.; Kageyama, T.; Taguchi-Atarashi, N.; Kinoshita-Daitoku, R.; Kuroda, E.; Di Santo, J.P.; Mimuro, H.; Moro, K.; et al. Bacteria-Induced Group 2 Innate Lymphoid Cells in the Stomach Provide Immune Protection through Induction of IgA. Immunity 2020, 52, 635–649.e4. [Google Scholar] [CrossRef] [PubMed]
- Morita, N.; Umemoto, E.; Fujita, S.; Hayashi, A.; Kikuta, J.; Kimura, I.; Haneda, T.; Imai, T.; Inoue, A.; Mimuro, H.; et al. GPR31-dependent dendrite protrusion of intestinal CX3CR1+ cells by bacterial metabolites. Nat. Cell Biol. 2019, 566, 110–114. [Google Scholar] [CrossRef]
- Mori, H.; Suzuki, H.; Matsuzaki, J.; Taniguchi, K.; Shimizu, T.; Yamane, T.; Masaoka, T.; Kanai, T. Gender Difference of Gastric Emptying in Healthy Volunteers and Patients with Functional Dyspepsia. Digestion 2017, 95, 72–78. [Google Scholar] [CrossRef]


| Characteristics | ITT Total (n = 28) | Group | p-Value | ||
|---|---|---|---|---|---|
| Placebo (n = 14) | LG21 Strain (n = 14) | ||||
| Age (years), mean ± SD † | 41.1 ± 11.0 | 40.8 ± 9.7 | 41.4 ± 12.5 | 0.880 | |
| Gender (n [%]) § | Female | 24 (85.7%) | 12 (85.7%) | 12 (85.7%) | 1.000 |
| Male | 4 (14.3%) | 2 (14.3%) | 2 (14.3%) | ||
| Classification by BMI (n [%]) § | <18.5 | 4 (14.3%) | 2 (14.3%) | 2 (14.3%) | 1.000 |
| 18.5–25.0 | 22 (78.6%) | 11 (78.6%) | 11 (78.6%) | ||
| ≥25.0 | 2 (7.1%) | 1 (7.1%) | 1 (7.1%) | ||
| Tmax (min), median (25th–75th percentile) ‡ | 60 (55–65) | 60 (55–65) | 60 (58.8–65) | 0.791 | |
| Classification by degree of Tmax (n [%]) § | ≥55 and <65 | 19 (67.9%) | 9 (64.3%) | 10 (71.4%) | 1.000 |
| ≥65 and <75 | 9 (32.1%) | 5 (35.7%) | 4 (28.6%) | ||
| Severity of stomach upset (n [%]) § | Mild | 14 (50.0%) | 7 (50.0%) | 7 (50.0%) | 1.000 |
| Moderate | 11 (39.3%) | 5 (35.7%) | 6 (42.9%) | ||
| Slightly severe | 3 (10.7%) | 2 (14.3%) | 1 (7.1%) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohtsu, T.; Haruma, K.; Ide, Y.; Takagi, A. The Effect of Continuous Intake of Lactobacillus gasseri OLL2716 on Mild to Moderate Delayed Gastric Emptying: A Randomized Controlled Study. Nutrients 2021, 13, 1852. https://doi.org/10.3390/nu13061852
Ohtsu T, Haruma K, Ide Y, Takagi A. The Effect of Continuous Intake of Lactobacillus gasseri OLL2716 on Mild to Moderate Delayed Gastric Emptying: A Randomized Controlled Study. Nutrients. 2021; 13(6):1852. https://doi.org/10.3390/nu13061852
Chicago/Turabian StyleOhtsu, Toshihiro, Ken Haruma, Yumiko Ide, and Atsushi Takagi. 2021. "The Effect of Continuous Intake of Lactobacillus gasseri OLL2716 on Mild to Moderate Delayed Gastric Emptying: A Randomized Controlled Study" Nutrients 13, no. 6: 1852. https://doi.org/10.3390/nu13061852
APA StyleOhtsu, T., Haruma, K., Ide, Y., & Takagi, A. (2021). The Effect of Continuous Intake of Lactobacillus gasseri OLL2716 on Mild to Moderate Delayed Gastric Emptying: A Randomized Controlled Study. Nutrients, 13(6), 1852. https://doi.org/10.3390/nu13061852





