Effect of an Enriched Protein Drink on Muscle Mass and Glycemic Control during Combined Lifestyle Intervention in Older Adults with Obesity and Type 2 Diabetes: A Double-Blind RCT
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Design and Randomization Procedures
2.3. Hypocaloric Diet
2.4. Exercise Program
2.5. Study Products
2.6. Measurement of Body Composition and Anthropometry
2.7. Measurement of Glycemic Control
2.8. Measurement of Muscle Strength and Power and Physical Performance
2.9. Statistical Analysis
3. Results
3.1. Subjects, Safety, and Compliance to Study Product Intake and Exercise Program
3.2. Body Weight, Body Composition, and Anthropometry
3.3. Glycemic Control
3.4. Muscle Strength, Muscle Power, and Physical Performance
3.5. Dietary Intake
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Component | Test Product | Control Product |
|---|---|---|
| Energy (kcal) | 150 | 150 |
| Protein (g) | 20.7 | - |
| Leucine, total a (g) | 2.8 | - |
| EAA, total a (g) | 10.6 | - |
| Carbohydrates (g) | 9.4 | 24.5 |
| Sugars b (g) | 4.2 | 15.6 |
| Fat (g) | 3.0 | 5.8 |
| Saturated fat (g) | 0.8 | 3.2 |
| Mono-unsaturated fat (g) | 1.7 | 2.1 |
| Poly-unsaturated fat (g) | 0.6 | 0.6 |
| Fiber (g) | 1.25 | - |
| Vitamin D3 c (μg) | 20 | - |
| Calcium c (mg) | 500 | 0.7 |
References
- Park, S.W.; Goodpaster, B.H.; Lee, J.S.; Kuller, L.H.; Boudreau, R.; de Rekeneire, N.; Harris, T.B.; Kritchevsky, S.; Tylavsky, F.A.; Nevitt, M.; et al. For the Health, Aging, and Body Composition Study. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care 2009, 32, 1993–1997. [Google Scholar] [CrossRef]
- Wycherley, T.P.; Buckley, J.D.; Noakes, M.; Clifton, P.M.; Brinkworth, G.D. Comparison of the effects of weight loss from a high-protein versus standard-protein energy-restricted diet on strength and aerobic capacity in overweight and obese men. Eur. J. Nutr. 2013, 52, 317–325. [Google Scholar] [CrossRef]
- Villareal, D.T.; Apovian, C.M.; Kushner, R.F.; Klein, S. Obesity in older adults: Technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Obes. Res. 2005, 13, 1849–1863. [Google Scholar] [CrossRef]
- DeFronzo, R.A. Lilly lecture 1987. The triumvirate: Beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 1988, 37, 667–687. [Google Scholar] [CrossRef]
- Trouwborst, I.; Verreijen, A.; Memelink, R.; Massanet, P.; Boirie, Y.; Weijs, P.; Tieland, M. Exercise and nutrition strategies to counteract sarcopenic obesity. Nutrients 2018, 10, 605. [Google Scholar] [CrossRef]
- Frimel, T.N.; Sinacore, D.R.; Villareal, D.T. Exercise attenuates the weight-loss-induced reduction in muscle mass in frail obese older adults. Med. Sci. Sports Exerc. 2008, 40, 1213–1219. [Google Scholar] [CrossRef]
- Willey, K.A.; Singh, M.A. Battling insulin resistance in elderly obese people with type 2 diabetes: Bring on the heavy weights. Diabetes Care 2003, 26, 1580–1588. [Google Scholar] [CrossRef]
- Wycherley, T.P.; Noakes, M.; Clifton, P.M.; Cleanthous, X.; Keogh, J.B.; Brinkworth, G.D. A high-protein diet with resistance exercise training improves weight loss and body composition in overweight and obese patients with type 2 diabetes. Diabetes Care 2010, 33, 969–976. [Google Scholar] [CrossRef]
- Verreijen, A.M.; Verlaan, S.; Engberink, M.F.; Swinkels, S.; de Vogel-van den Bosch, J.; Weijs, P.J. A high whey protein-, leucine-, and vitamin D-enriched supplement preserves muscle mass during intentional weight loss in obese older adults: A double-blind randomized controlled trial. Am. J. Clin. Nutr. 2015, 101, 279–286. [Google Scholar] [CrossRef]
- de Bandt, J.P. Leucine and mammalian target of rapamycin-dependent activation of muscle protein synthesis in aging. J. Nutr. 2016, 146, 2616S–2624S. [Google Scholar] [CrossRef]
- Manders, R.J.; Little, J.P.; Forbes, S.C.; Candow, D.G. Insulinotropic and muscle protein synthetic effects of branched-chain amino acids: Potential therapy for type 2 diabetes and sarcopenia. Nutrients 2012, 4, 1664–1678. [Google Scholar] [CrossRef]
- Beaudart, C.; Buckinx, F.; Rabenda, V.; Gillain, S.; Cavalier, E.; Slomian, J.; Petermans, J.; Reginster, J.-Y.; Bruyère, O. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: A systematic review and meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 2014, 99, 4336–4345. [Google Scholar] [CrossRef]
- Naharci, I.; Bozoglu, E.; Kocak, N.; Doganci, S.; Doruk, H.; Serdar, M. Effect of vitamin D on insulin sensitivity in elderly patients with impaired fasting glucose. Geriatr. Gerontol. Int. 2012, 12, 454–460. [Google Scholar] [CrossRef]
- Proks, P.; Reimann, F.; Green, N.; Gribble, F.; Ashcroft, F. Sulfonylurea stimulation of insulin secretion. Diabetes 2002, 51 (Suppl. 3), S368–S376. [Google Scholar] [CrossRef]
- Dimitriadis, G.; Mitrou, P.; Lambadiari, V.; Maratou, E.; Raptis, S.A. Insulin effects in muscle and adipose tissue. Diabetes Res. Clin. Pract. 2011, 93 (Suppl. 1), S52–S59. [Google Scholar] [CrossRef]
- Dutch Institute for Healthcare Improvement. Richtlijn Diagnostiek en Behandeling van Obesitas bij Volwassenen en Kinderen (Dutch; “Guideline Diagnosis and Treatment of Obesity in Adults and Children”); CBO: Utrecht, The Netherlands, 2008. [Google Scholar]
- National Institute for Public Health and the Environment. NEVO-Online, Versie 2013/4.0 (Dutch; “Dutch Food Composition Database, Version 2013/4.0”); RIVM: Bilthoven, The Netherlands, 2013.
- Rozenberg, R.; Bussmann, J.B.; Lesaffre, E.; Stam, H.J.; Praet, S.F. A steep ramp test is valid for estimating maximal power and oxygen uptake during a standard ramp test in type 2 diabetes. Scand. J. Med. Sci. Sports 2015, 25, 595–602. [Google Scholar] [CrossRef]
- Praet, S.F.; Van Loon, L.J. Optimizing the therapeutic benefits of exercise in type 2 diabetes. J. Appl. Physiol. 2007, 103, 1113–1120. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- Neeter, C.; Gustavsson, A.; Thomeé, P.; Augustsson, J.; Thomeé, R.; Karlsson, J. Development of a strength test battery for evaluating leg muscle power after anterior cruciate ligament injury and reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2006, 14, 571–580. [Google Scholar] [CrossRef]
- Shumway-Cook, A.; Guralnik, J.M.; Phillips, C.L.; Coppin, A.K.; Ciol, M.A.; Bandinelli, S.; Ferrucci, L. Age-associated declines in complex walking task performance: The Walking InCHIANTI toolkit. J. Am. Geriatr. Soc. 2007, 55, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef] [PubMed]
- Villareal, D.T.; Chode, S.; Parimi, N.; Sinacore, D.R.; Hilton, T.; Armamento-Villareal, R.; Napoli, N.; Qualls, C.; Shah, K. Weight loss, exercise, or both and physical function in obese older adults. N. Engl. J. Med. 2011, 364, 1218–1229. [Google Scholar] [CrossRef] [PubMed]
- Verreijen, A.M.; Engberink, M.F.; Memelink, R.G.; van der Plas, S.E.; Visser, M.; Weijs, P.J.M. Effect of a high protein diet and/or resistance exercise on the preservation of fat free mass during weight loss in overweight and obese older adults: A randomized controlled trial. Nutr. J. 2017, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Díaz-López, A.; Ruiz-Canela, M.; Basora, J.; Fitó, M.; Corella, D.; Serra-Majem, L.; Wärnberg, J.; Romaguera, D.; Estruch, R.; et al. PREDIMED-Plus investigators. Effect of a lifestyle intervention program with energy-restricted mediterranean diet and exercise on weight loss and cardiovascular risk factors: One-year results of the PREDIMED-Plus Trial. Diabetes Care 2019, 42, 777–788. [Google Scholar] [PubMed]
- Pot, G.K.; Battjes-Fries, M.C.E.; Patijn, O.N.; Pijl, H.; Witkamp, R.F.; De Visser, M.; van der Zijl, N.; de Vries, M.; Voshol, P.J. Nutrition and lifestyle intervention in type 2 diabetes: Pilot study in the Netherlands showing improved glucose control and reduction in glucose lowering medication. BMJ Nutr. Prev. Health 2019, 2. [Google Scholar] [CrossRef]
- Braam, L.A.; Ocké, M.C.; Bueno-de-Mesquita, H.B.; Seidell, J.C. Determinants of obesity-related underreporting of energy intake. Am. J. Epidemiol. 1998, 147, 1081–1086. [Google Scholar] [CrossRef]
- Pownall, H.J.; Schwartz, A.V.; Bray, G.A.; Berkowitz, R.I.; Lewis, C.E.; Boyko, E.J.; Jakicic, J.M.; Chen, H.; Heshka, S.; Gregg, E.W.; et al. Look AHEAD Research Group. Changes in regional body composition over 8 years in a randomized lifestyle trial: The look AHEAD study. Obes. Silver Spring 2016, 24, 1899–1905. [Google Scholar] [CrossRef]
- Weijs, P.J.M.; Wolfe, R.R. Exploration of the protein requirement during weight loss in obese older adults. Clin. Nutr. 2016, 35, 394–398. [Google Scholar] [CrossRef]
- Olver, T.D.; Laughlin, M.H. Endurance, interval sprint, and resistance exercise training: Impact on microvascular dysfunction in type 2 diabetes. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H337–H350. [Google Scholar] [CrossRef]
- Groen, B.B.L.; Hamer, H.M.; Snijders, T.; van Kranenburg, J.; Frijns, D.; Vink, H.; van Loon, L.J.C. Skeletal muscle capillary density and microvascular function are compromised with aging and type 2 diabetes. J. Appl. Physiol. 1985 2014, 116, 998–1005. [Google Scholar] [CrossRef]
- Robinson, M.M.; Dasari, S.; Konopka, A.R.; Johnson, M.L.; Manjunatha, S.; Esponda, R.R.; Carter, R.E.; Lanza, I.R.; Sreekumaran Nair, K. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metab. 2017, 25, 581–592. [Google Scholar] [CrossRef]
- Bouchonville, M.; Armamento-Villareal, R.; Shah, K.; Napoli, N.; Sinacore, D.R.; Qualls, C.; Villareal, D.T. Weight loss, exercise or both and cardiometabolic risk factors in obese older adults: Results of a randomized controlled trial. Int. J. Obes. Lond. 2014, 38, 423–431. [Google Scholar] [CrossRef]
- Tay, J.; Thompson, C.H.; Luscombe-Marsh, N.D.; Wycherley, T.P.; Noakes, M.; Buckley, J.D.; Wittert, G.A.; Yancy, W.S., Jr.; Brinkworth, G.D. Effects of an energy-restricted low-carbohydrate, high unsaturated fat/low saturated fat diet versus a high-carbohydrate, low-fat diet in type 2 diabetes: A 2-year randomized clinical trial. Diabetes Obes. Metab. 2018, 20, 858–871. [Google Scholar] [CrossRef]
- Jelleyman, C.; Yates, T.; O’Donovan, G.; Gray, L.J.; King, J.A.; Khunti, K.; Davies, M.J. The effects of high-intensity interval training on glucose regulation and insulin resistance: A meta-analysis. Obes. Rev. 2015, 16, 942–961. [Google Scholar] [CrossRef]
- Pasman, W.J.; Memelink, R.G.; de Vogel-Van den Bosch, J.; Begieneman, M.P.V.; van den Brink, W.J.; Weijs, P.J.M.; Wopereis, S. Obese Older Type 2 Diabetes Mellitus Patients with Muscle Insulin Resistance Benefit from an Enriched Protein Drink during Combined Lifestyle Intervention: The PROBE Study. Nutrients 2020, 12, 2979. [Google Scholar] [CrossRef]
- Manders, R.J.; Wagenmakers, A.J.; Koopman, R.; Zorenc, A.H.; Menheere, P.P.; Schaper, N.C.; Saris, W.H.M.; van Loon, L.J.C. Co-ingestion of a protein hydrolysate and amino acid mixture with carbohydrate improves plasma glucose disposal in patients with type 2 diabetes. Am. J. Clin. Nutr. 2005, 82, 76–83. [Google Scholar] [CrossRef]
- Talaei, A.; Mohamadi, M.; Adgi, Z. The effect of vitamin D on insulin resistance in patients with type 2 diabetes. Diabetol. Metab. Syndr. 2013, 5, 8. [Google Scholar] [CrossRef]
- Tieland, M.; Trouwborst, I.; Clark, B.C. Skeletal muscle performance and ageing. J. Cachexia Sarcopenia Muscle 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Liao, C.D.; Tsauo, J.Y.; Wu, Y.T.; Cheng, C.P.; Chen, H.C.; Huang, Y.C.; Chen, H.C.; Liou, T.H. Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2017, 106, 1078–1091. [Google Scholar] [CrossRef]
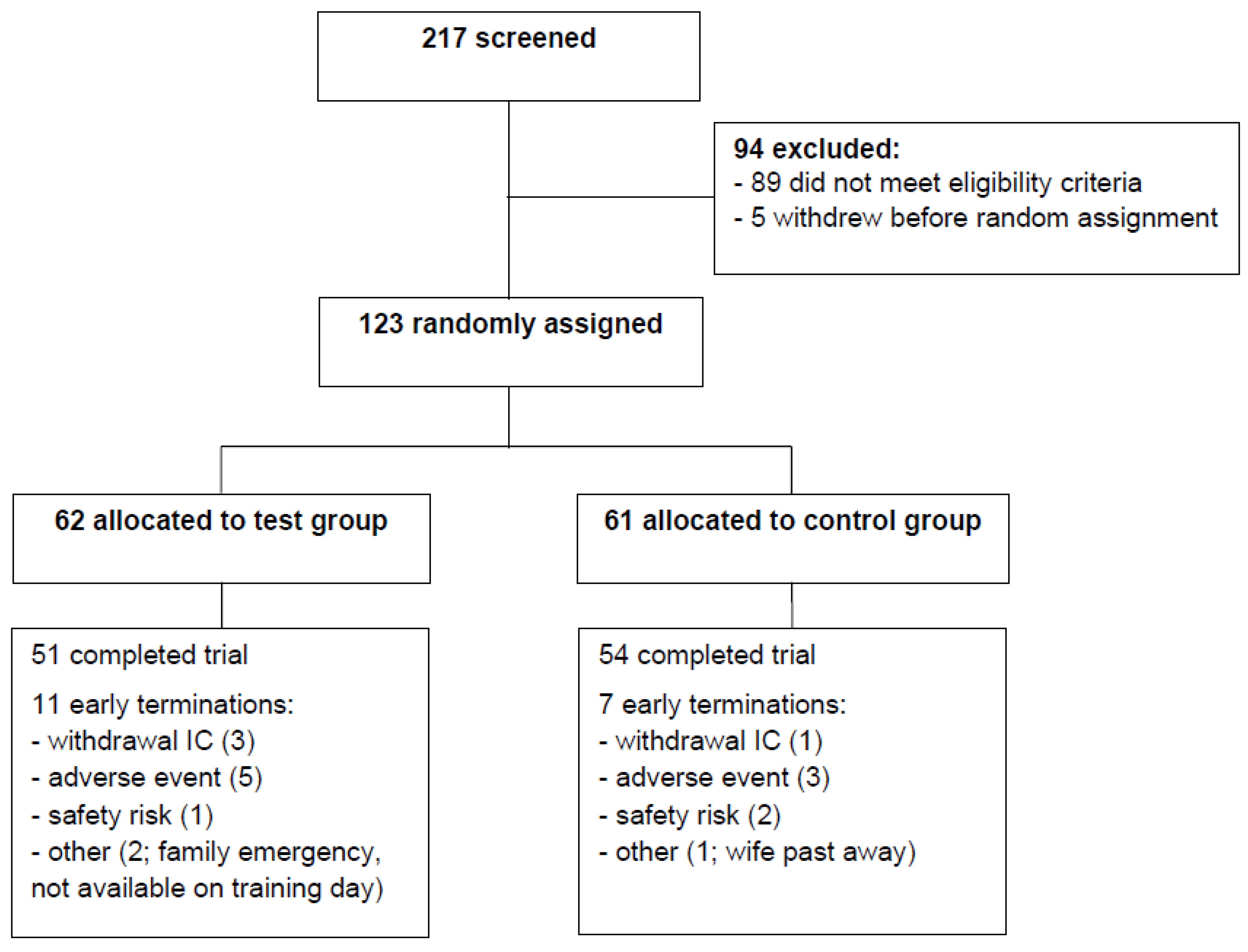
| Characteristic | n | Test Group | n | Control Group |
|---|---|---|---|---|
| Male sex, n (%) | 62 | 42 (68) | 61 | 38 (62) |
| Origin, % Caucasian | 62 | 86 | 61 | 85 |
| Age, years | 62 | 66.8 ± 6.0 | 61 | 65.8 ± 6.4 |
| Body weight, kg | 62 | 98.17 ± 14.99 | 61 | 100.07 ± 15.59 |
| BMI, kg/m2 | 62 | 32.8 ± 4.4 | 61 | 33.5 ± 4.6 |
| Waist circumference, cm | 61 | 114.1 ± 9.4 | 60 | 115.9 ± 10.7 |
| Fat mass, % | 61 | 33.5 ± 7.0 | 61 | 34.3 ± 6.0 |
| Leg muscle mass, kg | 61 | 19.59 ± 3.69 | 61 | 19.79 ± 3.45 |
| Appendicular muscle mass, kg | 59 | 26.47 ± 5.29 | 61 | 26.85 ± 5.10 |
| Total lean mass, kg | 60 | 63.17 ± 10.66 | 61 | 64.04 ± 10.96 |
| Skeletal muscle mass index, kg/m2 | 59 | 8.83 ± 1.21 | 61 | 8.93 ± 1.10 |
| Duration of diabetes, months | 58 | 94 ± 83 | 56 | 78 ± 57 |
| Use of diabetes medication, n (%) | 62 | 53 (86) | 61 | 55 (90) |
| Use of SU derivatives, n (%) | 21 (34) | 23 (38) | ||
| Use of metformin, n (%) | 49 (79) | 52 (85) | ||
| No medication, n (%) | 9 (15) | 6 (10) | ||
| Fasting glucose, mmol/L | 57 | 8.25 ± 1.76 | 58 | 8.24 ± 1.90 |
| HbA1c, mmol/mol | 60 | 51.08 ± 9.66 | 58 | 52.95 ± 10.86 |
| Fasting insulin, pmol/L | 60 | 116.1 ± 73.2 | 59 | 102.0 ± 41.5 |
| Serum calcidiol, nmol/L | 57 | 63.0 ± 28.1 | 58 | 60.4 ± 18.0 |
| Handgrip strength, kg | 60 | 36.3 ± 10.8 | 60 | 36.5 ± 10.3 |
| 400-m walk speed, m/s | 61 | 1.40 ± 0.22 | 58 | 1.49 ± 0.23 |
| Usual gait speed, m/s | 62 | 1.12 ± 0.22 | 60 | 1.18 ± 0.21 |
| Chair stand, s | 62 | 11.9 ± 2.5 | 60 | 11.5 ± 2.5 |
| PAL | 52 | 1.19 ± 0.07 | 57 | 1.20 ± 0.09 |
| Current smoker, n (%) | 62 | 7 (11) | 61 | 6 (10) |
| Alcohol user, n (%) | 62 | 46 (74) | 61 | 38 (62) |
| Test Group | Control Group | Intervention Effect | |
|---|---|---|---|
| Beta (95% CI) a | |||
| Body weight b, kg | |||
| Baseline (n) | 96.11 ± 1.97 (61) | 98.94 ± 1.91 (61) | |
| Change (n) | −2.23 ± 0.41 (50) | −2.92 ± 0.39 (54) | 0.69 (−0.44 to 1.82) |
| p value | <0.001 | <0.001 | 0.226 |
| BMI b, kg/m2 | |||
| Baseline (n) | 32.9 ± 0.6 (61) | 33.7 ± 0.6 (61) | |
| Change (n) | −0.7 ± 0.1 (50) | −1.0 ± 0.1 (54) | 0.2 (−0.2 to 0.6) |
| p value | <0.001 | <0.001 | 0.252 |
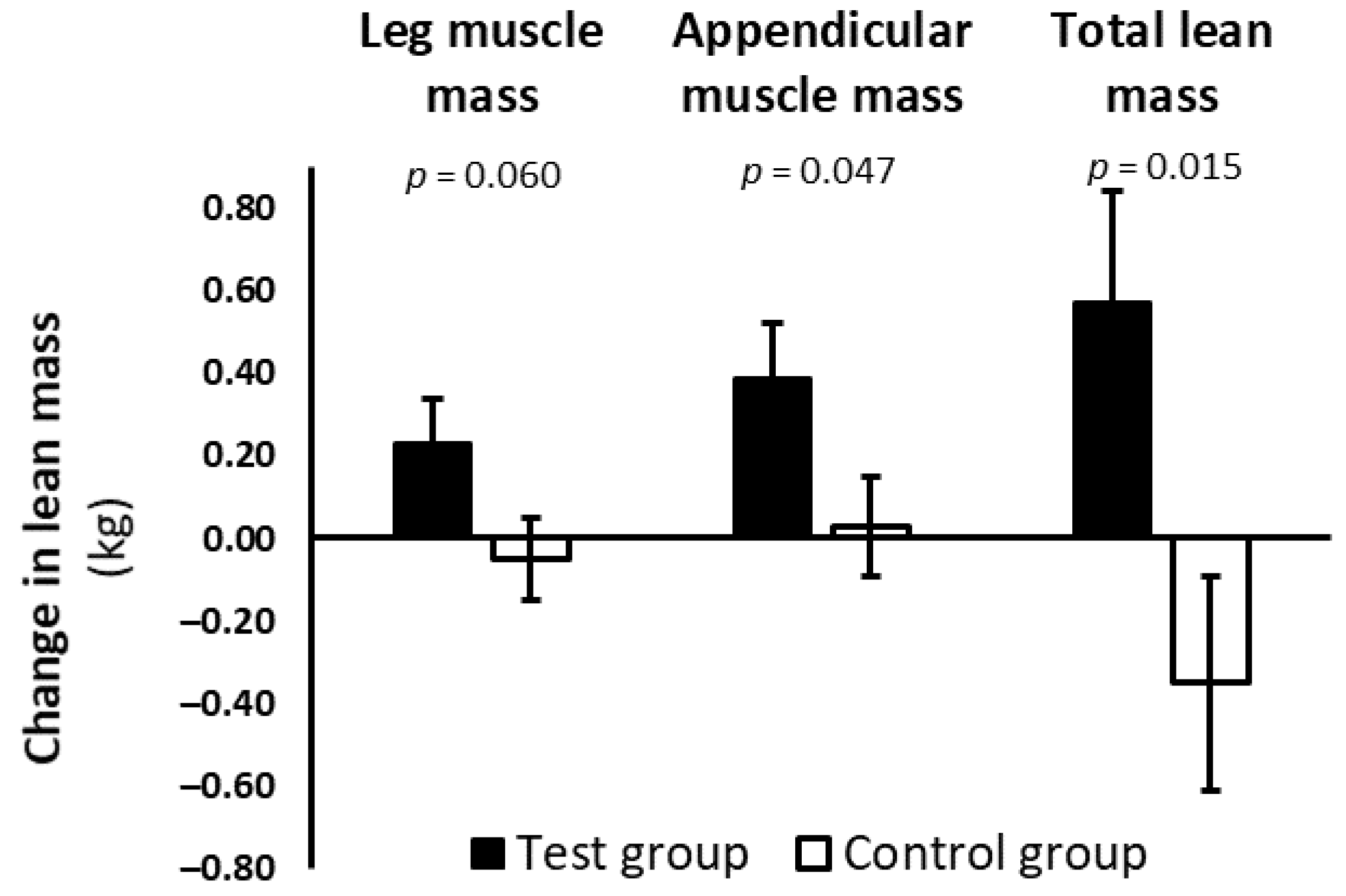
| Leg muscle mass b, kg | |||
| Baseline (n) | 18.69 ± 0.36 (60) | 19.19 ± 0.34 (61) | |
| Change (n) | 0.23 ± 0.11 (49) | −0.05 ± 0.10 (54) | 0.28 (−0.01 to 0.56) |
| p value | 0.030 | 0.655 | 0.060 |
| Appendicular muscle mass b, kg | |||
| Baseline (n) | 25.22 ± 0.48 (58) | 25.90 ± 0.46 (61) | |
| Change (n) | 0.39 ± 0.13 (47) | 0.03 ± 0.12 (51) | 0.36 (0.005 to 0.71) |
| p value | 0.003 | 0.795 | 0.047 |
| Total lean mass b, kg | |||
| Baseline (n) | 60.44 ± 1.01 (59) | 62.11 ± 0.98 (61) | |
| Change (n) | 0.57 ± 0.27 (48) | −0.35 ± 0.26 (52) | 0.92 (0.19 to 1.65) |
| p value | 0.034 | 0.179 | 0.015 |
| Fat mass b, kg | |||
| Baseline (n) | 34.30 ± 1.17 (60) | 35.24 ± 1.13 (61) | |
| Change (n) | −2.63 ± 0.33 (49) | −2.60 ± 0.32 (52) | −0.03 (−0.96 to 0.89) |
| p value | <0.001 | <0.001 | 0.941 |
| Waist circumference b, cm | |||
| Baseline (n) | 113.1 ± 1.4 (60) | 115.1 ± 1.3 (60) | |
| Change (n) | −3.4 ± 0.5 (49) | −3.7 ± 0.5 (52) | 0.2 (−1.2 to 1.7) |
| p value | <0.001 | <0.001 | 0.729 |
| VAT b, cm2 | |||
| Baseline (n) | 177.2 ± 7.1 (61) | 181.1 ± 6.8 (61) | |
| Change (n) | −18.9 ± 3.9 (50) | −17.3 ± 3.8 (54) | −1.6 (−12.5 to 9.3) |
| p value | <0.001 | <0.001 | 0.772 |
| Fasting plasma glucose, mmol/L | |||
| Baseline (n) | 8.38 ± 0.23 (57) | 8.34 ± 0.23 (58) | |
| Change (n) | −0.68 ± 0.23 (47) | −0.66 ± 0.23 (50) | −0.03 (−0.67 to 0.61) |
| p value | 0.004 | 0.004 | 0.936 |
| 2h plasma glucose, mmol/L | |||
| Baseline (n) | 15.82 ± 0.45 (57) | 15.61 ± 0.44 (57) | |
| Change (n) | −0.93 ± 0.37 (47) | −1.29 ± 0.37 (47) | 0.37 (−0.66 to 1.40) |
| p value | 0.013 | 0.001 | 0.477 |
| HbA1c, mmol/mol | |||
| Baseline (n) | 52.3 ± 1.2 (60) | 53.8 ± 1.2 (58) | |
| Change (n) | −4.4 ± 1.1 (49) | −5.7 ± 1.1 (51) | 1.3 (−1.7 to 4.4) |
| p value | <0.001 | <0.001 | 0.390 |
| Fasting plasma insulin, pmol/L | |||
| Baseline (n) | 119.4 ± 7.8 (60) | 104.8 ± 7.6 (59) | |
| Change (n) | −20.1 ± 6.5 (48) | 9.4 ± 6.4 (50) | −29.5 (−47.6 to −11.4) |
| p value | 0.003 | 0.147 | 0.002 |
| HOMA-IR | |||
| Baseline (n) | 6.32 ± 0.44 (57) | 5.52 ± 0.44 (57) | |
| Change (n) | −1.40 ± 0.41 (46) | 0.12 ± 0.40 (49) | −1.52 (−2.65 to −0.39) |
| p value | 0.001 | 0.769 | 0.009 |
| Matsuda index | |||
| Baseline (n) | 2.15 ± 0.18 (55) | 2.19 ± 0.17 (55) | |
| Change (n) | 0.52 ± 0.16 (43) | 0.00 ± 0.16 (44) | 0.52 (0.07 to 0.97) |
| p value | 0.002 | 0.980 | 0.023 |
| Serum calcidiol, nmol/L | |||
| Baseline (n) | 63.8 ± 2.9 (57) | 61.2 ± 2.8 (58) | |
| Change (n) | 18.7 ± 2.8 (45) | −3.3 ± 2.7 (48) | 22.0 (14.2 to 29.7) |
| p value | <0.001 | 0.236 | <0.001 |
| 10-RM leg press, kg | |||
| Baseline (n) | 125 ± 8 (55) | 121 ± 8 (54) | |
| Change (n) | 49 ± 7 (36) | 56 ± 6 (41) | −7 (−26 to 12) |
| p value | <0.001 | <0.001 | 0.462 |
| Knee extension power, Watt | |||
| Baseline (n) | 334 ± 17 (49) | 345 ± 16 (53) | |
| Change (n) | 30 ± 8 (34) | 35 ± 8 (39) | −5 (−27 to 17) |
| p value | <0.001 | <0.001 | 0.652 |
| 400-m walk speed, m/s | |||
| Baseline (n) | 1.37 ± 0.03 (61) | 1.46 ± 0.03 (58) | |
| Change (n) | 0.07 ± 0.02 (48) | 0.04 ± 0.02 (51) | 0.04 (−0.01 to 0.09) |
| p value | <0.001 | 0.044 | 0.166 |
| Usual gait speed, m/s | |||
| Baseline (n) | 1.11 ± 0.03 (62) | 1.17 ± 0.03 (60) | |
| Change (n) | 0.02 ± 0.03 (50) | −0.03 ± 0.03 (53) | 0.04 (−0.03 to 0.12) |
| p value | 0.594 | 0.325 | 0.286 |
| Chair stand, s | |||
| Baseline (n) | 12.1 ± 0.3 (62) | 11.7 ± 0.3 (58) | |
| Change (n) | −1.4 ± 0.3 (50) | −1.2 ± 0.3 (50) | −0.2 (−0.9 to 0.6) |
| p value | <0.001 | <0.001 | 0.677 |
| VO2peak, l/min | |||
| Baseline (n) | 1.60 ± 0.05 (61) | 1.76 ± 0.05 (60) | |
| Change (n) | 0.13 ± 0.04 (42) | 0.11 ± 0.03 (48) | 0.02 (−0.08 to 0.12) |
| p value | 0.001 | 0.002 | 0.665 |
| PAL | |||
| Baseline (n) | 1.18 ± 0.01 (52) | 1.19 ± 0.01 (57) | |
| Change (n) | 0.01 ± 0.01 (41) | 0.00 ± 0.01 (45) | 0.01 (−0.02 to 0.04) |
| p value | 0.335 | 0.833 | 0.580 |
| Test Group (n = 51) | Control Group (n = 54) | p Value a | |
|---|---|---|---|
| Energy intake, kcal/d | 1804 ± 430 | 1731 ± 445 | 0.411 |
| Protein, g/d | 110 ± 23.2 | 77.0 ± 26.4 | <0.001 |
| Protein, g/kg BW/d | 1.15 ± 0.31 | 0.82 ± 0.32 | <0.001 |
| Protein, % of energy | 24.7 ± 3.7 | 17.8 ± 3.8 | <0.001 |
| Carbohydrate, % of energy | 42.0 ± 5.7 | 46.9 ± 6.3 | <0.001 |
| Fat, % of energy | 28.1 ± 6.1 | 30.9 ± 6.8 | 0.037 |
| Saturated fat, % of energy | 9.9 ± 2.8 | 11.7 ± 2.9 | 0.002 |
| Mono-unsaturated fat, % of energy | 9.7 ± 2.9 | 10.6 ± 3.1 | 0.148 |
| Poly-unsaturated fat, % of energy | 6.0 ± 2.0 | 6.0 ± 2.0 | 0.972 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Memelink, R.G.; Pasman, W.J.; Bongers, A.; Tump, A.; van Ginkel, A.; Tromp, W.; Wopereis, S.; Verlaan, S.; de Vogel-van den Bosch, J.; Weijs, P.J.M. Effect of an Enriched Protein Drink on Muscle Mass and Glycemic Control during Combined Lifestyle Intervention in Older Adults with Obesity and Type 2 Diabetes: A Double-Blind RCT. Nutrients 2021, 13, 64. https://doi.org/10.3390/nu13010064
Memelink RG, Pasman WJ, Bongers A, Tump A, van Ginkel A, Tromp W, Wopereis S, Verlaan S, de Vogel-van den Bosch J, Weijs PJM. Effect of an Enriched Protein Drink on Muscle Mass and Glycemic Control during Combined Lifestyle Intervention in Older Adults with Obesity and Type 2 Diabetes: A Double-Blind RCT. Nutrients. 2021; 13(1):64. https://doi.org/10.3390/nu13010064
Chicago/Turabian StyleMemelink, Robert G., Wilrike J. Pasman, Anke Bongers, Anita Tump, Annemieke van Ginkel, Wim Tromp, Suzan Wopereis, Sjors Verlaan, Johan de Vogel-van den Bosch, and Peter J. M. Weijs. 2021. "Effect of an Enriched Protein Drink on Muscle Mass and Glycemic Control during Combined Lifestyle Intervention in Older Adults with Obesity and Type 2 Diabetes: A Double-Blind RCT" Nutrients 13, no. 1: 64. https://doi.org/10.3390/nu13010064
APA StyleMemelink, R. G., Pasman, W. J., Bongers, A., Tump, A., van Ginkel, A., Tromp, W., Wopereis, S., Verlaan, S., de Vogel-van den Bosch, J., & Weijs, P. J. M. (2021). Effect of an Enriched Protein Drink on Muscle Mass and Glycemic Control during Combined Lifestyle Intervention in Older Adults with Obesity and Type 2 Diabetes: A Double-Blind RCT. Nutrients, 13(1), 64. https://doi.org/10.3390/nu13010064





