Modified Fasting Compared to True Fasting Improves Blood Glucose Levels and Subjective Experiences of Hunger, Food Cravings and Mental Fatigue, But Not Cognitive Function: Results of an Acute Randomised Cross-Over Trial
Abstract
1. Introduction
2. Materials and Methods
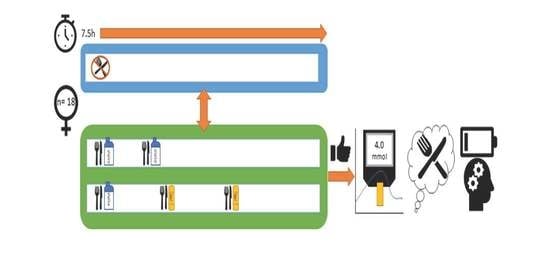
2.1. Design
2.2. Participants
2.3. Intervention
2.4. Blood Glucose Measurement
2.5. Satiety Questionnaire
2.6. Cognitive Fatigue Battery
2.6.1. Mackworth Clock Task (MCT)
2.6.2. Colour Multi-Source Interference Test (cMSIT)
2.6.3. Serial Subtraction
2.7. Subjective Fatigue Visual Analogue Scales (VAS)
2.8. Procedure
2.9. Power and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Friedrich, M. Global obesity epidemic worsening. JAMA 2017, 318, 603. [Google Scholar] [CrossRef] [PubMed]
- Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.-T.; Nguyet, N.T.M.; Dinh, T.C.; Lien, N.V.T.; Nguyen, K.-H.; Ngoc, V.T.N.; Tao, Y.; Le, D.-H.; Nga, V.B.; Jurgoński, A. An update on physical health and economic consequences of overweight and obesity. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 12, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Johns, D.J.; Hartmann-Boyce, J.; Jebb, S.A.; Aveyard, P. Diet or Exercise Interventions vs Combined Behavioral Weight Management Programs: A Systematic Review and Meta-Analysis of Direct Comparisons. J. Acad. Nutr. Diet. 2014, 114, 1557–1568. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.E.; Steptoe, A.; Beeken, R.J.; Kivimaki, M.; Wardle, J. Psychological Changes following Weight Loss in Overweight and Obese Adults: A Prospective Cohort Study. PLoS ONE 2014, 9, e104552. [Google Scholar] [CrossRef]
- Trepanowski, J.F.; Kroeger, C.M.; Barnosky, A.; Klempel, M.C.; Bhutani, S.; Hoddy, K.K.; Gabel, K.; Freels, S.; Rigdon, J.; Rood, J.; et al. Effect of alternate-day fasting on weight loss, weight maintenance, and cardioprotection among metabolically healthy obese adults: A randomized clinical trial. JAMA Intern. Med. 2017, 177, 930–938. [Google Scholar] [CrossRef]
- Headland, M.; Clifton, P.M.; Carter, S.; Keogh, J.B. Weight-Loss Outcomes: A Systematic Review and Meta-Analysis of Intermittent Energy Restriction Trials Lasting a Minimum of 6 Months. Nutrients 2016, 8, 354. [Google Scholar] [CrossRef]
- Klempel, M.C.; Kroeger, C.M.; Norkeviciute, E.; Goslawski, M.; Phillips, S.A.; Varady, K.A. Benefit of a low-fat over high-fat diet on vascular health during alternate day fasting. Nutr. Diabetes 2013, 3, e71. [Google Scholar] [CrossRef]
- Harvie, M.; Howell, A. Energy restriction and the prevention of breast cancer. Proc. Nutr. Soc. 2012, 71, 263–275. [Google Scholar] [CrossRef]
- Harvie, M.; Pegington, M.; Mattson, M.P.; Frystyk, J.; Dillon, B.; Evans, G.; Cuzick, J.; Jebb, S.A.; Martin, B.; Cutler, R.G.; et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: A randomized trial in young overweight women. Int. J. Obes. 2011, 35, 714–727. [Google Scholar] [CrossRef]
- Pender, S.; Gilbert, S.J.; Serpell, L. The Neuropsychology of Starvation: Set-Shifting and Central Coherence in a Fasted Nonclinical Sample. PLoS ONE 2014, 9, e110743. [Google Scholar] [CrossRef] [PubMed]
- Bolton, H.M.; Burgess, P.W.; Gilbert, S.J.; Serpell, L. Increased Set Shifting Costs in Fasted Healthy Volunteers. PLoS ONE 2014, 9, e101946. [Google Scholar] [CrossRef] [PubMed]
- Benau, E.M.; Orloff, N.C.; Janke, E.A.; Serpell, L.; Timko, C.A. A systematic review of the effects of experimental fasting on cognition. Appetite 2014, 77, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, H.R.; Caruso, C.M.; Niro, P.J.; Adam, G.E.; Kellogg, M.D.; Nindl, B.C.; Kramer, F.M. A double-blind, placebo-controlled test of 2 d of calorie deprivation: Effects on cognition, activity, sleep, and interstitial glucose concentrations. Am. J. Clin. Nutr. 2008, 88, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Solianik, R.; Sujeta, A. Two-day fasting evokes stress, but does not affect mood, brain activity, cognitive, psychomotor, and motor performance in overweight women. Behav. Brain Res. 2018, 338, 166–172. [Google Scholar] [CrossRef]
- de Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef]
- Benton, D.; Ruffin, M.P.; Lassel, T.; Nabb, S.; Messaoudi, M.; Vinoy, S.; Desor, D.; Lang, V. The delivery rate of dietary carbohydrates affects cognitive performance in both rats and humans. Psychopharmacology 2003, 166, 86–90. [Google Scholar] [CrossRef]
- Polonsky, W.H.; Jelsovsky, Z.; Panzera, S.; Parkin, C.G.; Wagner, R.S. Primary care physicians identify and act upon glycemic abnormalities found in structured, episodic blood glucose monitoring data from non-insulin-treated type 2 diabetes. Diabetes Technol. Ther. 2009, 11, 283–291. [Google Scholar] [CrossRef]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 38–48. [Google Scholar] [CrossRef]
- Belobrajdic, D.P.; Regina, A.; Klingner, B.; Zajac, I.; Chapron, S.; Berbezy, P.; Bird, A.R. High-Amylose Wheat Lowers the Postprandial Glycemic Response to Bread in Healthy Adults: A Randomized Controlled Crossover Trial. J. Nutr. 2019, 149, 1335–1345. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Haskell, C.F.; Robertson, B.; Reay, J.; Brewster-Maund, C.; Luedemann, J.; Maggini, S.; Ruf, M.; Zangara, A.; Scholey, A.B. Improved cognitive performance and mental fatigue following a multi-vitamin and mineral supplement with added guarana (Paullinia cupana). Appetite 2008, 50, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Lichstein, K.L.; Riedel, B.W.; Richman, S.L. The Mackworth Clock Test: A computerized version. J. Psychol. 2000, 134, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, M.; Kubiak, T.; Conner, T.S. Positive affect and self-control: Attention to self-control demands mediates the influence of positive affect on consecutive self-control. Cogn. Emot. 2014, 28, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Bond, A.; Lader, M. The use of analogue scales in rating subjective feelings. Brit. J. Med. Psychol. 1974. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org (accessed on 28 December 2020).
- Bakdash, J.; Marusich, L. Repeated measures correlation. Front. Psychol. 2016, 8. [Google Scholar] [CrossRef]
- Fitzmaurice, G.M.; Laird, N.M.; Ware, J.H. Modeling the mean: Analyzing response profiles. In Applied Longitudinal Analysis, 2nd ed.; John Wiley & Sons, Incorporated: Hoboken, NJ, USA, 2011. [Google Scholar]
- Bell, M.L.; King, M.T.; Fairclough, D.L. Bias in area under the curve for longitudinal clinical trials with missing patient reported outcome data: Summary measures versus summary statistics. SAGE Open 2014, 4. [Google Scholar] [CrossRef]
- Brindal, E.; Baird, D.; Danthiir, V.; Wilson, C.; Bowen, J.; Slater, A.; Noakes, M. Ingesting breakfast meals of different glycaemic load does not alter cognition and satiety in children. Eur. J. Clin. Nutr. 2012, 66, 1166–1171. [Google Scholar] [CrossRef][Green Version]
- Sünram-Lea, S.I.; Owen, L. The impact of diet-based glycaemic response and glucose regulation on cognition: Evidence across the lifespan. Proc. Nutr. Soc. 2017, 76, 466–477. [Google Scholar] [CrossRef]
- Kemps, E.; Tiggemann, M. Working memory performance and preoccupying thoughts in female dieters: Evidence for a selective central executive impairment. Br. J. Clin. Psychol. 2005, 44, 357–366. [Google Scholar] [CrossRef]
- Higgs, S. Cognitive processing of food rewards. Appetite 2016, 104, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Stamataki, N.S.; Elliott, R.; McKie, S.; McLaughlin, J.T. Attentional bias to food varies as a function of metabolic state independent of weight status. Appetite 2019, 143, 104388. [Google Scholar] [CrossRef] [PubMed]
- Jonker, N.C.; Bennik, E.C.; De Lang, T.A.; De Jong, P.J. Influence of hunger on attentional engagement with and disengagement from pictorial food cues in women with a healthy weight. Appetite 2020, 151, 104686. [Google Scholar] [CrossRef] [PubMed]
- Prickett, C.; Brennan, L.; Stolwyk, R. Examining the relationship between obesity and cognitive function: A systematic literature review. Obes. Res. Clin. Pract. 2015, 9, 93–113. [Google Scholar] [CrossRef]


| Nutritional Information | Liquid Meal Replacement (LMR) 1 | Snack Replacement Bar (SRB) |
|---|---|---|
| Weight (g) | 276 | 35 |
| Energy (kJ) | 1071 | 555 |
| Energy (cal) | 256 | 133 |
| Protein (g) | 25.1 | 10.1 |
| Fat (g) | 4.1 | 5.4 |
| Carb (g) | 26.1 | 4.1 |
| Fibre (g) | 6.2 | 4.4 |
| Weight (g) | 276 | 35 |
| Condition | Time | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 07:30 | 08:30 | 09:30 | 10:00 | 11:00 | 11:30 | 12:00 | 12:30 | 13:00 | 14:00 | 15:00 | 15:30 | 16:00 | |
| Bulking | LMR | LMR | |||||||||||
| Extended | LMR | SRB | SRB | ||||||||||
| Fasting | Nil Intake | ||||||||||||
| Assessments | Practice | Baseline | Time 1 | Time 2 | Time 3 | Time 4 | Time 5 | Time 6 | Time 7 | ||||
| (SFS, CFB) | (SFS, CFB, BG, SQ) | (SFS, CFB, BG, SQ) | (BG, SQ) | (SFS, CFB, BG, SQ) | (BG, SQ) | (SFS, CFB, BG, SQ) | (BG, SQ) | (SFS, CFB, BG, SQ) | |||||
| Outcome Variables | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | 13. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Blood glucose | −0.09 | −0.14 * | 0.10 | −0.04 | −0.01 | 0.01 | 0.00 | 0.00 | 0.05 | 0.00 | 0.06 | 0.07 | |
| 2. Mental fatigue | −0.09 | 0.20 * | −0.17 | 0.13 | 0.05 | 0.15 | 0.10 | 0.02 | 0.09 | 0.05 | −0.05 | −0.03 | |
| 3. Hunger | −0.14 ** | 0.20 ** | −0.77 *** | 0.21 *** | 0.19 ** | 0.45 *** | 0.26 *** | 0.03 | 0.04 | 0.03 | −0.13 | −0.09 | |
| 4. Fullness | 0.10 | −0.17 * | −0.77 *** | −0.12 | −0.17 ** | −0.36 *** | −0.25 *** | −0.07 | 0.00 | 0.04 | 0.11 | 0.12 | |
| 5. Sweet craving | −0.04 | 0.13 | 0.21 *** | −0.12 * | 0.31 *** | 0.31 *** | 0.21 *** | 0.09 | 0.04 | 0.07 | 0.02 | −0.05 | |
| 6. Salty craving | −0.01 | 0.05 | 0.19 *** | −0.17 ** | 0.31 *** | 0.37 *** | 0.33 *** | 0.08 | −0.09 | −0.06 | 0.09 | 0.08 | |
| 7. Savoury craving | 0.01 | 0.15 * | 0.45 *** | −0.36 *** | 0.31 *** | 0.37 *** | 0.24 *** | 0.11 | −0.04 | −0.06 | −0.02 | 0.09 | |
| 8. Fatty craving | 0.00 | 0.10 | 0.26 *** | −0.25 *** | 0.21 *** | 0.33 *** | 0.24 *** | 0.07 | 0.04 | 0.09 | −0.04 | −0.15 | |
| 9. MCT | 0.00 | 0.02 | 0.03 | −0.07 | 0.09 | 0.08 | 0.11 | 0.07 | 0.02 | 0.07 | 0.27 *** | 0.19 * | |
| 10. MSIT effect | 0.05 | 0.09 | 0.04 | 0.00 | 0.04 | −0.09 | −0.04 | 0.04 | 0.02 | −0.03 | 0.00 | 0.03 | |
| 11. MSIT attention effect | 0.00 | 0.05 | 0.03 | 0.04 | 0.07 | −0.06 | −0.06 | 0.09 | 0.07 | −0.03 | −0.03 | −0.10 | |
| 12. Serial 3s correct | 0.06 | −0.05 | −0.13 | 0.11 | 0.02 | 0.09 | −0.02 | −0.04 | 0.27 *** | 0.00 | −0.03 | 0.46 *** | |
| 13. Serial 7s correct | 0.07 | −0.03 | −0.09 | 0.12 | −0.05 | 0.08 | 0.09 | −0.15 * | 0.19 ** | 0.03 | −0.10 | 0.46 *** |
| Model Estimates | Blood Glucose (mmol/L) | Mental Fatigue | Hunger | Fullness |
|---|---|---|---|---|
| Fixed effects (B ± SE) | ||||
| (Intercept) | 4.95 ± 0.12 *** | −7.07 ± 5.78 | 0.03 ± 0.20 | −0.30 ± 0.22 |
| Time: T1 | −0.25 ± 0.11 * | 2.41 ± 3.02 | −0.03 ± 0.19 | 0.22 ± 0.20 |
| Time: T2 | −0.46 ± 0.11 *** | 0.18 ± 0.20 | −0.05 ± 0.20 | |
| Time: T3 | −0.40 ± 0.12 ** | 5.79 ± 3.99 | 0.51 ± 0.21 * | −0.15 ± 0.21 |
| Time: T4 | −0.52 ± 0.12 *** | 0.61 ± 0.22 ** | −0.29 ± 0.22 | |
| Time: T5 | −0.42 ± 0.12 ** | 10.72 ± 5.23 * | 0.69 ± 0.23 ** | −0.18 ± 0.23 |
| Time: T6 | −0.62 ± 0.12 *** | 0.92 ± 0.24 *** | −0.54 ± 0.24 * | |
| Time: T7 | −0.55 ± 0.13 *** | 10.88 ± 6.44 | 0.90 ± 0.25 *** | −0.54 ± 0.25 * |
| Bx × Bulking | 0.01 ± 0.12 | −4.24 ± 4.26 | −0.28 ± 0.21 | 0.23 ± 0.24 |
| T1 × Bulking | 0.15 ± 0.12 | −7.10 ± 3.87 | −0.96 ± 0.21 *** | 1.07 ± 0.23 *** |
| T2 × Bulking | 0.20 ± 0.12 | −0.69 ± 0.21 ** | 0.78 ± 0.23 ** | |
| T3 × Bulking | 0.32 ± 0.12 * | −9.18 ± 3.56 * | −1.48 ± 0.22 *** | 1.36 ± 0.23 *** |
| T4 × Bulking | 0.38 ± 0.13 ** | −1.21 ± 0.22 *** | 1.30 ± 0.23 *** | |
| T5 × Bulking | 0.15 ± 0.13 | −7.80 ± 3.46 * | −0.92 ± 0.23 *** | 0.67 ± 0.23 ** |
| T6 × Bulking | 0.06 ± 0.13 | −0.85 ± 0.23 *** | 0.62 ± 0.23 ** | |
| T7 × Bulking | 0.12 ± 0.13 | −2.42 ± 3.53 | −0.52 ± 0.24 * | 0.38 ± 0.24 |
| Bx × Extended | 0.12 ± 0.12 | 2.09 ± 4.26 | −0.12 ± 0.21 | 0.21 ± 0.24 |
| T1 × Extended | 0.08 ± 0.12 | −1.10 ± 3.87 | −0.84 ± 0.21 *** | 1.03 ± 0.23 *** |
| T2 × Extended | 0.35 ± 0.12 ** | −0.52 ± 0.21 * | 0.66 ± 0.23 ** | |
| T3 × Extended | 0.19 ± 0.12 | −7.92 ± 3.56 * | −0.78 ± 0.22 *** | 0.86 ± 0.23 *** |
| T4 × Extended | 0.55 ± 0.13 *** | −1.02 ± 0.22 *** | 0.93 ± 0.23 *** | |
| T5 × Extended | 0.18 ± 0.13 | −3.54 ± 3.46 | −0.63 ± 0.23 ** | 0.34 ± 0.23 |
| T6 × Extended | 0.16 ± 0.13 | −0.38 ± 0.23 | 0.27 ± 0.23 | |
| T7 × Extended | 0.79 ± 0.13 *** | −1.19 ± 3.53 | −0.87 ± 0.24 *** | 1.02 ± 0.24 *** |
| Random effects (SD) | ||||
| Participant | ||||
| (Intercept) | 0.35 | 20.50 | 0.56 | 0.57 |
| Time (hrs) | 0.03 | 2.84 | 0.07 | 0.07 |
| Participant × Session | ||||
| (Intercept) | 0.13 | 9.70 | 0.27 | 0.41 |
| Time (h) | 0.01 | 1.20 | 0.04 | 0.04 |
| Residual | 0.32 | 7.62 | 0.53 | 0.55 |
| AUC differential (± SE) | ||||
| Bulking—Fasting | 1.33 ± 0.47 ** | −27.40 ± 10.30 ** | −6.52 ± 0.91 *** | 6.10 ± 0.97 *** |
| Extended—Fasting | 1.97 ± 0.47 *** | −12.11 ± 10.30 | −4.66 ± 0.91 *** | 4.71 ± 0.97 *** |
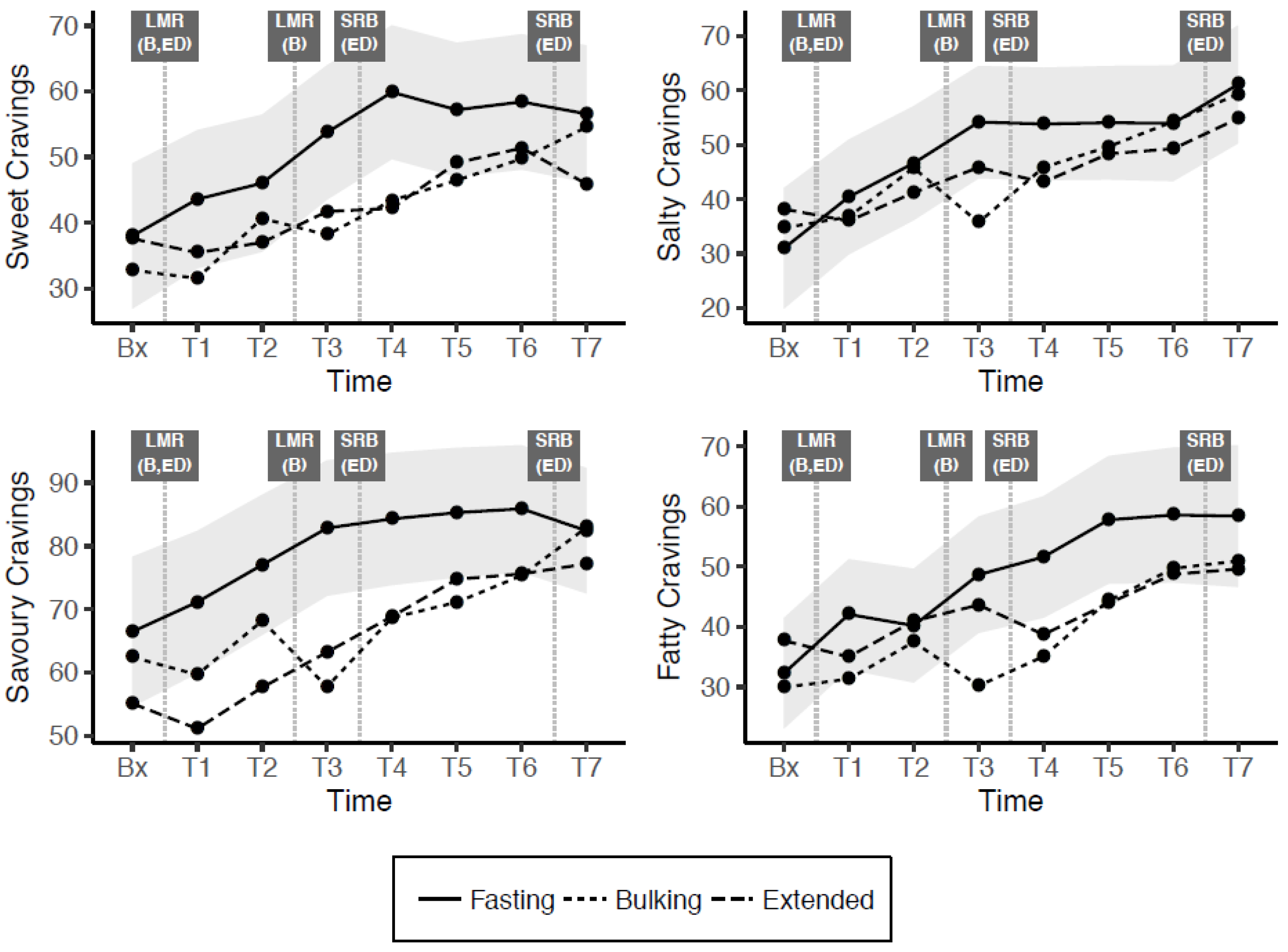
| Model Estimates | Sweet Craving | Salty Craving | Savoury Craving | Fatty Craving |
|---|---|---|---|---|
| Fixed effects (B ± SE) | ||||
| (Intercept) | 37.99 ± 6.92 *** | 30.98 ± 7.02 *** | 66.44 ± 5.37 *** | 32.30 ± 7.71 *** |
| Time: T1 | 5.60 ± 3.95 | 9.44 ± 3.81 * | 4.69 ± 4.16 | 9.79 ± 3.98 * |
| Time: T2 | 8.05 ± 4.22 | 15.64 ± 4.10 *** | 10.57 ± 4.38 * | 7.92 ± 4.37 |
| Time: T3 | 15.77 ± 4.46 *** | 23.17 ± 4.36 *** | 16.39 ± 4.54 *** | 16.37 ± 4.79 ** |
| Time: T4 | 21.90 ± 4.80 *** | 22.84 ± 4.72 *** | 17.85 ± 4.81 *** | 19.31 ± 5.31 *** |
| Time: T5 | 19.22 ± 5.18 *** | 23.05 ± 5.13 *** | 18.84 ± 5.12 *** | 25.47 ± 5.88 *** |
| Time: T6 | 20.43 ± 5.61 *** | 22.96 ± 5.58 *** | 19.45 ± 5.46 *** | 26.28 ± 6.50 *** |
| Time: T7 | 18.60 ± 5.98 ** | 30.12 ± 5.97 *** | 15.97 ± 5.76 ** | 26.11 ± 7.03 *** |
| Bx × Bulking | −5.20 ± 5.65 | 3.75 ± 5.66 | −4.01 ± 6.06 | −2.31 ± 4.69 |
| T1 × Bulking | −12.04 ± 5.38 * | −3.65 ± 5.39 | −11.49 ± 5.75 * | −10.75 ± 4.68 * |
| T2 × Bulking | −5.37 ± 5.34 | −0.87 ± 5.35 | −8.85 ± 5.66 | −2.72 ± 4.83 |
| T3 × Bulking | −15.60 ± 5.23 ** | −18.43 ± 5.27 ** | −25.11 ± 5.47 *** | −18.44 ± 4.94 *** |
| T4 × Bulking | −16.48 ± 5.20 ** | −7.99 ± 5.28 | −15.64 ± 5.35 ** | −16.52 ± 5.15 ** |
| T5 × Bulking | −10.73 ± 5.22 * | −4.39 ± 5.33 | −14.18 ± 5.24 ** | −13.41 ± 5.42 * |
| T6 × Bulking | −8.67 ± 5.27 | 0.33 ± 5.43 | −10.55 ± 5.14 * | −8.88 ± 5.73 |
| T7 × Bulking | −2.02 ± 5.34 | −1.80 ± 5.54 | 0.53 ± 5.07 | −7.47 ± 6.01 |
| Bx × Extended | −0.40 ± 5.65 | 7.21 ± 5.66 | −11.35 ± 6.06 | 5.42 ± 4.69 |
| T1 × Extended | −8.15 ± 5.38 | −4.42 ± 5.39 | −20.02 ± 5.75 ** | −7.05 ± 4.68 |
| T2 × Extended | −9.03 ± 5.34 | −5.41 ± 5.35 | −19.38 ± 5.66 ** | 0.76 ± 4.83 |
| T3 × Extended | −12.05 ± 5.23 * | −8.31 ± 5.27 | −19.63 ± 5.47 *** | −5.02 ± 4.94 |
| T4 × Extended | −17.68 ± 5.20 ** | −10.72 ± 5.28 * | −15.44 ± 5.35 ** | −12.87 ± 5.16 * |
| T5 × Extended | −8.07 ± 5.22 | −5.69 ± 5.33 | −10.54 ± 5.24 * | −13.87 ± 5.42 * |
| T6 × Extended | −7.04 ± 5.27 | −4.68 ± 5.43 | −10.29 ± 5.14 * | −9.85 ± 5.73 |
| T7 × Extended | −10.68 ± 5.34 * | −6.27 ± 5.54 | −5.24 ± 5.07 | −8.80 ± 6.01 |
| Random effects (SD) | ||||
| Participant | ||||
| (Intercept) | 22.59 | 23.06 | 12.99 | 27.89 |
| Time (h) | 2.03 | 2.01 | 1.96 | 2.63 |
| Participant × Session | ||||
| (Intercept) | 11.95 | 12.37 | 12.86 | 8.26 |
| Time (hrs) | 1.22 | 1.37 | 0.78 | 1.59 |
| Residual | 10.66 | 10.22 | 11.37 | 10.38 |
| AUC differential (± SE) | ||||
| Bulking—Fasting | −72.50 ± 26.18 ** | −34.02 ± 27.53 | −87.55 ± 26.61 ** | −75.61 ± 24.99 ** |
| Extended—Fasting | −67.54 ± 26.18 ** | −38.76 ± 27.53 | −103.60 ± 26.62 *** | −49.59 ± 24.98 * |
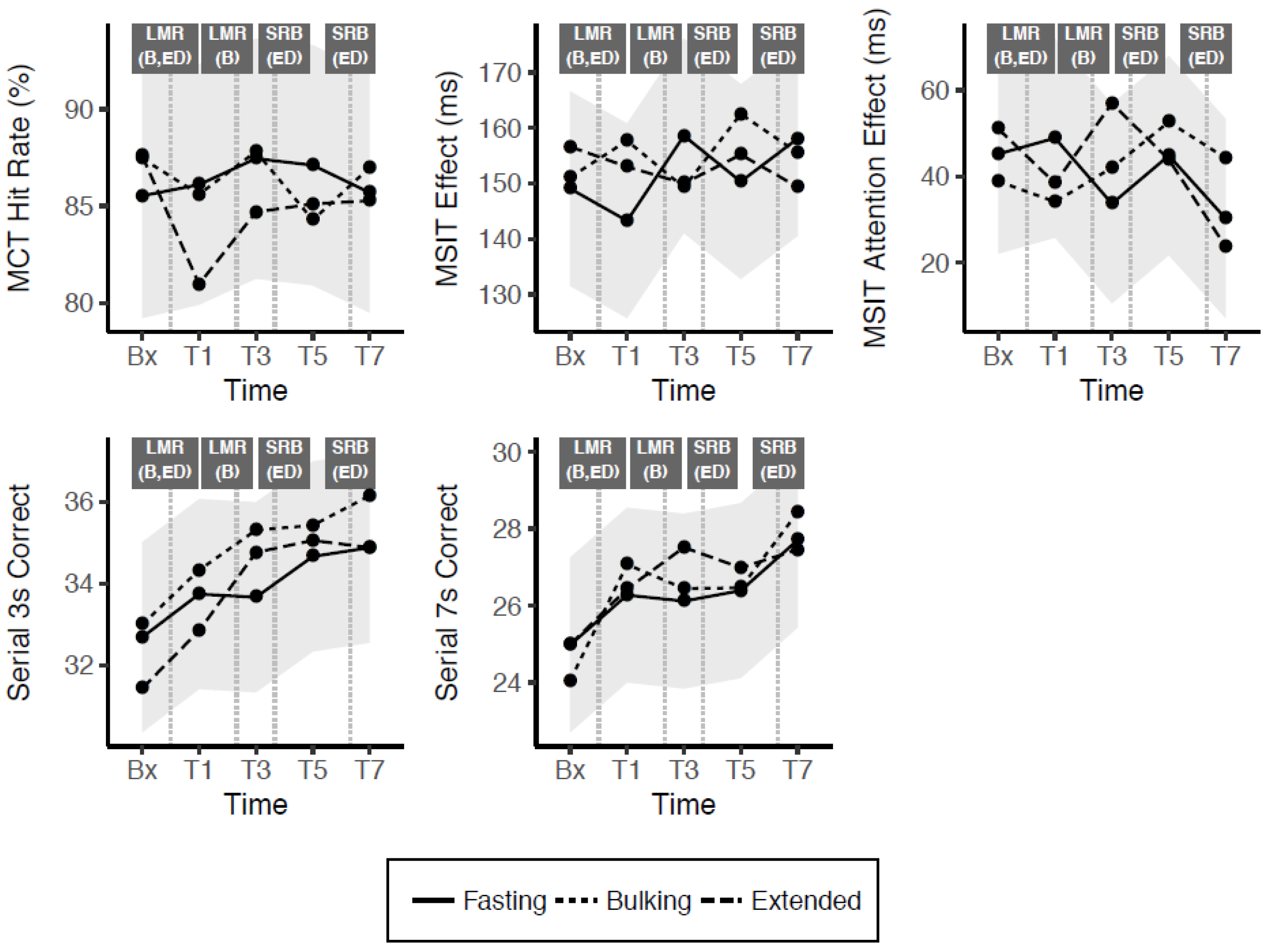
| Model Estimates | MCT Hit Rate (%) | MSIT Effect (ms) | MSIT Attention Effect (ms) | Serial 3s Correct Trials a | Serial 7s Correct Trials a |
|---|---|---|---|---|---|
| Fixed effects (B ± SE) | |||||
| (Intercept) | 92.74 ± 8.69 *** | 201.64 ± 21.33 *** | 38.13 ± 17.89 * | 39.65 ± 6.06 *** | 29.93 ± 6.56 *** |
| Time: T1 | 0.58 ± 3.19 | 2.06 ± 8.96 | −6.32 ± 11.78 | 1.06 ± 0.85 | 1.29 ± 0.86 |
| Time: T3 | 1.93 ± 3.26 | 7.56 ± 8.96 | 5.85 ± 11.78 | 0.98 ± 1.04 | 1.14 ± 1.03 |
| Time: T5 | 1.58 ± 3.38 | −5.80 ± 8.96 | 3.69 ± 11.78 | 1.98 ± 1.12 | 1.41 ± 1.10 |
| Time: T7 | 0.17 ± 3.52 | 8.65 ± 8.96 | −11.06 ± 11.78 | 2.20 ± 1.16 | 2.73 ± 1.13 |
| Age | 0.02 ± 0.24 | −0.66 ± 0.59 | 0.16 ± 0.42 | −0.34 ± 0.18 | −0.37 ± 0.19 |
| Week | −2.61 ± 0.71 *** | −10.45 ± 2.01 *** | 0.67 ± 2.64 | 1.31 ± 0.38 ** | 2.24 ± 0.37 *** |
| Bx × Bulking | 1.94 ± 3.17 | 3.96 ± 8.96 | −6.77 ± 11.78 | 0.31 ± 1.19 | −0.93 ± 1.16 |
| T1 × Bulking | −0.50 ± 3.17 | 7.48 ± 8.96 | −5.18 ± 11.78 | 0.59 ± 1.19 | 0.80 ± 1.16 |
| T3 × Bulking | 0.36 ± 3.17 | −7.35 ± 8.96 | −9.04 ± 11.78 | 1.65 ± 1.19 | 0.33 ± 1.16 |
| T5 × Bulking | −2.83 ± 3.17 | 6.75 ± 8.96 | 8.03 ± 11.78 | 0.76 ± 1.19 | 0.07 ± 1.16 |
| T7 × Bulking | 1.29 ± 3.17 | −7.37 ± 8.96 | 10.70 ± 11.78 | 1.27 ± 1.19 | 0.72 ± 1.16 |
| Bx × Extended | 2.07 ± 3.22 b | 13.47 ± 8.96 | 7.43 ± 11.78 | −1.25 ± 1.19 | 0.01 ± 1.16 |
| T1 × Extended | −5.14 ± 3.17 | 4.08 ± 8.96 | 5.06 ± 11.78 | −0.90 ± 1.19 | 0.16 ± 1.16 |
| T3 × Extended | −2.76 ± 3.17 | 1.48 ± 8.96 | −20.79 ± 11.78 | 1.10 ± 1.19 | 1.40 ± 1.16 |
| T5 × Extended | −2.00 ± 3.17 | 12.27 ± 8.96 | −4.63 ± 11.78 | 0.39 ± 1.19 | 0.59 ± 1.16 |
| T7 × Extended | −0.42 ± 3.17 | −8.34 ± 8.96 | −10.43 ± 11.78 | 0.00 ± 1.19 | −0.25 ± 1.16 |
| Random effects (SD) | |||||
| Participant | |||||
| (Intercept) | 16.60 | 20.28 | 12.48 | 6.31 | 6.91 |
| Time (hrs) | 0.81 | ||||
| Residual | 8.92 | 25.23 | 33.19 | 3.35 | 3.27 |
| AUC differential (±SE) | |||||
| Bulking—Fasting | −1.35 ± 5.73 | 23.33 ± 19.35 | −4.22 ± 21.30 | 3.79 ± 3.16 | 1.10 ± 3.02 |
| Extended—Fasting | −9.08 ± 5.73 | 8.22 ± 19.35 | −21.86 ± 21.30 | −0.03 ± 3.16 | 2.03 ± 3.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zajac, I.; Herreen, D.; Hunkin, H.; James-Martin, G.; Doyen, M.; Kakoschke, N.; Brindal, E. Modified Fasting Compared to True Fasting Improves Blood Glucose Levels and Subjective Experiences of Hunger, Food Cravings and Mental Fatigue, But Not Cognitive Function: Results of an Acute Randomised Cross-Over Trial. Nutrients 2021, 13, 65. https://doi.org/10.3390/nu13010065
Zajac I, Herreen D, Hunkin H, James-Martin G, Doyen M, Kakoschke N, Brindal E. Modified Fasting Compared to True Fasting Improves Blood Glucose Levels and Subjective Experiences of Hunger, Food Cravings and Mental Fatigue, But Not Cognitive Function: Results of an Acute Randomised Cross-Over Trial. Nutrients. 2021; 13(1):65. https://doi.org/10.3390/nu13010065
Chicago/Turabian StyleZajac, Ian, Danielle Herreen, Hugh Hunkin, Genevieve James-Martin, Mathilde Doyen, Naomi Kakoschke, and Emily Brindal. 2021. "Modified Fasting Compared to True Fasting Improves Blood Glucose Levels and Subjective Experiences of Hunger, Food Cravings and Mental Fatigue, But Not Cognitive Function: Results of an Acute Randomised Cross-Over Trial" Nutrients 13, no. 1: 65. https://doi.org/10.3390/nu13010065
APA StyleZajac, I., Herreen, D., Hunkin, H., James-Martin, G., Doyen, M., Kakoschke, N., & Brindal, E. (2021). Modified Fasting Compared to True Fasting Improves Blood Glucose Levels and Subjective Experiences of Hunger, Food Cravings and Mental Fatigue, But Not Cognitive Function: Results of an Acute Randomised Cross-Over Trial. Nutrients, 13(1), 65. https://doi.org/10.3390/nu13010065