Parenteral Fish-Oil Containing Lipid Emulsions Limit Initial Lipopolysaccharide-Induced Host Immune Responses in Preterm Pigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care and Surgery
2.2. Nutrition Protocol and Study Design
2.3. LPS Infusion Protocol
2.4. Fatty Acid Analysis
2.5. Cytokine Measurements
2.6. Oxylipin Analysis
2.7. Statistical Analysis
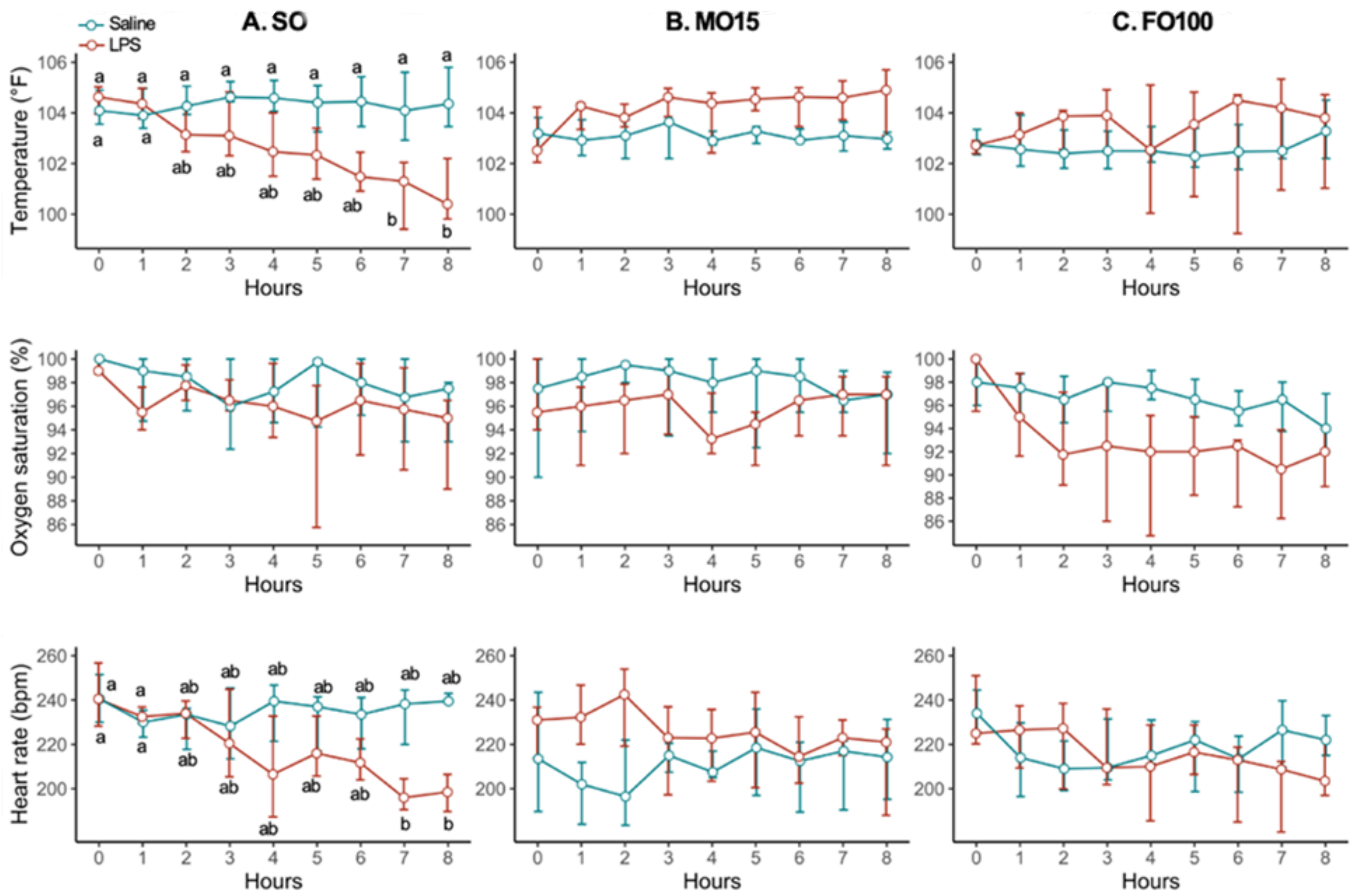
3. Results
3.1. Pig Cohort Characteristics
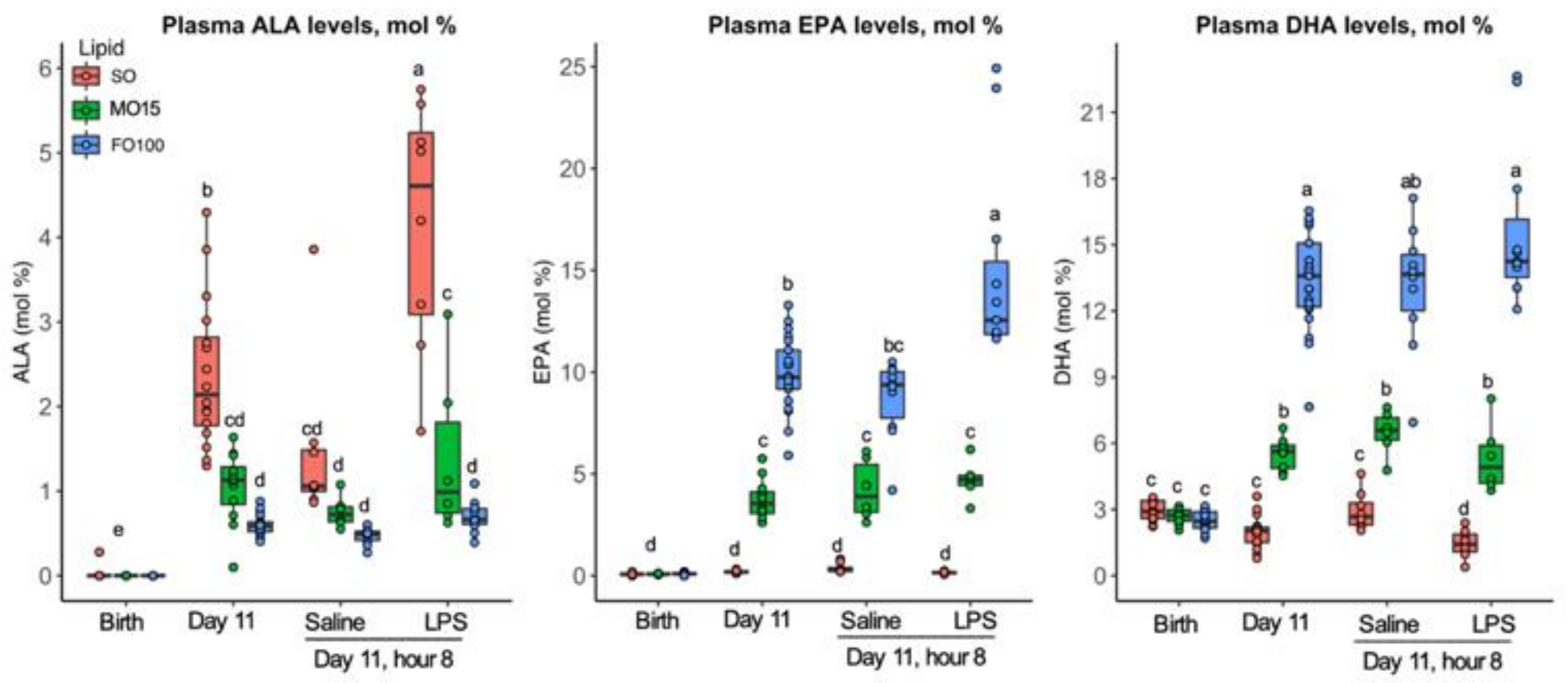
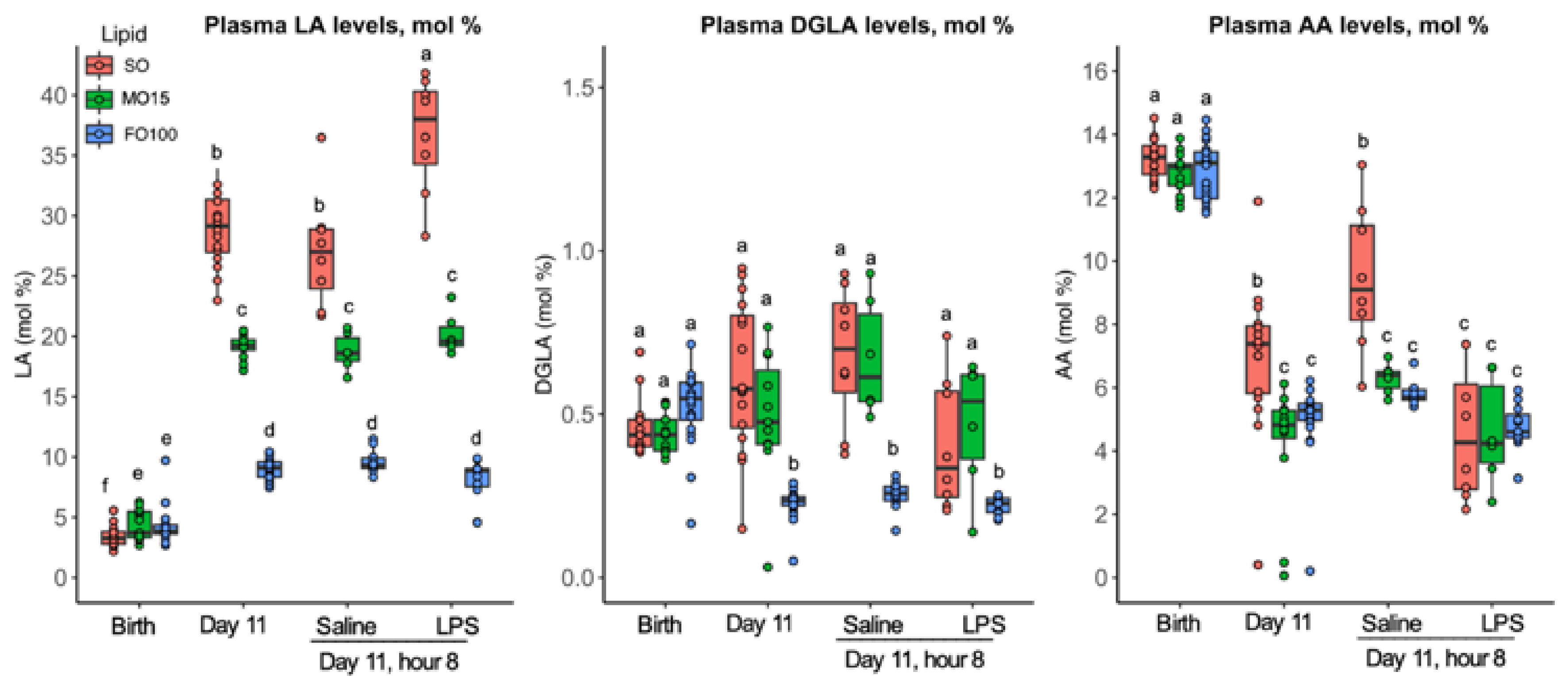
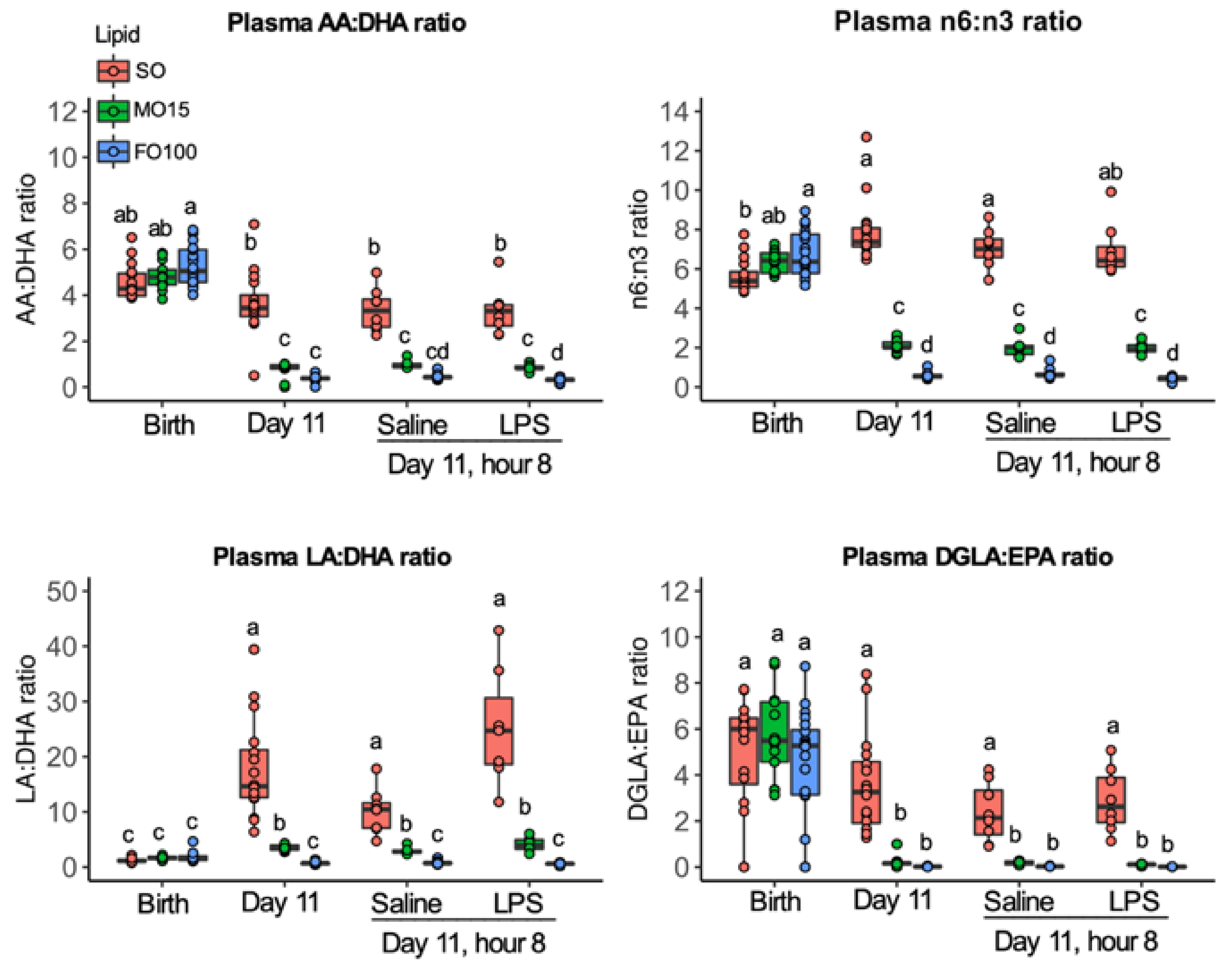
3.2. Fatty Acid Changes
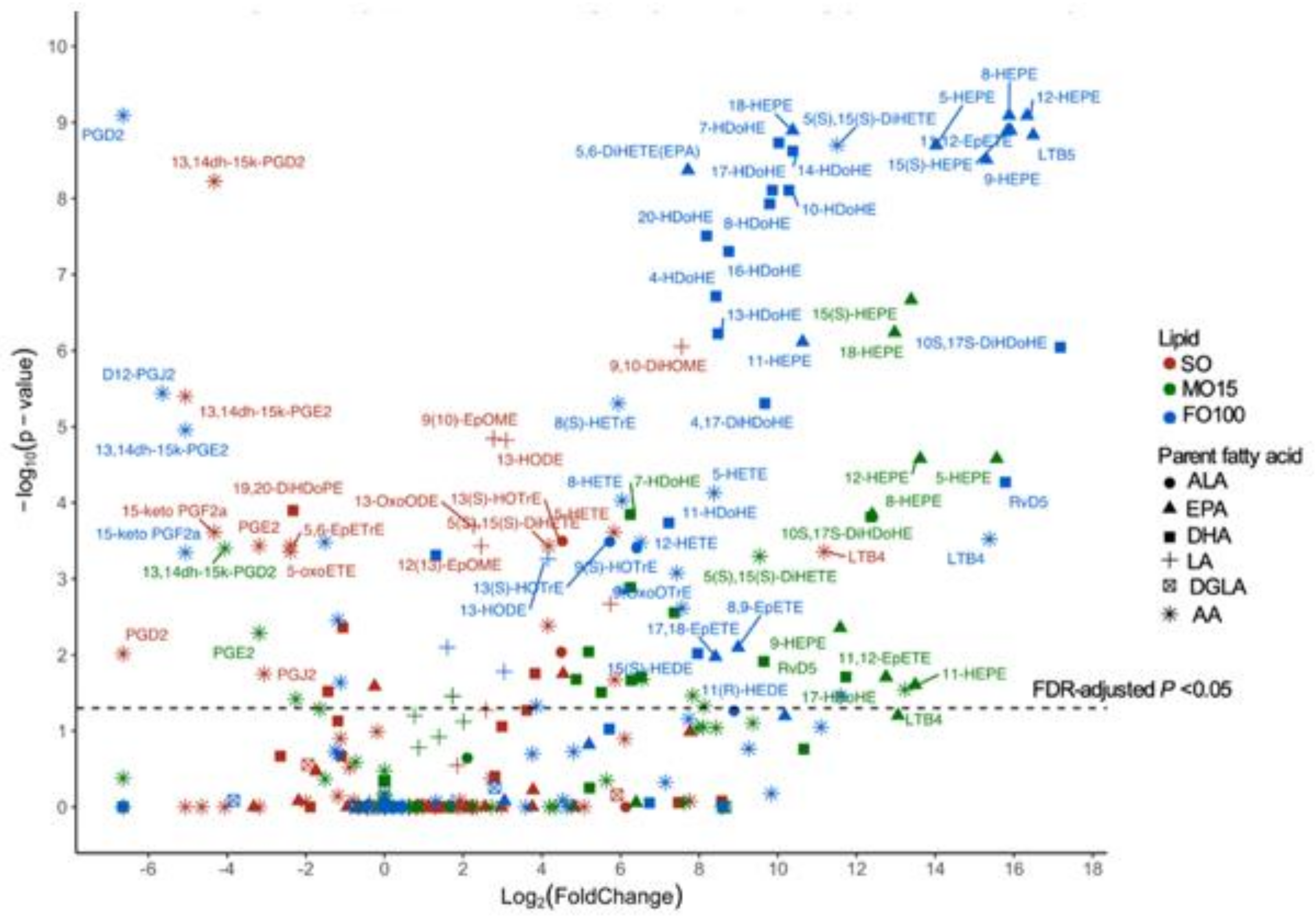
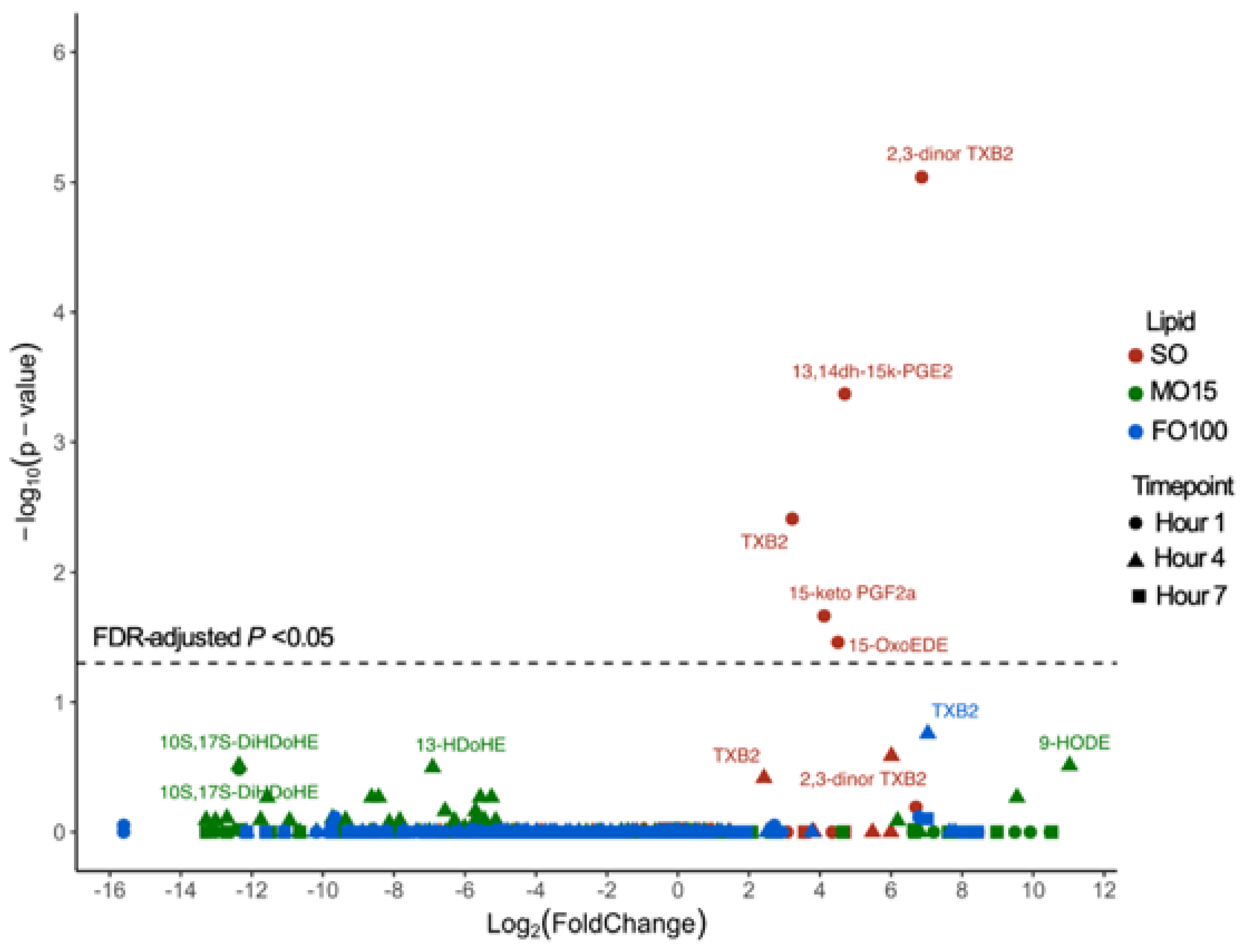
3.3. Plasma Oxylipin Changes
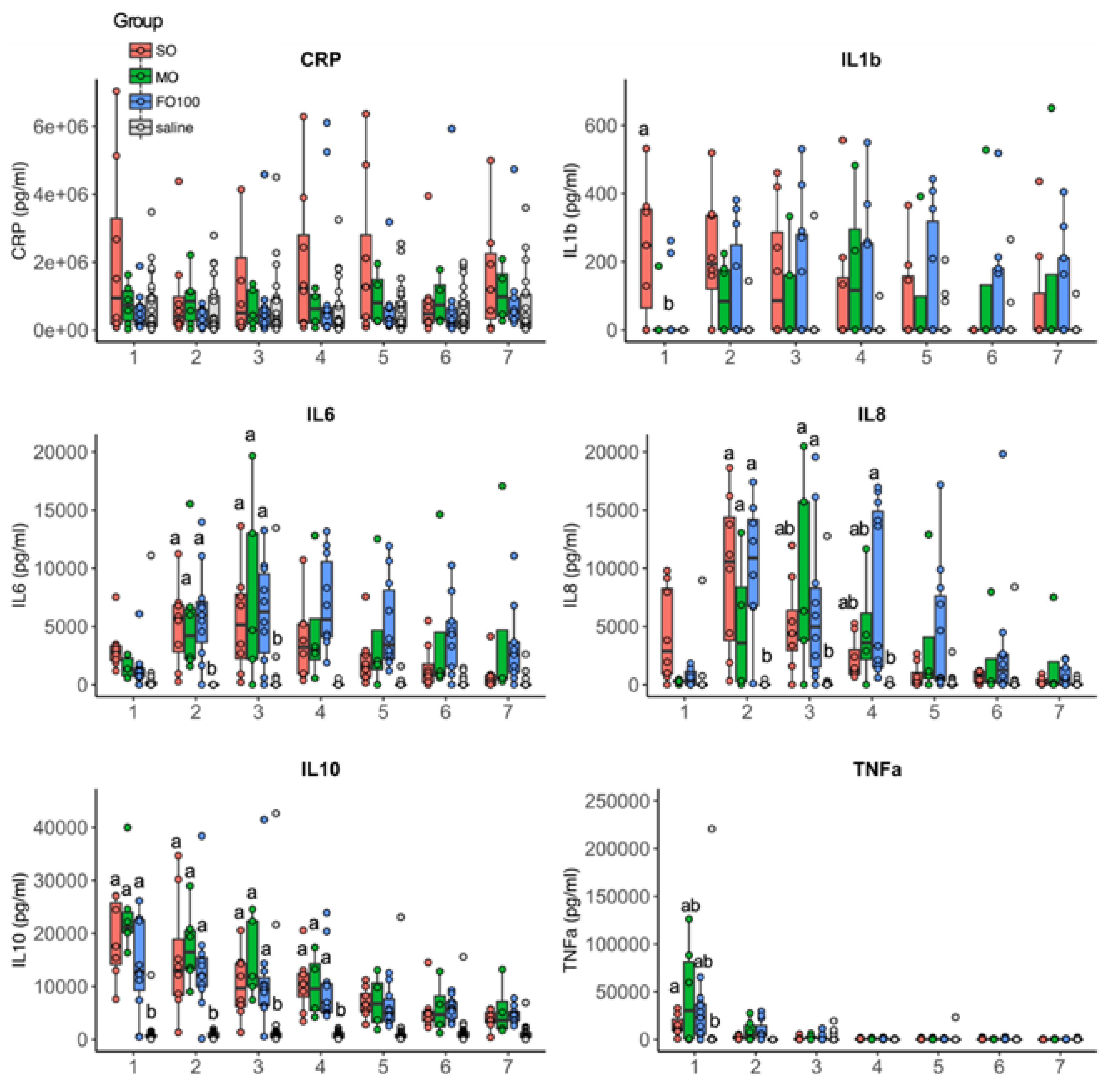
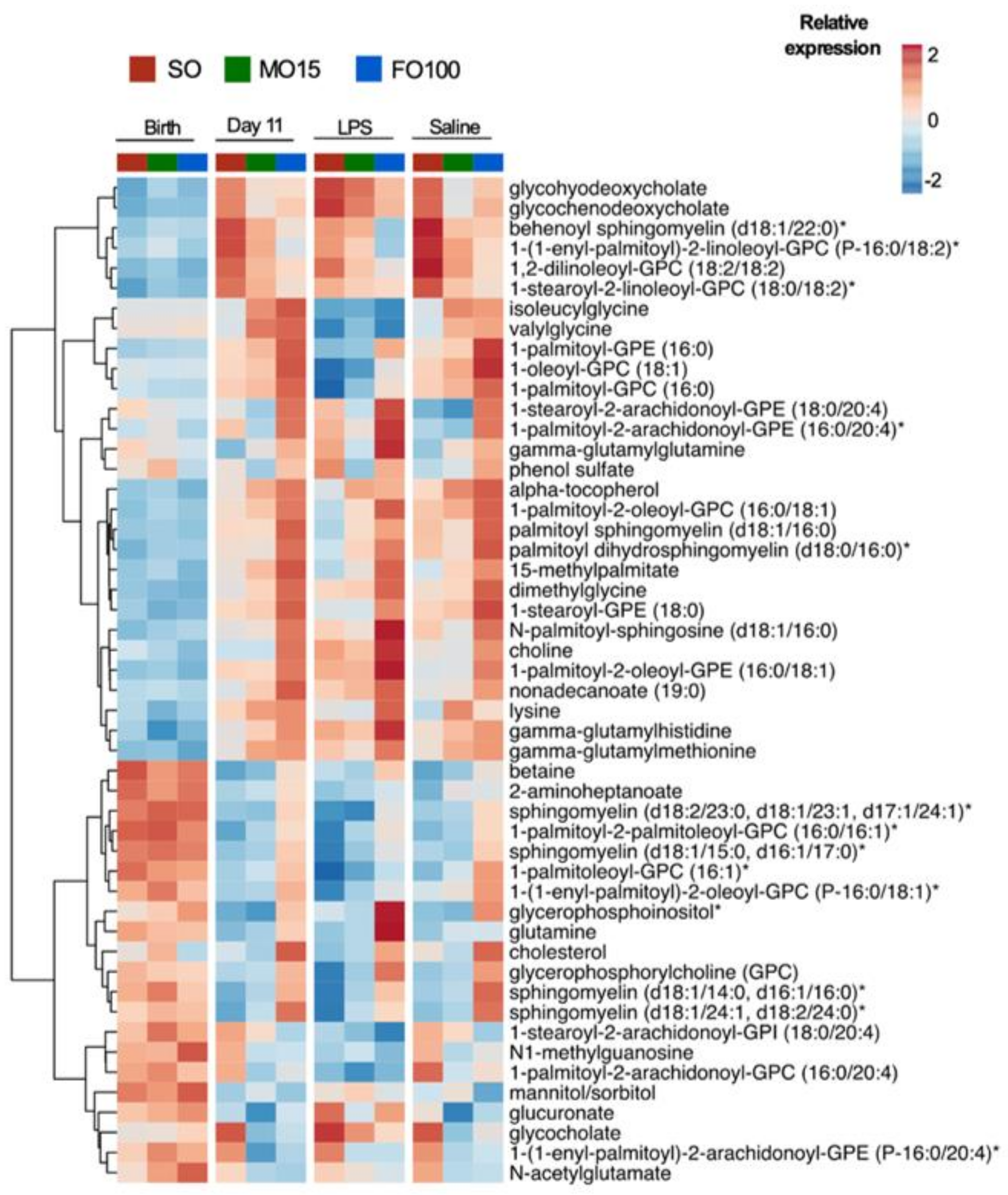
3.4. Cytokine and Metabolomic Changes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Andescavage, N.N.; Plessis, A.; Carter, R.; Serag, A.; Evangelou, I.; Vezina, G.; Robertson, R.; Limperopoulos, C. Complex Trajectories of Brain Development in the Healthy Human Fetus. Cereb. Cortex 2017, 27, 5274–5283. [Google Scholar] [CrossRef]
- Bouyssi, K.M.; Plessis, A.J.; Carter, R.; Brossard, R.M.; Murnick, J.; Tinkleman, L.; Robertson, R.L.; Limperopoulos, C. Third Trimester Brain Growth in Preterm Infants Compared with in Utero Healthy Fetuses. Pediatrics 2016, 138. [Google Scholar] [CrossRef]
- Darmaun, D.; Lapillonne, A.; Simeoni, U.; Picaud, J.C.; Roze, J.C.; Saliba, E.; Bocquet, A.; Chouraqui, J.P.; Dupont, C.; Feillet, F.; et al. Parenteral nutrition for preterm infants: Issues and strategy. Arch. Pediatr. 2018, 25, 286–294. [Google Scholar] [CrossRef]
- Martin, C.R.; Dasilva, D.A.; Cluette, B.J.E.; Dimonda, C.; Hamill, A.; Bhutta, A.Q.; Coronel, E.; Wilschanski, M.; Stephens, A.J.; Driscoll, D.F.; et al. Decreased postnatal docosahexaenoic and arachidonic acid blood levels in premature infants are associated with neonatal morbidities. J. Pediatr. 2011, 159, 743–749. [Google Scholar] [CrossRef]
- Martin, C.R.; Cheesman, A.; Brown, J.; Makda, M.; Kutner, A.J.; Silva, D.; Zaman, M.; Freedman, S.D. Factors Determining Optimal Fatty Acid Absorption in Preterm Infants. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 130–136. [Google Scholar] [CrossRef]
- Larsen, B.M.; Goonewardene, L.A.; Joffe, A.R.; Aerde, J.E.; Field, C.J.; Olstad, D.L.; Clandinin, M.T. Pre-treatment with an intravenous lipid emulsion containing fish oil (eicosapentaenoic and docosahexaenoic acid) decreases inflammatory markers after open-heart surgery in infants: A randomized, controlled trial. Clin. Nutr. 2012, 31, 322–329. [Google Scholar] [CrossRef]
- Diamond, I.R.; Sterescu, A.; Pencharz, P.B.; Wales, P.W. The rationale for the use of parenteral omega-3 lipids in children with short bowel syndrome and liver disease. Pediatr. Surg. Int. 2008, 24, 773–778. [Google Scholar] [CrossRef]
- Belza, C.; Wales, J.C.; Courtney, M.G.; Silva, N.; Avitzur, Y.; Wales, P.W. An Observational Study of Smoflipid vs Intralipid on the Evolution of Intestinal Failure-Associated Liver Disease in Infants with Intestinal Failure. JPEN J. Parenter. Enter. Nutr. 2019. [Google Scholar] [CrossRef]
- Gura, K.M.; Duggan, C.P.; Collier, S.B.; Jennings, R.W.; Folkman, J.; Bistrian, B.R.; Puder, M. Reversal of parenteral nutrition-associated liver disease in two infants with short bowel syndrome using parenteral fish oil: Implications for future management. Pediatrics 2006, 118, 197–201. [Google Scholar] [CrossRef]
- Gura, K.M.; Lee, S.; Valim, C.; Zhou, J.; Kim, S.; Modi, B.P.; Arsenault, D.A.; Strijbosch, R.A.; Lopes, S.; Duggan, C.; et al. Safety and efficacy of a fish-oil-based fat emulsion in the treatment of parenteral nutrition-associated liver disease. Pediatrics 2008, 121, 678–686. [Google Scholar] [CrossRef]
- Meijer, V.E.; Gura, K.M.; Le, H.D.; Meisel, J.A.; Puder, M. Fish oil-based lipid emulsions prevent and reverse parenteral nutrition-associated liver disease: The Boston experience. JPEN J. Parenter. Enter. Nutr. 2009, 33, 541–547. [Google Scholar] [CrossRef]
- Najm, S.; Lofqvist, C.; Hellgren, G.; Engstrom, E.; Lundgren, P.; Hard, A.L.; Lapillonne, A.; Savman, K.; Nilsson, A.K.; Andersson, M.X.; et al. Effects of a lipid emulsion containing fish oil on polyunsaturated fatty acid profiles, growth and morbidities in extremely premature infants: A randomized controlled trial. Clin. Nutr. ESPEN 2017, 20, 17–23. [Google Scholar] [CrossRef]
- Lofqvist, C.A.; Najm, S.; Hellgren, G.; Engstrom, E.; Savman, K.; Nilsson, A.K.; Andersson, M.X.; Hard, A.L.; Smith, L.E.H.; Hellstrom, A. Association of Retinopathy of Prematurity with Low Levels of Arachidonic Acid: A Secondary Analysis of a Randomized Clinical Trial. JAMA Ophthalmol. 2018, 136, 271–277. [Google Scholar] [CrossRef]
- Nathan, C.; Ding, A. Nonresolving inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef]
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef]
- Sansbury, B.E.; Spite, M. Resolution of Acute Inflammation and the Role of Resolvins in Immunity, Thrombosis, and Vascular Biology. Circ. Res. 2016, 119, 113–130. [Google Scholar] [CrossRef]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 2014, 40, 315–327. [Google Scholar] [CrossRef]
- Vlaardingerbroek, H.; Ng, K.; Stoll, B.; Benight, N.; Chacko, S.; Kluijtmans, L.A.; Kulik, W.; Squires, E.J.; Olutoye, O.; Schady, D.; et al. New generation lipid emulsions prevent PNALD in chronic parenterally fed preterm pigs. J. Lipid Res. 2014, 55, 466–477. [Google Scholar] [CrossRef]
- Ng, K.; Stoll, B.; Chacko, S.; Saenz, P.M.; Lauridsen, C.; Gray, M.; Squires, E.J.; Marini, J.; Zamora, I.J.; Olutoye, O.O.; et al. Vitamin E in New-Generation Lipid Emulsions Protects Against Parenteral Nutrition-Associated Liver Disease in Parenteral Nutrition-Fed Preterm Pigs. JPEN J. Parenter. Enter. Nutr. 2015, 40, 656–671. [Google Scholar] [CrossRef]
- Sangild, P.T.; Thymann, T.; Schmidt, M.; Stoll, B.; Burrin, D.G.; Buddington, R.K. Invited review: The preterm pig as a model in pediatric gastroenterology. J. Anim. Sci. 2013, 91, 4713–4729. [Google Scholar] [CrossRef]
- Eiby, Y.A.; Wright, L.L.; Kalanjati, V.P.; Miller, S.M.; Bjorkman, S.T.; Keates, H.L.; Lumbers, E.R.; Colditz, P.B.; Lingwood, B.E. A pig model of the preterm neonate: Anthropometric and physiological characteristics. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Sangild, P.T.; Petersen, Y.M.; Schmidt, M.; Elnif, J.; Petersen, T.K.; Buddington, R.K.; Greisen, G.; Michaelsen, K.F.; Burrin, D.G. Preterm birth affects the intestinal response to parenteral and enteral nutrition in newborn pigs. J. Nutr. 2002, 132, 2673–2681. [Google Scholar] [CrossRef]
- Lavallee, C.M.; Pherson, J.A.R.; Zhou, M.; Gao, Y.; Wizzard, P.R.; Wales, P.W.; Turner, J.M.; Willing, B.P. Lipid Emulsion Formulation of Parenteral Nutrition Affects Intestinal Microbiota and Host Responses in Neonatal Piglets. JPEN J. Parenter. Enter. Nutr. 2017, 41, 1301–1309. [Google Scholar] [CrossRef]
- Guthrie, G.; Stoll, B.; Chacko, S.; Lauridsen, C.; Plat, J.; Burrin, D. Rifampicin, not vitamin E, suppresses parenteral nutrition-associated liver disease development through the pregnane X receptor pathway in piglets. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, 41–52. [Google Scholar] [CrossRef]
- Stoll, B.; Horst, D.A.; Cui, L.; Chang, X.; Ellis, K.J.; Hadsell, D.L.; Suryawan, A.; Kurundkar, A.; Maheshwari, A.; Davis, T.A.; et al. Chronic parenteral nutrition induces hepatic inflammation, steatosis, and insulin resistance in neonatal pigs. J. Nutr. 2010, 140, 2193–2200. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane, S.G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- King, E.; Brien, J.T.; Donaghy, P.; Morris, C.; Barnett, N.; Olsen, K.; Martin, R.C.; Taylor, J.P.; Thomas, A.J. Peripheral inflammation in prodromal Alzheimer’s and Lewy body dementias. J. Neurol. Neurosurg. Psychiatry 2018, 89, 339–345. [Google Scholar] [CrossRef]
- Vexler, A.; Tao, G.; Chen, X. A toolkit for clinical statisticians to fix problems based on biomarker measurements subject to instrumental limitations: From repeated measurement techniques to a hybrid pooled-unpooled design. Methods Mol. Biol. 2015, 1208, 439–460. [Google Scholar] [CrossRef]
- Maddipati, K.R.; Romero, R.; Chaiworapongsa, T.; Zhou, S.L.; Xu, Z.; Tarca, A.L.; Kusanovic, J.P.; Munoz, H.; Honn, K.V. Eicosanomic profiling reveals dominance of the epoxygenase pathway in human amniotic fluid at term in spontaneous labor. FASEB J. 2014, 28, 4835–4846. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Kolde, R. pheatmap: Pretty heatmaps [Software]. 2015. [Google Scholar]
- Meijer, V.E.; Le, H.D.; Meisel, J.A.; Gura, K.M.; Puder, M. Parenteral fish oil as monotherapy prevents essential fatty acid deficiency in parenteral nutrition-dependent patients. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 212–218. [Google Scholar] [CrossRef]
- Carnielli, V.P.; Simonato, M.; Verlato, G.; Luijendijk, I.; Curtis, M.; Sauer, P.J.; Cogo, P.E. Synthesis of long-chain polyunsaturated fatty acids in preterm newborns fed formula with long-chain polyunsaturated fatty acids. Am. J. Clin. Nutr. 2007, 86, 1323–1330. [Google Scholar] [CrossRef]
- Nagano, N.; Okada, T.; Kayama, K.; Hosono, S.; Kitamura, Y.; Takahashi, S. Delta-6 desaturase activity during the first year of life in preterm infants. Prostaglandins Leukot. Essent. Fatty Acids 2016, 115, 8–11. [Google Scholar] [CrossRef]
- Uauy, R.; Mena, P.; Wegher, B.; Nieto, S.; Salem, N., Jr. Long chain polyunsaturated fatty acid formation in neonates: Effect of gestational age and intrauterine growth. Pediatr. Res. 2000, 47, 127–135. [Google Scholar] [CrossRef]
- Szitanyi, P.; Koletzko, B.; Mydlilova, A.; Demmelmair, H. Metabolism of 13C-labeled linoleic acid in newborn infants during the first week of life. Pediatr. Res. 1999, 45, 669–673. [Google Scholar] [CrossRef]
- Akinsulire, O.; Perides, G.; Anez, B.L.; Cluette, B.J.; Nedder, A.; Pollack, E.; Singh, P.; Liu, Y.; Sanchez-Fernandez, L.L.; Obregon, E.; et al. Early Enteral Administration of a Complex Lipid Emulsion Supplement Prevents Postnatal Deficits in Docosahexaenoic and Arachidonic Acids and Increases Tissue Accretion of Lipophilic Nutrients in Preterm Piglets. JPEN J. Parenter. Enter. Nutr. 2020, 44, 69–79. [Google Scholar] [CrossRef]
- Needleman, P.; Raz, A.; Minkes, M.S.; Ferrendelli, J.A.; Sprecher, H. Triene prostaglandins: Prostacyclin and thromboxane biosynthesis and unique biological properties. Proc. Natl. Acad. Sci. USA 1979, 76, 944–948. [Google Scholar] [CrossRef]
- Hawcroft, G.; Loadman, P.M.; Belluzzi, A.; Hull, M.A. Effect of eicosapentaenoic acid on E-type prostaglandin synthesis and EP4 receptor signaling in human colorectal cancer cells. Neoplasia 2010, 12, 618–627. [Google Scholar] [CrossRef]
- Kramer, H.J.; Stevens, J.; Grimminger, F.; Seeger, W. Fish oil fatty acids and human platelets: Dose-dependent decrease in dienoic and increase in trienoic thromboxane generation. Biochem. Pharmacol. 1996, 52, 1211–1217. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv. Nutr. 2015, 6, 513–540. [Google Scholar] [CrossRef]
- Corey, E.J.; Shih, C.; Cashman, J.R. Docosahexaenoic acid is a strong inhibitor of prostaglandin but not leukotriene biosynthesis. Proc. Natl. Acad. Sci. USA 1983, 80, 3581–3584. [Google Scholar] [CrossRef]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef]
- Hadley, K.B.; Ryan, A.S.; Forsyth, S.; Gautier, S.; Salem, N., Jr. The Essentiality of Arachidonic Acid in Infant Development. Nutrients 2016, 8, 216. [Google Scholar] [CrossRef]
- Harauma, A.; Yasuda, H.; Hatanaka, E.; Nakamura, M.T.; Salem, N., Jr.; Moriguchi, T. The essentiality of arachidonic acid in addition to docosahexaenoic acid for brain growth and function. Prostaglandins Leukot. Essent. Fatty Acids 2017, 116, 9–18. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, X.; Tse, H.F.; Rong, J. Gallic Acid-L-Leucine Conjugate Protects Mice against LPS-Induced Inflammation and Sepsis via Correcting Proinflammatory Lipid Mediator Profiles and Oxidative Stress. Oxid. Med. Cell. Longev. 2018, 2018, 1081287. [Google Scholar] [CrossRef]
- He, L.K.; Liu, L.H.; Hahn, E.; Gamelli, R.L. The expression of cyclooxygenase and the production of prostaglandin E2 in neutrophils after burn injury and infection. J. Burn Care Rehabil. 2001, 22, 58–64. [Google Scholar] [CrossRef]
- Alba, L.T.C.; Martins, E.F.; Miyasaka, C.K.; Lopes, L.R.; Landgraf, R.G.; Jancar, S.; Curi, R.; Sannomiya, P. Evidence that arachidonic acid derived from neutrophils and prostaglandin E2 are associated with the induction of acute lung inflammation by lipopolysaccharide of Escherichia coli. Inflamm. Res. 2004, 53, 658–663. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B. Success of prostaglandin E2 in structure-function is a challenge for structure-based therapeutics. Proc. Natl. Acad. Sci. USA 2003, 100, 8609–8611. [Google Scholar] [CrossRef]
- Sheppe, A.E.F.; Kummari, E.; Walker, A.; Richards, A.; Hui, W.W.; Lee, J.H.; Mangum, L.; Borazjani, A.; Ross, M.K.; Edelmann, M.J. PGE2 Augments Inflammasome Activation and M1 Polarization in Macrophages Infected with Salmonella Typhimurium and Yersinia enterocolitica. Front. Microbiol. 2018, 9, 2447. [Google Scholar] [CrossRef]
- Levy, B.D.; Clish, C.B.; Schmidt, B.; Gronert, K.; Serhan, C.N. Lipid mediator class switching during acute inflammation: Signals in resolution. Nat. Immunol. 2001, 2, 612–619. [Google Scholar] [CrossRef]
- Loynes, C.A.; Lee, J.A.; Robertson, A.L.; Steel, M.J.; Ellett, F.; Feng, Y.; Levy, B.D.; Whyte, M.K.B.; Renshaw, S.A. PGE2 production at sites of tissue injury promotes an anti-inflammatory neutrophil phenotype and determines the outcome of inflammation resolution in vivo. Sci. Adv. 2018, 4. [Google Scholar] [CrossRef]
- Strassmann, G.; Patil, K.V.; Finkelman, F.; Fong, M.; Kambayashi, T. Evidence for the involvement of interleukin 10 in the differential deactivation of murine peritoneal macrophages by prostaglandin E2. J. Exp. Med. 1994, 180, 2365–2370. [Google Scholar] [CrossRef]
- Drobnik, W.; Liebisch, G.; Audebert, F.X.; Frohlich, D.; Gluck, T.; Vogel, P.; Rothe, G.; Schmitz, G. Plasma ceramide and lysophosphatidylcholine inversely correlate with mortality in sepsis patients. J. Lipid Res. 2003, 44, 754–761. [Google Scholar] [CrossRef]
- Li, Z.; Fan, Y.; Liu, J.; Li, Y.; Huan, C.; Bui, H.H.; Kuo, M.S.; Park, T.S.; Cao, G.; Jiang, X.C. Impact of sphingomyelin synthase 1 deficiency on sphingolipid metabolism and atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1577–1584. [Google Scholar] [CrossRef]
- Hailemariam, T.K.; Huan, C.; Liu, J.; Li, Z.; Roman, C.; Kalbfeisch, M.; Bui, H.H.; Peake, D.A.; Kuo, M.S.; Cao, G.; et al. Sphingomyelin synthase 2 deficiency attenuates NFkappaB activation. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1519–1526. [Google Scholar] [CrossRef]
- Lou, B.; Dong, J.; Li, Y.; Ding, T.; Bi, T.; Li, Y.; Deng, X.; Ye, D.; Jiang, X.C. Pharmacologic inhibition of sphingomyelin synthase (SMS) activity reduces apolipoprotein-B secretion from hepatocytes and attenuates endotoxin-mediated macrophage inflammation. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Wright, S.D.; Kolesnick, R.N. Does endotoxin stimulate cells by mimicking ceramide? Immunol. Today 1995, 16, 297–302. [Google Scholar] [CrossRef]
- Wu, D.; Marko, M.; Claycombe, K.; Paulson, K.E.; Meydani, S.N. Ceramide-induced and age-associated increase in macrophage COX-2 expression is mediated through up-regulation of NF-kappa B activity. J. Biol. Chem. 2003, 278, 10983–10992. [Google Scholar] [CrossRef]
- Opreanu, M.; Lydic, T.A.; Reid, G.E.; Sorley, K.M.; Esselman, W.J.; Busik, J.V. Inhibition of cytokine signaling in human retinal endothelial cells through downregulation of sphingomyelinases by docosahexaenoic acid. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3253–3263. [Google Scholar] [CrossRef]
- Wong, M.L.; Xie, B.; Beatini, N.; Phu, P.; Marathe, S.; Johns, A.; Gold, P.W.; Hirsch, E.; Williams, K.J.; Licinio, J.; et al. Acute systemic inflammation up-regulates secretory sphingomyelinase in vivo: A possible link between inflammatory cytokines and atherogenesis. Proc. Natl. Acad. Sci. USA 2000, 97, 8681–8686. [Google Scholar] [CrossRef]
- Anez, B.L.; Dao, D.T.; Baker, M.A.; Fell, G.L.; Puder, M.; Gura, K.M. Intravenous Fat Emulsion Formulations for the Adult and Pediatric Patient: Understanding the Differences. Nutr. Clin. Pract. 2016, 31, 596–609. [Google Scholar] [CrossRef]
- Wanten, G.J.; Calder, P.C. Immune modulation by parenteral lipid emulsions. Am. J. Clin. Nutr. 2007, 85, 1171–1184. [Google Scholar] [CrossRef]
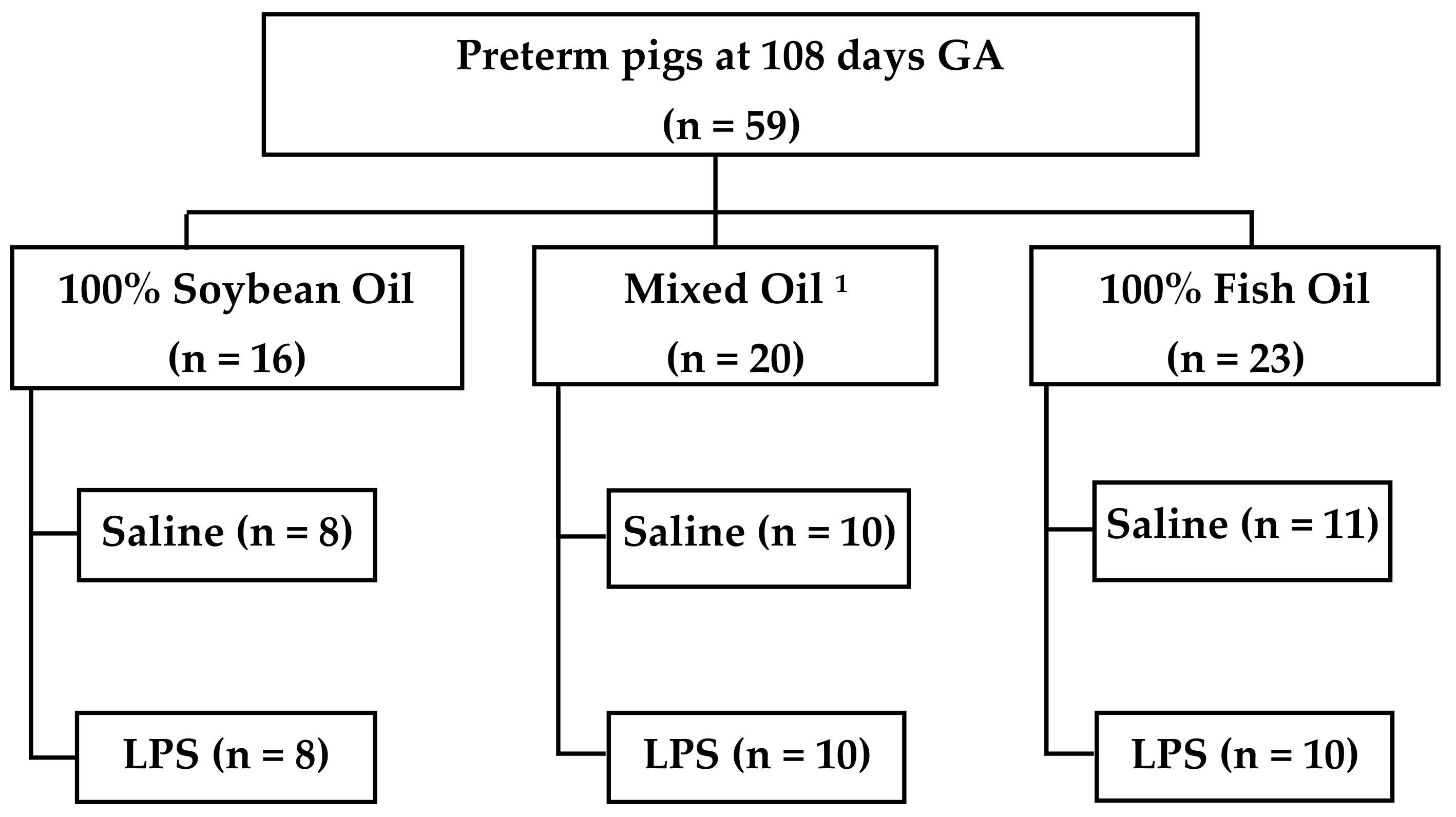
| Lipid | Treatment | n | Male | Birth Weight (g) | Final Weight (g) | Growth Velocity (g/kg/d) | Mortality |
|---|---|---|---|---|---|---|---|
| 100% Soybean Oil (SO) | Total | 16 | 68.8% | 1273 (362) | 2521 (605) | 98.4 (9.73 | 1 (6.3%) |
| Saline | 8 | 1380 (382) | 2569 (603) | 96.3 (11.1) | 1 (12.5%) | ||
| LPS | 8 | 1243 (356) | 2515 (614) | 98.8 (7.77) | 0 (0.0%) | ||
| Mixed Oil (MO15) | Total | 20 | 60.0% | 1199 (274) | 2387 (474) | 101.0 (12.8) | 2 (10.0%) |
| Saline | 10 | 1189 (240) | 2387 (364) | 105.0 (9.55) | 1 (10.0%) | ||
| LPS | 10 | 1211 (298) | 2378 (523) | 97.0 (16.7) | 1 (10.0%) | ||
| 100% Fish Oil (FO100) | Total | 23 | 52.2% | 1249 (177) | 2438 (373) | 98.7 (19.0) | 3 (13.0%) |
| Saline | 12 | 1228 (150) | 2438 (450) | 97.9 (16.7) | 0 (0.0%) | ||
| LPS | 11 | 1265 (142) | 2444 (313) | 100.5 (17.4) | 3 (25.0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakah, W.; Ramiro-Cortijo, D.; Singh, P.; Brown, J.; Stoll, B.; Kulkarni, M.; Oosterloo, B.C.; Burrin, D.; Maddipati, K.R.; Fichorova, R.N.; et al. Parenteral Fish-Oil Containing Lipid Emulsions Limit Initial Lipopolysaccharide-Induced Host Immune Responses in Preterm Pigs. Nutrients 2021, 13, 205. https://doi.org/10.3390/nu13010205
Yakah W, Ramiro-Cortijo D, Singh P, Brown J, Stoll B, Kulkarni M, Oosterloo BC, Burrin D, Maddipati KR, Fichorova RN, et al. Parenteral Fish-Oil Containing Lipid Emulsions Limit Initial Lipopolysaccharide-Induced Host Immune Responses in Preterm Pigs. Nutrients. 2021; 13(1):205. https://doi.org/10.3390/nu13010205
Chicago/Turabian StyleYakah, William, David Ramiro-Cortijo, Pratibha Singh, Joanne Brown, Barbara Stoll, Madhulika Kulkarni, Berthe C. Oosterloo, Doug Burrin, Krishna Rao Maddipati, Raina N. Fichorova, and et al. 2021. "Parenteral Fish-Oil Containing Lipid Emulsions Limit Initial Lipopolysaccharide-Induced Host Immune Responses in Preterm Pigs" Nutrients 13, no. 1: 205. https://doi.org/10.3390/nu13010205
APA StyleYakah, W., Ramiro-Cortijo, D., Singh, P., Brown, J., Stoll, B., Kulkarni, M., Oosterloo, B. C., Burrin, D., Maddipati, K. R., Fichorova, R. N., Freedman, S. D., & Martin, C. R. (2021). Parenteral Fish-Oil Containing Lipid Emulsions Limit Initial Lipopolysaccharide-Induced Host Immune Responses in Preterm Pigs. Nutrients, 13(1), 205. https://doi.org/10.3390/nu13010205







