Abstract
Polyphenols (PPs) are the naturally occurring bioactive components in fruits and vegetables, and they are the most abundant antioxidant in the human diet. Studies are suggesting that ingestion of PPs might be helpful to ameliorate metabolic syndromes that may contribute in the prevention of several chronic disorders like diabetes, obesity, hypertension, and colon cancer. PPs have structural diversity which impacts their bioavailability as they accumulate in the large intestine and are extensively metabolized through gut microbiota (GM). Intestinal microbiota transforms PPs into their metabolites to make them bioactive. Interestingly, not only GM act on PPs to metabolize them but PPs also modulate the composition of GM. Thus, change in GM from pathogenic to beneficial ones may be helpful to ameliorate gut health and associated diseases. However, to overcome the low bioavailability of PPs, various approaches have been developed to improve their solubility and transportation through the gut. In this review, we present evidence supporting the structural changes that occur after metabolic reactions in PPs (curcumin, quercetin, and catechins) and their effect on GM composition that leads to improving overall gut health and helping to ameliorate metabolic disorders.
1. Introduction
Plant polyphenols (PPs) are secondary metabolites and are ubiquitously found in various parts of plants like roots, stems, leaves, flowers, and pulp. PPs are considered to be very necessary for plant survival in the environment but not directly responsible for the development and growth of plants. PPs are produced from primary metabolites and intermediates through unique biosynthetic pathways [1]. They are a class of non-essential phytonutrients and are abundant in fruits, cereals, and vegetables [2,3]. Molecules of PPs display at least one aromatic ring carrying one or more hydroxyl groups [4] and they are also found as conjugate with organic acids or sugars or as polymers (flavonoids). Additionally, PPs are hydrolyzable and condensed tannins represent a special group that interacts with proteins [5]. According to Costa et al. [6], around 8000 polyphenolic structures have been recognized. They are subdivided according to their chemical structure (depending on the number of hydroxyls in the molecule and on the nature and the position of other substituents) into the following structural classes: lignans, phenolic acids, stilbenes, condensed or hydrolyzable tannins, and flavonoids (containing anthocyanins and isoflavonoids). It has been documented that PPs are primarily distributed as glycosides in plants and their elementary structure is aglycones. However, the phenolic acids comprise hydroxycinnamic acids (caffeic, ferulic, chlorogenic acid, p-coumaric, and sinapic) and hydroxybenzoic (gallic, syringic acid, vanillic, and protocatechuic acid) in the non-flavonoid group. Phenolic acids exist in plants as soluble-free, conjugate, and insoluble bound forms. Flavonoids are originated from the acetate/malonate pathways that are stored as glycosides in plants. They constitute a large group of phenolic compounds [7]. The majority of flavonoids contain diphenyl propane which is a closed pyran—a variation in the central pyran ring that is due to oxidation and hydroxylation pattern [8]. The presence of conjugated chromophore is considered to be responsible for yellow and red color development in flavonoids. Anthocyanidins (like cyanidin) are an example which illustrates red and magenta color [9]. Flavonoids and their subgroups (flavones, flavanones, flavonols, and flavanonols) are ubiquitous in the plant kingdom [8]. Other PPs such as lignans (e.g., lariciresinol and pinoresinol) are non-flavonoid diphenolic components that are composed of phenylpropanoid units. Stilbenes represents non-flavonoids (including trans-resveratrol), abundantly present in grapes and red wine [10]. Tannins are further subdivided into two groups: (1) Hydrolysed tannins are the esters of gallic acid and egalic acids, while (2) condensed tannins are the polymers of catechin and epicatechin [8]. The aim of this review is to provide an overview of the biological role of PPs against metabolic syndromes to improve gut health, and their extraction techniques. Specifically, we discussed the role of curcumin, quercetin, and catechins in the gut with their biotransformation into metabolites through gut microbiota (GM) and changes that occur after that.
2. Biological Role of PPs
It has been stated that PPs play a vital role in human nutrition as they have antioxidative ability and can decrease the reactive oxygen species (ROS). Moreover, they can be utilized to ameliorate metabolic disorders like obesity, diabetes, cancer, and cardio-metabolic diseases [11]. Among PPs, phenolic acids can lower the risk of chronic diseases like cardiovascular disease, cancer, etc. Since various diseases have been associated with oxidative stress, dietary PPs reduce the effects caused by excessive ROS or other nitrogen species. By neutralizing the free radicals via donating an electron and as direct radical scavengers of the lipid peroxidation chain reactions, PPs eradicate the oxidative stress [12]. Phenolic acids and flavonoids, including anthocyanins, procyanidins, quercetin, catechins, curcumin, and ellagic acids, have their role in obesity and weight management [13]. Despite their role in disease management, PPs lose their therapeutic properties due to glycosylation and become less available to the target cell. Due to the glycosylated form, their absorption mainly takes place in the colon. Gowd et al. [14] briefly documented the metabolism of PPs in humans and reported that PPs have high molecular weight and complex structure; that is why only 5–10% are absorbed in the small intestine. About 90–95% reach the colon and their GM play a significant role in the breaking down of these complexes and converting them into absorbable metabolites. After conversion into phenolic metabolites, they reach the liver through a portal vein upon absorption. Additionally, they undergo extensive degradation via metabolic reactions to form active metabolites. Then, these metabolites enter into systematic circulation reach target cells and tissues where they can show physiological significance. Unused and remaining metabolites are excreted through urine. The schematic ingestion, digestion, absorption, and excretion of PPs are exhibited in Figure 1.
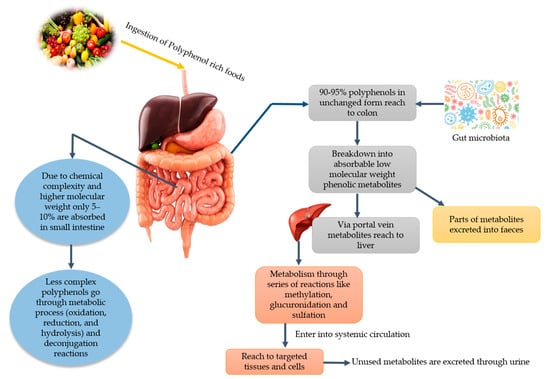
Figure 1.
Major dietary sources of polyphenols and potential gut microbiota-associated benefits.
3. Extraction of PPs
Although 90–95% of PPs are absorbed in the colon, however, their efficacy (in terms of its therapeutic effects) is about 15–20%. Extraction of PPs is a crucial step, and various techniques can be employed that can help to enhance the bioactivity and bioavailability of extracted PPs. These extracted PPs can be used for many medical and pharmaceutical purposes to utilize their therapeutic values effectively. Being hydrophilic and phenolic in nature, they can be extracted by various solvents, including methanol, acetone, acetonitrile, and ethanol. Among these solvents, methanol is found to be more efficient for the extraction of lower molecular weight PPs while aqueous acetone—for higher molecular weight PPs [15]. Different extraction techniques are utilized for PPs including maceration, heat-assisted, ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), homogeniser-assisted extraction (HAE), and rapid solid–liquid dynamic extraction (RSLDE). Maceration is a simple and traditional method of extraction that is performed in glass containers at room temperature [16,17]. Heat-assisted extraction is carried out at a high temperature in glass containers with continuous mixing in a water bath or the incubator shaker, allowing unattended operation in a temperature-controlled environment. The use of thermal energy improves the efficiency of the extraction by disruption of cellular structures, the increment of cell membrane permeability, and breakdown of PPs–lipoprotein interactions, which cause enhancement of PPs solubility and mass transfer [18]. Continuous mixing for minutes or hours is required and the choice of extraction solvent in maceration and heat-assisted extraction depends on the chemical and physical properties of the targeted compound and extraction method. MAE is performed with a microwave extractor system that is equipped with a digital control system. Procedure time (1–25 min), stirring (250 rpm), and temperature (20–50 °C) are used. UAE is carried out in an ultrasound bath or probe and plant tissues are destroyed through ultrasonic waves, as they cause mechanical vibrations that lead to expansion and compression cycles during movement through the extraction medium and provoke the rise of temperature and negative pressure [19,20]. These mechanical and thermal effects cause the degradation of cell walls, the release of cell contents, greater penetration of solvent into plant material, the increment of mass transfer, and thus, the increase of PPs yield [21]. HAE is usually operated at high speed which requires homogenization and centrifugation in the presence of a solvent. The high-shear rate applied promotes the rapid rupture of the plant material, releasing the constituents into the extraction solvent [22]. Besides, RSLDE uses the Naviglio extractor generation in which a suitable solvent is used under the pressure gradient between the outside and the inside of a solid matrix containing extractable material, followed by a sudden restoration of the initial equilibrium conditions, induces forced extraction [23]. Recently, Yilmaz et al. [19] reported a study for the extraction of PPs using maceration, MAE, and UAE methods from Stevia rebaudiana Bertoni. In that study, MAE and UAE required less processing time and showed a higher yield as compared to maceration [19]. Galan et al. [24] carried out a comparison study between MAE and conventional health-assisted extraction; they stated that MAE possessed higher yield and antioxidant activity for PPs. Another comparison study among maceration, HAE, UAE, MAE, and RSLDE was conducted and revealed that better extraction was found with 100% methanol as an extraction solvent than methanol/water (50:50, v/v). The highest phenolic content was found with HAE, followed by UAE then MAE. In another similar study, da Rosa et al. [25] compared MAE, UAE, and maceration and reported that MAE is more efficient in yield with short extraction time, followed by UAE and maceration. Additionally, regarding in vitro antioxidant activity: ferric reducing antioxidant power was found highest with UAE in which water and methanol are used as a solvent. In contrast, oxygen radical absorbance capacity was found highest with HAE with 100% methanol as solvent extraction [26]. Jovanović et al. [27] stated a comparison of extraction techniques and compiled that both maceration and heat-assisted extraction are simple and traditional methods. However, the usage of high solvent in these techniques demands high cost and may lead to environmental problems, whereas extracted PPs yield is also low. On the other hand, UAE and MAE require less time and less solvent. Moreover, they show a higher yield with less environmental issues. From the above-mentioned data, it can be concluded that the selection of the right extraction technique is essential for wider and correct compound characterization. All of the extraction techniques promote the recovery of phenolic compounds but with different efficiencies. So, the use of non-conventional (other than maceration and heat-associated) extraction technologies is suggested.
4. Metabolic Syndrome
Hippocrates has been quoted as saying “death sits in the bowels” and “bad digestion is the root of all evil” in 400 B.C., emphasizing on the importance of the diet and human intestines in health and disease, which was recognized long ago [28]. On the other hand, gut metabolic syndromes are multiple risk factors like dyslipidemia, hyperglycemia, oxidative stress, insulin resistance, hypertension, fatty liver, etc. These syndromes result in metabolic diseases such as diabetes, obesity, hepatopathy, nephropathy, inflammation, cardiomyopathy, neurodegeneration, and osteoarthritis [29,30,31]. Metabolic syndromes are one of the concerning issues in the world as their prevalence is increasing day by day. It has been estimated that their prevalence will increase by up to 53% by the year 2035 [32]. Human intestines comprise an intricate ecological colony of dwelling bacteria, known as GM [33]. GM is the colony of collective microbes (mainly bacteria) residing in the gastrointestinal tract (GIT) and about 100 trillion microorganisms live in the human gut [28]. Other than bacteria, protozoa, virus, and eukaryotic organisms, including fungi, are also inhabiting but in a minimal number. The small intestine (duodenum, jejunum, and ileum) comprises several bacteria ranging from 104 bacteria/mL content to 106–107 bacteria/mL at the ileocecal junction. On the other hand, most of the non-sporing anaerobes reside in the large intestine and it has a number of bacteria ranging between 1011 and 1012/g [34]. On average, 90% of the bacteria in the gut of an adult are phyla Firmicutes (Gram-positive) and phyla Bacteroidetes (Gram-negative); many others are also present but in much lower abundance, such as Verrucomicrobia (Gram-negative), namely Akkermansia muciniphila (Gram-negative), and Actinobacteria (Gram-positive), namely Bifidobacterium, Proteobacteria (Gram-negative) [35]. GM play a major role in metabolic health (digestion and metabolism), vitamin synthesis, maintains gut homeostasis (a balance of host responses to the beneficial enteric microbial community and the pathogenic stimuli that can arise [36]) and, when aberrant, to the pathogenesis of various common metabolic disorders [37,38]. Further, GM regulate the gut endocrine function and neurological signaling, host immunity, modify drug action and metabolism, eradicate toxins, and produce various composites that affect the host [39]. The qualitative composition of the GM differs, depending on age and eating patterns, e.g., Bifidobacteria, and Proteobacteria are found abundantly in the gut of breastfed infants [40]. However, Bacteroides and Clostridia prevail abundantly in babies fed with formula milk [41]. While during weaning, Bacteroidetes and Firmicutes compositions seem to increase with the decrease in Proteobacteria and Actinobacteria compositions, conversely, in elders, Bacteroidetes and Proteobacteria tend to upsurge [42].
Moreover, Kumar et al. [33] stated that bacteria in amniotic fluid, genetic background, breastfeeding, solid foods, adulthood dietary habits, aging, exercise, stress, drug (such as antibiotics), and xenobiotics are the major modulators of GM in humans. These modulators are responsible for an impairment in GM composition or function that is known as GM dysbiosis (GMD) [43]. Many studies evidently stated that there is an association between diseases and GMD, including those of the GI tract, such as ulcerative colitis, inflammatory disease, colorectal cancer, obesity, type 2 diabetes, metabolic liver disease, cardiometabolic diseases, Alzheimer’s disease, and Parkinson’s [38,42,44,45,46]. Therefore, researchers are trying to find nutraceutical or therapeutic interventions to develop a healthy GM equilibrium to retard the harmful bacteria and pathobionts without affecting the beneficial or symbionts ones.
5. GM and PPs
Studies are suggesting that complex and dynamic interplay occurs between PPs and GM during metabolism, contributing a lot to the overall health of individuals. Thus, retention of PPs in intestines for a long time can promote beneficial effect on GM. On the other hand, GM enhance the biological activity of PPs by biotransforming them into active metabolites (phenolics) [37]. Bacterial species, such Bifidobacterium sp., Lactobacillus sp., Escherichia coli, Bacteroides sp., Eubacterium sp., Enterococcus caccae, Bifidobacterium catenulatum, Ruminococcus gauvreauii, etc., during catabolic pathways, catalyze the phenolics metabolism [47,48]. Therefore, deviation in daily intake of PPs may lead to differences in metabolites of phenolics. Moreover, variations in GM composition are also documented to affect the bioavailability and bioactive effect of PPs and their metabolites [49]. Studies have revealed that PPs can modulate the GM colony by employing antimicrobial activity or prebiotic-like effect against harmful bacteria residing in the gut [50]. In the last decade, the impact of PPs on gut ecology has been studied a lot [51]. Schematic illustrations of sources of PPs and potential GM-associated benefits in humans are depicted in Figure 2. Additionally, the effects of some of the PPs on GM modulation and their effects on metabolic disorders are shown in Table 1.
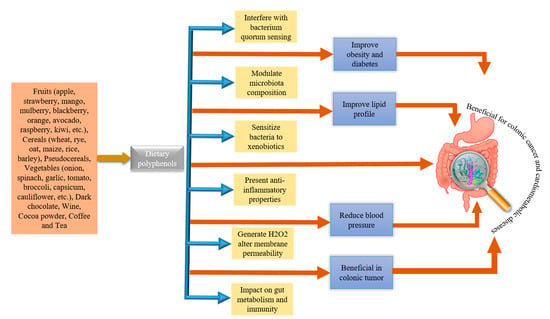
Figure 2.
Major dietary sources of polyphenols and potential gut microbiota-associated benefits.

Table 1.
Effect of polyphenols on gut microbiota (GM) modulation and their major effects on gut health with applied models.
6. Curcumin
Turmeric, also known as Curcuma longa L. belongs to Zingiberaceae (or ginger family) and is a golden-colored spice. Curcumin ((1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) is the principle curcuminoid of turmeric used in traditional medicine to cure various kinds of malady, as well as being a food additive and coloring agent in Asian cuisines and in beverage industries [96]. Hewlings and Kalman [97] stated the beneficial effects of curcumin in the treatment of chronic diseases, such as gastrointestinal, neurological disorders, cardiovascular disease, diabetes, and several types of cancer [98,99,100,101]. Although curcumin has therapeutic properties against many disorders, it has poor bioavailability and low gastrointestinal absorption that is mainly attributed to water insolubility, rapid metabolism, and excretion [101,102]. Enzymes of the large intestine metabolise curcumin, and it is carried out in two phases. In phase-1 metabolism, it yields three metabolites, 1,7-bis(4-hydroxy-3-methoxyphenyl)heptane-3,5-dione (tetrahydrocurcumin), 5-hydroxy-1,7-bis(4-hydroxy-3-methoxyphenyl)-3-heptanone (hexahydrocurcumin), and 1,7-bis(4-hydroxy-3-methoxyphenyl)heptane-3,5-diol (octahydrocurcumin) under reduction. After that, curcumin and its metabolites subject to conjugation through phase-II metabolism to yield sulfate and glucuronide O-conjugated metabolites [103,104]. Transformation not only occurs through enzymes produced by hepatocytes or enterocytes, but also by the enzymes of GM residing in the colon, which can generate many active metabolites [104]. Curcumin metabolites have properties and potency similar to curcumin and exhibit the same physiological and pharmacological properties [105]. It has been stated that curcumin and GM have bidirectional interactions such as GM regulation by curcumin and biotransformation of curcumin by GM [101,105,106]. The reciprocal interaction between curcumin and GM is illustrated in Figure 3. Carmody et al. [107] reported that the biological properties of curcumin depend on the activity of metabolites produced by GM digestion. The curcumin metabolic pathways by GM include reduction, methylation, demethoxylation, hydroxylation, and acetylation, and the main products are 1,7-bis(4-hydroxy-3-methoxyphenyl)heptane-3,5-dione (tetrahydrocurcumin), 3-(4-Hydroxy-3-methoxyphenyl)propanoic acid (dihydroferulic acid), and 1-(4-hydroxy-3-methoxyphenyl)-2-propanol. Furthermore, curcumin can also be metabolized by Pichia pastoris into four major metabolites, include 1,7-bis(4-hydroxy-3methoxyphenyl) heptan-3,5-diol, 5-hydroxy-1,7-bis(4-hydroxy-3-methoxyphenyl) heptan-3-one, 5-hydroxy-1,7-bis(4-hydroxyphenyl) heptane-3-one, and 5-hydroxy-7-(4-hydroxy-3-methoxyphenyl)-1-(4-hydroxyphenyl) heptan-3-one [101,103]. Many GM, such as E. coli, E. fergusonii (ATCC 35469) Blautia sp. (mrg-pmf1), Bifidobacterium (Bifidobacteria longum BB536, Bifidobacteria pseudocatenulaum G4), Lactobacillus (Lactobacillus casei and Lactobacillus acidophilus), Enterococcus faecalis JCM 5803, Pichia anomala, and Bacillus megateriumdcmb-002, are found biologically relevant in the biotransformation and degradation of curcumin [103,108,109].
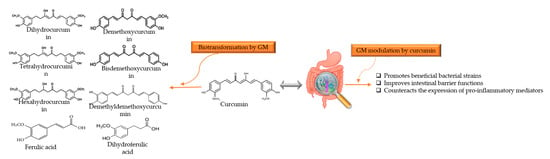
Figure 3.
Reciprocal interaction between curcumin and GM. Biotransformation of curcumin occurs due to GM that convert it into several metabolites through pathways like demethylation, reduction, acetylation, hydroxylation, and demethoxylation. These metabolites associated with showing biological activities such as antioxidant, anti-inflammatory, anti-tumoral, and neuroprotective activity. Whereas, GM modulation alters the microbial abundance, diversity, and composition, which also exerts health benefits, indirectly. GM: gut microbiota.
Curcumin and Gut Health
After oral administration, curcumin is distributed in the intestines, and then curcumin exerts its effects on the GM (such as microbial richness, diversity, and composition) [95]. A study conducted by Shen et al. [110] stated that curcumin administration exerts significant effects on GM family such as Bacteroidaceae, Rikenellaceae, and Prevotellaceae. Another study revealed that curcumin administration was effective in weight loss in ovariectomized rats. Moreover, curcumin significantly promoted GM, including Anaerotruncus, Exiguobacterium, Helicobacter, Papillibacter, Pseudomonas, Serratia, and Shewanella. This significance partially reversed the deficiency of estrogen induced by ovariectomy [111]. A very recent study conducted by Al-Saud [112] showed that intragastrical administration of curcumin to type 2 diabetic and obese male albino Wistar rats for 8 weeks (80 mg/kg/day) exhibited anti-obese and anti-diabetic properties, as well as enhancement in the expressions of GLUT4 gene. Curcumin revealed glucose-lowering effects, dyslipidaemia, decrease in insulin resistance, and malondialdehyde levels in the liver and pancreas. Koboziev et al. [113] supplemented a very high in fat diet with curcumin to mice prone to diet-induced metabolic dysfunction. They found animals to be protected against obesity and osteoarthritis without significant changes in knee cartilage integrity, glucose clearance, and white adipocyte size. Curcumin also ameliorates the intestinal barrier function (by modulating intracellular signaling and the organization of tight junctions) in metabolic diseases, as indicated by a reduced rate of bacterial translocation to the blood, liver, kidneys, and spleen [114]. A study showed that administration of curcumin significantly reduced the Western-diet-induced blood lipopolysaccharide and ameliorated the intestinal barrier [115]. Thus, it can be concluded that curcumin prevents metabolic diseases through a mechanism involved in the regulation of the intestinal barrier.
Many studies suggest that curcumin can actively hinder intestinal inflammation by modulating the homeostasis of the gut-brain axis, and could also exhibit neuroprotective beneficial [104]. Curcumin inhibits lipopolysaccharide-induced nuclear factor-κB (NF-κB) p65 translocation and mitogen-activated protein kinase phosphorylation in dendritic cells that lead to inflammation reduction [116]. As GMD, priming the innate immune system by microbiota, determines a neuroinflammatory response that causes misfolding of neuronal amyloid-β and α-synuclein [117]. Further, curcumin treatment decreases the microbial abundance of cancer-related species like Prevotella, Coriobacterales, and Ruminococcus [118]. Wu et al. [119] stated that curcumin regulates signaling pathways, such as NF-κB, and nuclear factor erythroid-2-related factor 2, epigenomics/epigenetics pathways of histones modifications, and DNA methylation. These help to exhibit antioxidative and anticancer properties. Another study on curcumin encapsulated with nanoparticles found it effective against colitis as it modulates GM and regulates T-cells [120]. The use of curcumin with randomized clinical trials showed therapeutic effects against ulcerative colitis and Crohn’s disease, but meta-analyses showed controversial results about the therapeutic approach [121]. Tetrahydrocurcumin can decrease the blood glucose level, increase the expression of pancreatic glucagon-like peptide-1, protect islet β cells, and the secretions of insulin in diabetic rats. Furthermore, it restores the intestinal dysbiosis as it lowers the relative abundance of Actinobacteria, Proteobacteria, and Firmicutes/Bacteroidetes ratio [122].
7. Quercetin
Quercetin (3,5,7-trihydroxy-2-(3,4-dihydroxyphenyl)-4Hchromen-4-one) is one of the most common flavonoids present in consumer foods and it belongs to the family of flavonols (myricetin and kaempferol). It is commonly found in green tea, lettuce, radish leaves, cranberry, apple, onion, buckwheat, coriander, lovage, etc. In plants, it is usually bound as ethers or phenolic acids or glycoside/aglycone (with or without linked sugars), etc. Even though it has various forms in nature, the form quercetin-3-O-glucoside is found in plants (as sugar moieties like rutinose or rhamnose), which generally acts as a pigment and give color to a multitude of vegetables and fruits [123,124,125]. Daily intake dose of quercetin is ranging from 1 to 250 mg/day [126]. Upon ingestion, quercetin can interact with salivary proteins and form soluble protein–quercetin binary aggregates [127], and in the stomach, quercetin is exposed to the lower pH conditions which may break phenolic acids by bacteria ring fusion [128]. After reaching the small intestine, it is deglycosylated by lactate pholrizin hydrolase (a family of 1 β-glucosidase), yielding quercetin aglycon. About 65–81% of quercetin goes to the liver through the epithelium, where it is metabolized and becomes bioavailable [129]. Complex metabolic reactions in the small intestine and stomach make it bioavailable, with bioavailability reported to be less than 10% [130]. GM transform quercetin into homoprocatechuic acid (3,4-dihydroxyphenylacetic acid), Protocatechuic acid (3,4-dihydroxybenzoic acid), 4-hydroxybenzoic acid, and 3-(3-hydroxyphenyl)propionic acid [131]. Di Pede et al. [132] observed the influence of different formulations on the microbial metabolism of quercetin in a time-dependent manner. It has been documented that Bacteroides fragilis, Eubacterium ramulus, Clostridium perfringens, Bacteroides JY-6, Bifidobacterium B-9, Lactobacillus L-2, and Streptococcus S-2 are the bacterial strains responsible for the transformation of quercetin into the metabolites [133,134]. Biotransformation of quercetin into metabolites by GM and their benefits in the gut is illustrated in Figure 4.
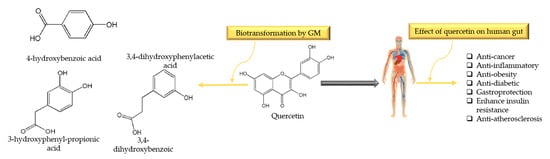
Figure 4.
Biotransformation of quercetin into metabolites by gut microbiota (mainly by Bacteroides fragilis, Eubacterium ramulus, C. perfringens) and their benefits in gut.
Quercetin and Gut Health
It has been mentioned in studies that quercetin has antioxidant, anti-inflammatory, antiviral, anti-obesity, antidepressant properties, as well as preventing cancer, diabetes, asthma, hypertension, and cardiovascular diseases [135,136]. A study reported by Ju et al. [137] stated that quercetin decreases the abundance of E. coli and proteobacteria. The genera Coprococcus_1, Anaerovorax, Ruminiclostridium_9, Mucisprillum, Roseburia, and Tyzzerella are also reported to increase in mice after the treatment of quercetin. Another study stated that it promoted the populations of Bifidobacterium, Bacteroides, Clostridia, and Lactobacillus and significantly suppressed Enterococcus and Fusobacterium [135], thus, it promotes gut homeostasis. A recent study by Lin et al. [138] reported that supplementation of quercetin to mice ameliorated the effects of Citrobacter rodentium-induced colitis, terminated the production of pro-inflammatory cytokines including interleukin (IL)-17, IL-6, tumor necrosis factor-α, and enhanced the production of IL-10 in the colon tissues. It also ameliorated the intestinal barrier function with the reduction in the activity of serum diamine oxidase and content of serum D-lactic acid. [135]. A study conducted on diabetic rats suggested that quercetin improved dyslipidemia, decreased serum blood glucose levels, enhanced insulin levels, and decreased oxidative stress injury [139]. Quercetin promotes cell survival and reduces ethanol-induced liver injury, and suppresses autophagic flux in both in vitro and in vivo studies [140,141]. Oral administration of quercetin to rats increased the sexual activity, intromission frequency, mount frequency, sperm count, and motility, and reduced the testicular damage induced by diabetes [142,143]. Intravenous administration of quercetin reported a lowering in blood pressure of hypersensitive rats [144]. Li et al. [145] documented that quercetin can reduce the Streptococcus suis-mediated inflammation by inhibiting the suilysin activity both in vitro and in vivo. Other studies on liver disorders suggested that quercetin can protect the liver from ethanol-induced liver fat accumulation and rotenone-induced liver-metabolic imbalances [146,147]. Further, quercetin can decrease lipid peroxidation in both serum and liver tissues and can exert free-radical and ROS-scavenging activity [148,149]. It also enhances superoxide dismutase, glutathione peroxidase, and catalase activities. On the other hand, it decreases lipid peroxidation of bone marrow and spleen tissues [150].
Quercetin is considered beneficial against different types of cancers, including pancreatic cancer, osteosarcoma, breast cancer, cervical cancer, leukemia, colon cancer, gastrointestinal cancer, ovarian cancer, and oral cancer [151]. It has been reported by Zhou et al. [152] that quercetin increased the cytotoxicity of doxorubicin in SW620/Ad300 cells and repressed the transport activity of P-glycoprotein which resulted in overcoming the colon cancer cells’ resistance to chemotherapy by inhibiting solute carrier family 1, member 5 transporter. Quercetin possesses morphological changes, decreases total viability via apoptotic and Bcl-x, and enhances the pro-apoptotic protein Bcl-2 family proteins, such as Bad, Bid, and Bax in AGS human gastrointestinal cancer cells (a human gastric adenocarcinoma cell-line) [153]. Moreover, it can protect intestinal porcine enterocyte cells against H2O2-induced apoptosis through the hindrance of the mitochondrial apoptosis pathway [154]. Forney et al. [155] revealed that quercetin has anti-inflammatory effects as it ameliorates adipose tissue expansion by reducing the levels of monocyte chemoattractant protein-1 mRNA and serum IL-6 in white adipose tissue of obese mice. Additionally, quercetin ameliorates glucosamine-induced inflammation and apoptosis in human umbilical vein endothelial cells that characterize a model of vascular endothelial injury in the initial stages of atherosclerosis [156].
8. Catechins
Catechins are widely distributed in many foods and herbs such as tea, cacaos, apples, persimmons, berries, and grapes [157]. Catechins are one of the main antioxidant agents that are biologically active and present in green tea (Camellia sinesis) [158]. Catechins include epigallocatechin-3-gallate (EGCG), epigallocatechin (EGC), epicatechin, epicatechin-3-gallate, gallocatechin gallate (GCG), and gallocatechins, among which EGCG is the most abundant and biologically active [159]. Biotransformation of tea catechins into their metabolites is mainly dependent on the GM. Ingested catechins pass through the small intestine (with very less degradation) and reach the colon where they are metabolized from the microflora, giving rise to both phenylvalerolactones and phenylvaleric acids [160]. Some portions of catechins may undergo extensive phase-I (which include oxidation, reduction and hydrolysis) and phase-II (conjugation) biotransformation in intestinal cells and then form the hepatocytes that result in the rapid release of a series of water-soluble conjugate metabolites such as methyl, glucuronide, and sulfate derivatives. Furthermore, they are converted into small molecular compounds which enter the hepatoenteral circulation or systemic circulation to exert various physiological functions [161]. Kutschera et al. [47] reported two bacterial strains, Flavonifractor plautii and Eggerthella lenta, which are responsible for the biotransformation of dietary catechins into hydroxyvaleric acid and valerolactones metabolites (illustrated in Figure 5). Particularly, Eggerthella lenta convert catechins into 1-(3,4-dihydroxyphenyl)-3-(2,4,6-trihydroxyphenyl)propan-2-ol, and Flavonifractor plautii transform it further into 5-(3,4-dihydroxyphenyl)-γ-valerolactone and 4-hydroxy-5-(3,4-hydroxyphenyl)valeric acid [47]. Microbial dihydroxylation reactions could further convert 4-hydroxy-5-(3,4-hydroxyphenyl)valeric acid to 5-(dihydroxyphenyl)-valeric acid and then to 3-hydroxyphenyl-valeric acid [162]. Ultimately, they are absorbed and undergo glucuronidation: 3′-O-glucuronide conjugate of (dihydroxyphenyl)-γ-valerolactone is the most abundant valerolactone species in urine after green tea intake [160]. The (dihydroxyphenyl)-γ-valerolactone metabolite showed remarkable antioxidant activity in vitro [163].
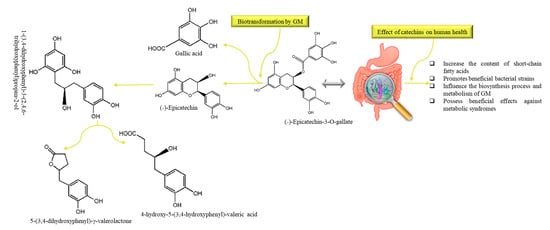
Figure 5.
The metabolic pathway and common metabolites of (˗)-Epicatechin-3-O-gallate and their modulatory effects on human gut: Eggerthella lenta and Flavonifractor plautii are mainly responsible for the biotransformation of dietary catechins. GM: gut microbiota.
Catechins and Gut Health
Catechins act as anti-inflammatories, antimicrobials, immunomodulators, regulators of ROS production, antioxidants, free radical scavengers, neuroprotective agents, anti-ageing, protectors of the circulatory system and cardiac tissues [164,165]. Catechins can inhibit the growth rate of H. pylori, Staphylococcus aureus, E. coli O157:H7, Salmonella typhimurium DT104, Pseudomonas aeruginosa, etc. [166]. Mainly, EGCG and GCG inhibit the growth of Bacteroides-Prevotella, Clostridium histolyticum, and Eubacterium-Clostridium groups [167]. Liao et al. [168] revealed that tea polyphenols could increase the Bifidobacteria and reduce total serum cholesterol and low-density lipoprotein cholesterol levels in mice. Kim et al. [169] treated 3T3-L1 mouse adipocytes with ECG and gallic acid during differentiation adipocytes and found that ECG enhanced adiponectin and uncoupled protein 1 transcription in mature adipocytes. Besides, supplementation of green tea catechins to obese subjects and patients with non-alcoholic fatty liver disease showed a decrease in body fat [170]. Catechins have strong anti-adipogenesis and anti-differentiation effects on mature adipocytes and 3T3-L1 preadipocytes via regulating the cyclic AMP/protein kinase A and C/EBPs/PPARγ/SREBP1C signaling pathways, which can thus exhibit the dual effect of preventing obesity and reducing fat [171]. Other than these, catechins also exhibit antimicrobial activities, as it has been reported that they can inhibit Salmonella enterica serovar Typhimurium type III protein secretion and invasion of host cells [172], syntaxin-1 expression [173], and induce endogenous oxidative stress in E. coli [174]. Catechin of green tea, EGCG, showed promising results in the inhibition of colon, prostate, lung, pancreatic, intestinal, and stomach cancers [158]. Many studies evidently confirmed the induction of apoptosis and cell cycle arrest by EGCG in colon cancer HCT-116 cells [175]. Additionally, EGCG prevents the activation of the epidermal growth factor receptor, HER2 genes and receptors, and multiple downstream signaling pathways in colon cancer cell lines [176]. Sur et al. [177] documented that EGCG and theaflavin inhibit mouse liver carcinogenesis through modulation of self-renewal Wnt and hedgehog pathways. Grzesik et al. [178] conducted a comparison study among EGCG, epicatechin, epicatechin gallate, gallocatechin gallate, curcumin, and hydrocinnamic acid to check their antioxidant properties. They found that EGCG and epicatechin gallate were the most efficient antioxidants as compared to others. Hence, catechins and their metabolites can regulate intestinal microecological balance by the modulation of the component of intestinal flora.
All of the above-mentioned studies reported protective effects of curcumin, quercetin, and catechins as they can promote beneficial strains by suppressing pathogenic ones. All of the data suggested by these studies cannot be compared because of different formulations, doses, study conditions, time of treatment, route of administration, and interventions being used, and the prebiotic effect of these PPs is probably due to an indirect effect. In most cases, the metabolism of PPs provides a “direct” fitness advantage to gut and GM. Thus, with the support of this data, the ability of curcumin, quercetin, and catechins to positively modulate GM, GMD, and gut may help to understand their therapeutic benefits better. Still, clinical studies are needed to estimate the specific effects of these PPs on the human gut microbiome in patients with metabolic syndromes.
9. Bioavailability of Polyphenols
The fraction of a nutrient/non-nutrient that is available to the human body for physiological functions/storage can be referred to as bioavailability [179]. Several factors affect the bioavailability of PPs after ingestion, e.g., low water solubility. After oral administration, the drastic degradation processes start due to the transit in the different organs of the GIT, where the unaffected compounds need to be released from the food matrix to be absorbable. Further, low metabolism in the small intestine and permeation through the intestinal barrier renders them unable to distribute further into the bloodstream [180] (Figure 6). Application of PPs in functional foods and nutraceuticals as a drug molecule is limited due to their low aqueous solubility, inefficient systemic delivery, extensive first-pass metabolism, and poor oral bioavailability [126]. Catechin (especially EGCG), curcumin, and quercetin are the well-known bioactive PPs but they have low bioavailability. To overcome the bioavailability issues and utilization of beneficial properties of PPs, nanoencapsulation can be a promising technique [125]. Substances are encapsulated at the nanoscale with continuous films of coating material that improve the target specificity, stability, solubility, and drug release. They protect the substances during the GIT interaction, intestinal permeation, and increase residence time in intestines. Nano-delivery systems such as lipid-based carriers, polymer nanoparticles, inclusion complexes, micelles, and conjugates-based encapsulation are being used [181]. Other than nanotechnology, microencapsulation is also a new strategy to enhance the availability and their therapeutic effects [180]. Molecules are encapsulated in microscopic capsules that can measure from millimeter to micrometer [182]. Comparatively, the nanoencapsulation technique is considered better than microencapsulation because of the ultrathin layers that help to enhance the mass transport of substances to the islets and reduce the volume of material. Cell and animal models have shown positive results, but studies related to humans are still lacking.
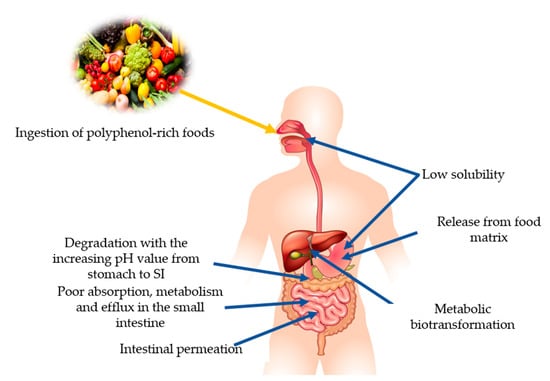
Figure 6.
Summary of the factors affecting the bioavailability of polyphenols.
Moreover, Annunziata et al. [183] proposed that fermentation is a natural strategy with minimum environmental impacts to increase the bioavailability of bioactive compounds (e.g., polyphenols) that can help to produce functional foods with higher nutritional values and health-promoting compounds. Fermentation mainly enhances the solubility and converts them into activated form before utilizing them into functional foods. So, the application of one of these strategies might be helpful to overcome metabolic disorders in clinical trials.
10. Conclusions and Perspectives
PPs are naturally occurring bioactive compounds which have an important role in human nutrition due to their antioxidative ability and their ability to decrease ROS. Therefore, they are known as promising candidates that can prevent and combat several metabolic syndromes. Regular consumption of PPs can help to ameliorate metabolic disorders such as obesity, diabetes, cancer, and cardio-metabolic disorders. Metabolism of PPs occurs in the intestines; thus, retention of PPs in intestines for a long time can promote beneficial effect on GM, as well as GM enhancing the biological activity of PPs by biotransforming them into active metabolites that help to improve the overall gut health. However, there are still some crucial challenges, such as poor bioavailability, making it difficult to achieve profitable results in in vitro and in vivo studies, which is why their use in functional and nutraceutical foods for therapeutic uses is limited. For this reason, after extraction of PPs, suitable approaches can be used to enhance their bioavailability, e.g., fermentation, micro- and nanoencapsulation. Another challenge is to determine the dosage level, as the concentration of PPs varies in foods, as it is altered by the food preparation methods. PPs interact with GM which influences how many doses are needed to achieve the optimal therapeutic effects. Moreover, without clinical studies, it is quite difficult to use PPs-based treatments, even though animal studies are available.
On the other hand, animal studies are also controversial as they cannot be compared perfectly because of different dosage levels, study conditions, time of treatment, route of administration, and interventions. Besides, the usage of PPs as prebiotics is also a challenge, their role as prebiotics in the human gut varies, depending on the residing probiotic strains; in many studies, the number of patients is also very small. Apart from this, supplementation of probiotics into PPs formulations can be useful to enhance their therapeutic effects synergistically, with improved efficiency. If scientists overcome these issues in both in vitro and in vivo studies, not only metabolic diseases, by promoting the gut homeostasis, but also many associated diseases will possibly get treated.
Author Contributions
U.S.: writing—original draft, writing—review & editing, conceptualization, M.R., E.B.-M.D., R.C. and A.J.: writing—review & editing, D.-H.O.: supervision, funding acquisition, conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This work was supported by a grant from the Brain Korea (BK) 21 Plus Project (Grant No. 22A20153713433) funded by the Korean Government, Republic of Korea.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| PPs | Plant polyphenols |
| GM | Gut microbiota |
| GMD | Gut microbiota dysbiosis |
| ROS | Reactive oxygen species |
| MAE | Microwave-assisted extraction |
| UAE | Ultrasonic-assisted extraction |
| HAE | Homogeniser-assisted extraction |
| RSLDE | Rapid solid-liquid dynamic extraction |
| GIT | Gastrointestinal tract |
| EGCG | Epigallocatechin-3-gallate |
| EGC | Epigallocatechin |
| GCG | Gallocatechin gallate |
| IL | Interleukin |
| NF-κB | nuclear factor-κB |
References
- Li, S.; Tan, H.Y.; Wang, N.; Cheung, F.; Hong, M.; Feng, Y. The potential and action mechanism of polyphenols in the treatment of liver diseases. Oxidative Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zheng, Y.; Tang, W.; Yan, W.; Nie, H.; Fang, J.; Liu, G. Dietary polyphenols in lipid metabolism: A role of gut microbiome. Anim. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Rubab, M.; Chelliah, R.; Saravanakumar, K.; Kim, J.-R.; Yoo, D.; Wang, M.-H.; Oh, D.-H. Phytochemical characterization, and antioxidant and antimicrobial activities of white cabbage extract on the quality and shelf life of raw beef during refrigerated storage. RSC Adv. 2020, 10, 41430–41442. [Google Scholar] [CrossRef]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy effects of plant polyphenols: Molecular mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, B.; Simon, J.; Kitunen, V.; Adamczyk, S.; Smolander, A. Tannins and their complex interaction with different organic nitrogen compounds and enzymes: Old paradigms versus recent advances. ChemistryOpen 2017, 6, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Tsatsakis, A.; Mamoulakis, C.; Teodoro, M.; Briguglio, G.; Caruso, E.; Tsoukalas, D.; Margina, D.; Dardiotis, E.; Kouretas, D. Current evidence on the effect of dietary polyphenols intake on chronic diseases. Food Chem. Toxicol. 2017, 110, 286–299. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Junior, M.R.M. Phenolic compounds: Structure, classification, and antioxidant power. In Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–50. [Google Scholar]
- Tsimogiannis, D.; Oreopoulou, V. Classification of phenolic compounds in plants. In Polyphenols in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 263–284. [Google Scholar]
- Shabbir, U.; Khalid, S.; Abbas, M.; Suleria, H.A.R. Natural carotenoids: Weapon against life-style-related disorders. In Phytochemicals from Medicinal Plants: Scope, Applications, and Potential Health Claims, 1st ed.; Suleria, H.A.R., Goyal, M.R., Butt, M.S., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 159–178. [Google Scholar]
- Poltronieri, P.; Xu, B.; Giovinazzo, G. Resveratrol and other Stilbenes: Effects on Dysregulated Gene Expression in Cancers and Novel Delivery Systems. Anti-Cancer Agents Med. Chem. 2020. [Google Scholar] [CrossRef]
- Maurya, R.; Bishnoi, M.; Kondepudi, K.K. Plant Polyphenols and Gut Bacteria: Role in Obesity-Induced Metabolic Endotoxaemia and Inflammation. In Advances in Agri-Food Biotechnology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 221–238. [Google Scholar]
- Caro-Gómez, E.; Sierra, J.A.; Escobar, J.S.; Álvarez-Quintero, R.; Naranjo, M.; Medina, S.; Velásquez-Mejía, E.P.; Tabares-Guevara, J.H.; Jaramillo, J.C.; León-Varela, Y.M. Green Coffee Extract Improves Cardiometabolic Parameters and Modulates Gut Microbiota in High-Fat-Diet-Fed ApoE-/- Mice. Nutrients 2019, 11, 497. [Google Scholar] [CrossRef]
- Lin, S.; Wang, Z.; Lam, K.-L.; Zeng, S.; Tan, B.K.; Hu, J. Role of intestinal microecology in the regulation of energy metabolism by dietary polyphenols and their metabolites. Food Nutr. Res. 2019, 63. [Google Scholar] [CrossRef]
- Gowd, V.; Karim, N.; Shishir, M.R.I.; Xie, L.; Chen, W. Dietary polyphenols to combat the metabolic diseases via altering gut microbiota. Trends Food Sci. Technol. 2019, 93, 81–93. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Đorđević, V.B.; Zdunić, G.M.; Pljevljakušić, D.S.; Šavikin, K.P.; Gođevac, D.M.; Bugarski, B.M. Optimization of the extraction process of polyphenols from Thymus serpyllum L. herb using maceration, heat-and ultrasound-assisted techniques. Sep. Purif. Technol. 2017, 179, 369–380. [Google Scholar]
- Vuleta, G.; Milic, J.; Savic, S. Farmaceutska Tehnologija [Pharmaceutical Technology]; Faculty of Pharmacy, University of Belgrade: Belgrade, Serbia, 2012. [Google Scholar]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Yılmaz, F.M.; Görgüç, A.; Uygun, Ö.; Bircan, C. Steviol glycosides and polyphenols extraction from Stevia rebaudiana Bertoni leaves using maceration, microwave-, and ultrasound-assisted techniques. Sep. Sci. Technol. 2020, 1–13. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, Y.; Padilla-Zakour, O.; Yang, G. Polyphenols, antioxidant and antimicrobial activities of leaf and bark extracts of Solidago canadensis L. Ind. Crop. Prod. 2015, 74, 803–809. [Google Scholar] [CrossRef]
- Eyiz, V.; Tontul, I.; Turker, S. Optimization of green extraction of phytochemicals from red grape pomace by homogenizer assisted extraction. J. Food Meas. Charact. 2020, 14, 39–47. [Google Scholar] [CrossRef]
- Naviglio, D.; Scarano, P.; Ciaravolo, M.; Gallo, M. Rapid Solid-Liquid Dynamic Extraction (RSLDE): A powerful and greener alternative to the latest solid-liquid extraction techniques. Foods 2019, 8, 245. [Google Scholar] [CrossRef]
- Galan, A.-M.; Calinescu, I.; Trifan, A.; Winkworth-Smith, C.; Calvo-Carrascal, M.; Dodds, C.; Binner, E. New insights into the role of selective and volumetric heating during microwave extraction: Investigation of the extraction of polyphenolic compounds from sea buckthorn leaves using microwave-assisted extraction and conventional solvent extraction. Chem. Eng. Process. Process. Intensif. 2017, 116, 29–39. [Google Scholar] [CrossRef]
- da Rosa, G.S.; Vanga, S.K.; Gariepy, Y.; Raghavan, V. Comparison of microwave, ultrasonic and conventional techniques for extraction of bioactive compounds from olive leaves (Olea europaea L.). Innov. Food Sci. Emerg. Technol. 2019, 58, 102234. [Google Scholar] [CrossRef]
- Rocchetti, G.; Blasi, F.; Montesano, D.; Ghisoni, S.; Marcotullio, M.C.; Sabatini, S.; Cossignani, L.; Lucini, L. Impact of conventional/non-conventional extraction methods on the untargeted phenolic profile of Moringa oleifera leaves. Food Res. Int. 2019, 115, 319–327. [Google Scholar] [CrossRef]
- Jovanović, A.; Petrović, P.; Đorđević, V.; Zdunić, G.; Šavikin, K.; Bugarski, B. Polyphenols extraction from plant sources. Lek. Sirovine 2017, 37, 45–49. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef]
- Grundy, S.M. Metabolic Syndrome; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Karim, N.; Jia, Z.; Zheng, X.; Cui, S.; Chen, W. A recent review of citrus flavanone naringenin on metabolic diseases and its potential sources for high yield-production. Trends Food Sci. Technol. 2018, 79, 35–54. [Google Scholar] [CrossRef]
- John, O.D.; Du Preez, R.; Panchal, S.K.; Brown, L. Tropical foods as functional foods for metabolic syndrome. Food Funct. 2020, 11, 6946–6960. [Google Scholar] [CrossRef]
- Engin, A. The definition and prevalence of obesity and metabolic syndrome. In Obesity and Lipotoxicity; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–17. [Google Scholar]
- Kumar Singh, A.; Cabral, C.; Kumar, R.; Ganguly, R.; Kumar Rana, H.; Gupta, A.; Rosaria Lauro, M.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial effects of dietary polyphenols on gut microbiota and strategies to improve delivery efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef]
- Westfall, S.; Lomis, N.; Kahouli, I.; Dia, S.Y.; Singh, S.P.; Prakash, S. Microbiome, probiotics and neurodegenerative diseases: Deciphering the gut brain axis. Cell. Mol. Life Sci. 2017, 74, 3769–3787. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Lopes, T.C.M.; Mosser, D.M.; Gonçalves, R. Macrophage polarization in intestinal inflammation and gut homeostasis. Inflamm. Res. 2020, 69, 1–10. [Google Scholar]
- Jamar, G.; Estadella, D.; Pisani, L.P. Contribution of anthocyanin-rich foods in obesity control through gut microbiota interactions. BioFactors 2017, 43, 507–516. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2020, 19, 1–17. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The human intestinal microbiome in health and disease. New Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Fan, W.; Huo, G.; Li, X.; Yang, L.; Duan, C. Impact of diet in shaping gut microbiota revealed by a comparative study in infants during the first six months of life. J. Microbiol. Biotechnol 2014, 24, 133–143. [Google Scholar] [CrossRef]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.-z.; Abe, F.; Osawa, R. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 2016, 16, 1–12. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Alzheimer’s disease and gut microbiota modifications: The long way between preclinical studies and clinical evidence. Pharmacol. Res. 2018, 129, 329–336. [Google Scholar] [CrossRef]
- Madan, S.; Mehra, M.R. Gut dysbiosis and heart failure: Navigating the universe within. Eur. J. Heart Fail. 2020, 22, 629–637. [Google Scholar] [CrossRef]
- Zuo, T.; Ng, S.C. The gut microbiota in the pathogenesis and therapeutics of inflammatory bowel disease. Front. Microbiol. 2018, 9, 2247. [Google Scholar] [CrossRef]
- Pascale, A.; Marchesi, N.; Marelli, C.; Coppola, A.; Luzi, L.; Govoni, S.; Giustina, A.; Gazzaruso, C. Microbiota and metabolic diseases. Endocrine 2018, 61, 357–371. [Google Scholar] [CrossRef]
- Brial, F.; Le Lay, A.; Dumas, M.-E.; Gauguier, D. Implication of gut microbiota metabolites in cardiovascular and metabolic diseases. Cell. Mol. Life Sci. 2018, 75, 3977–3990. [Google Scholar] [CrossRef]
- Kutschera, M.; Engst, W.; Blaut, M.; Braune, A. Isolation of catechin-converting human intestinal bacteria. J. Appl. Microbiol. 2011, 111, 165–175. [Google Scholar] [CrossRef]
- Duda-Chodak, A. The inhibitory effect of polyphenols on human gut microbiota. J. Physiol Pharm. 2012, 63, 497–503. [Google Scholar]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly) phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary polyphenols promote growth of the gut bacterium Akkermansia muciniphila and attenuate high-fat diet–induced metabolic syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef]
- Kuhn, P.; Kalariya, H.M.; Poulev, A.; Ribnicky, D.M.; Jaja-Chimedza, A.; Roopchand, D.E.; Raskin, I. Grape polyphenols reduce gut-localized reactive oxygen species associated with the development of metabolic syndrome in mice. PLoS ONE 2018, 13, e0198716. [Google Scholar] [CrossRef]
- Zorraquín, I.; Sánchez-Hernández, E.; Ayuda-Durán, B.; Silva, M.; González-Paramás, A.M.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolomé, B. Current and future experimental approaches in the study of grape and wine polyphenols interacting gut microbiota. J. Sci. Food Agric. 2020. [Google Scholar] [CrossRef]
- Moreno-Indias, I.; Sánchez-Alcoholado, L.; Pérez-Martínez, P.; Andrés-Lacueva, C.; Cardona, F.; Tinahones, F.; Queipo-Ortuño, M.I. Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients. Food Funct. 2016, 7, 1775–1787. [Google Scholar] [CrossRef]
- Jin, G.; Asou, Y.; Ishiyama, K.; Okawa, A.; Kanno, T.; Niwano, Y. Proanthocyanidin-rich grape seed extract modulates intestinal microbiota in ovariectomized mice. J. Food Sci. 2018, 83, 1149–1152. [Google Scholar] [CrossRef]
- Kemperman, R.A.; Gross, G.; Mondot, S.; Possemiers, S.; Marzorati, M.; Van de Wiele, T.; Doré, J.; Vaughan, E.E. Impact of polyphenols from black tea and red wine/grape juice on a gut model microbiome. Food Res. Int. 2013, 53, 659–669. [Google Scholar] [CrossRef]
- Snopek, L.; Mlcek, J.; Sochorova, L.; Baron, M.; Hlavacova, I.; Jurikova, T.; Kizek, R.; Sedlackova, E.; Sochor, J. Contribution of red wine consumption to human health protection. Molecules 2018, 23, 1684. [Google Scholar] [CrossRef] [PubMed]
- Yamakoshi, J.; Tokutake, S.; Kikuchi, M.; Kubota, Y.; Konishi, H.; Mitsuoka, T. Effect of proanthocyanidin-rich extract from grape seeds on human fecal flora and fecal odor. Microb. Ecol. Health Dis. 2001, 13, 25–31. [Google Scholar]
- Liu, Y.-C.; Li, X.-Y.; Shen, L. Modulation effect of tea consumption on gut microbiota. Appl. Microbiol. Biotechnol. 2020, 104, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, A.B.; Sun, S.; Ajami, N.J.; Ross, M.C.; Wang, H.; Zhang, L.; Reuhl, K.; Kobayashi, K.; Onishi, J.C. Green Tea Polyphenols Modify the Gut Microbiome in db/db Mice as Co-Abundance Groups Correlating with the Blood Glucose Lowering Effect. Mol. Nutr. Food Res. 2019, 63, 1801064. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, L.; Shen, C.-L.; Wang, J.-S. Green tea polyphenols boost gut-microbiota-dependent mitochondrial TCA and urea cycles in Sprague–Dawley rats. J. Nutr. Biochem. 2020, 81, 108395. [Google Scholar] [CrossRef]
- Bond, T.; Derbyshire, E. Tea Compounds and the Gut Microbiome: Findings from Trials and Mechanistic Studies. Nutrients 2019, 11, 2364. [Google Scholar] [CrossRef]
- Tombola, F.; Campello, S.; De Luca, L.; Ruggiero, P.; Del Giudice, G.; Papini, E.; Zoratti, M. Plant polyphenols inhibit VacA, a toxin secreted by the gastric pathogen Helicobacter pylori. FEBS Lett. 2003, 543, 184–189. [Google Scholar] [CrossRef]
- Rastmanesh, R. High polyphenol, low probiotic diet for weight loss because of intestinal microbiota interaction. Chem. Biol. Interact. 2011, 189, 1–8. [Google Scholar] [CrossRef]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef]
- Anhê, F.F.; Nachbar, R.T.; Varin, T.V.; Vilela, V.; Dudonné, S.; Pilon, G.; Fournier, M.; Lecours, M.-A.; Desjardins, Y.; Roy, D. A polyphenol-rich cranberry extract reverses insulin resistance and hepatic steatosis independently of body weight loss. Mol. Metab. 2017, 6, 1563–1573. [Google Scholar] [CrossRef]
- Li, H.; Christman, L.M.; Li, R.; Gu, L. Synergic Interactions between Polyphenols and Gut Microbiota in Mitigating Inflammatory Bowel Diseases. Food Funct. 2020. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.S.-Y.; Varin, T.V.; St-Pierre, P.; Pilon, G.; Tremblay, A.; Marette, A. A polyphenol-rich cranberry extract protects against endogenous exposure to persistent organic pollutants during weight loss in mice. Food Chem. Toxicol. 2020, 146, 111832. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Huang, K.; Zhao, C.; Xu, W.; Sheng, Y.; Luo, Y.; He, X. Procyanidin attenuates weight gain and modifies the gut microbiota in high fat diet induced obese mice. J. Funct. Foods 2018, 49, 362–368. [Google Scholar] [CrossRef]
- Lobo, A.; Liu, Y.; Song, Y.; Liu, S.; Zhang, R.; Liang, H.; Xin, H. Effect of procyanidins on lipid metabolism and inflammation in rats exposed to alcohol and iron. Heliyon 2020, 6, e04847. [Google Scholar] [CrossRef] [PubMed]
- Koutzoumis, D.N.; Vergara, M.; Pino, J.; Buddendorff, J.; Khoshbouei, H.; Mandel, R.J.; Torres, G.E. Alterations of the gut microbiota with antibiotics protects dopamine neuron loss and improve motor deficits in a pharmacological rodent model of Parkinson’s disease. Exp. Neurol. 2020, 325, 113159. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Wang, Y.; Lin, Y.; Lang, Y.; Li, E.; Zhang, X.; Zhang, Q.; Feng, Y.; Meng, X.; Li, B. Blueberry polyphenols extract as a potential prebiotic with anti-obesity effects on C57BL/6 J mice by modulating the gut microbiota. J. Nutr. Biochem. 2019, 64, 88–100. [Google Scholar] [CrossRef]
- Rodríguez-Daza, M.-C.; Roquim, M.; Dudonné, S.; Pilon, G.; Levy, E.; Marette, A.; Roy, D.; Desjardins, Y. Berry polyphenols and fibers modulate distinct microbial metabolic functions and gut microbiota enterotype-like clustering in obese mice. Front. Microbiol. 2020, 11, 2032. [Google Scholar] [CrossRef]
- Lee, S.; Keirsey, K.I.; Kirkland, R.; Grunewald, Z.I.; Fischer, J.G.; de La Serre, C.B. Blueberry supplementation influences the gut microbiota, inflammation, and insulin resistance in high-fat-diet–fed rats. J. Nutr. 2018, 148, 209–219. [Google Scholar] [CrossRef]
- Lavefve, L.; Howard, L.R.; Carbonero, F. Berry polyphenols metabolism and impact on human gut microbiota and health. Food Funct. 2020, 11, 45–65. [Google Scholar] [CrossRef]
- Lima, A.C.D.; Cecatti, C.; Fidélix, M.P.; Adorno, M.A.T.; Sakamoto, I.K.; Cesar, T.B.; Sivieri, K. Effect of daily consumption of orange juice on the levels of blood glucose, lipids, and gut microbiota metabolites: Controlled clinical trials. J. Med. Food 2019, 22, 202–210. [Google Scholar] [CrossRef]
- Brasili, E.; Hassimotto, N.M.A.; Del Chierico, F.; Marini, F.; Quagliariello, A.; Sciubba, F.; Miccheli, A.; Putignani, L.; Lajolo, F. Daily consumption of orange juice from Citrus sinensis L. Osbeck cv. Cara Cara and cv. Bahia differently affects gut microbiota profiling as unveiled by an integrated meta-omics approach. J. Agric. Food Chem. 2019, 67, 1381–1391. [Google Scholar] [CrossRef]
- Fidélix, M.; Milenkovic, D.; Sivieri, K.; Cesar, T. Microbiota modulation and effects on metabolic biomarkers by orange juice: A controlled clinical trial. Food Funct. 2020, 11, 1599–1610. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Su, Q.; Liu, Y. Sinapine reduces non-alcoholic fatty liver disease in mice by modulating the composition of the gut microbiota. Food Funct. 2019, 10, 3637–3649. [Google Scholar] [CrossRef]
- Ashley, D.; Marasini, D.; Brownmiller, C.; Lee, J.; Carbonero, F.; Lee, S.-O. Impact of grain sorghum polyphenols on microbiota of normal weight and overweight/obese subjects during in vitro fecal fermentation. Nutrients 2019, 11, 217. [Google Scholar] [CrossRef]
- Ritchie, L.E.; Sturino, J.M.; Carroll, R.J.; Rooney, L.W.; Azcarate-Peril, M.A.; Turner, N.D. Polyphenol-rich sorghum brans alter colon microbiota and impact species diversity and species richness after multiple bouts of dextran sodium sulfate-induced colitis. Fems Microbiol. Ecol. 2015, 91. [Google Scholar] [CrossRef]
- De Sousa, A.R.; de Castro Moreira, M.E.; Grancieri, M.; Toledo, R.C.L.; de Oliveira Araújo, F.; Mantovani, H.C.; Queiroz, V.A.V.; Martino, H.S.D. Extruded sorghum (Sorghum bicolor L.) improves gut microbiota, reduces inflammation, and oxidative stress in obese rats fed a high-fat diet. J. Funct. Foods 2019, 58, 282–291. [Google Scholar] [CrossRef]
- Qiao, Y.; Sun, J.; Xia, S.; Tang, X.; Shi, Y.; Le, G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 2014, 5, 1241–1249. [Google Scholar] [CrossRef]
- Sengottuvelan, M.; Nalini, N. Dietary supplementation of resveratrol suppresses colonic tumour incidence in 1, 2-dimethylhydrazine-treated rats by modulating biotransforming enzymes and aberrant crypt foci development. Br. J. Nutr. 2006, 96, 145–153. [Google Scholar] [CrossRef]
- Larrosa, M.; Yañéz-Gascón, M.J.; Selma, M.V.; Gonzalez-Sarrias, A.; Toti, S.; Cerón, J.J.; Tomas-Barberan, F.; Dolara, P.; Espín, J.C. Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. J. Agric. Food Chem. 2009, 57, 2211–2220. [Google Scholar] [CrossRef]
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.; Portillo, M.; Martínez, J.; Milagro, F. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef]
- Tamura, M.; Hoshi, C.; Kobori, M.; Takahashi, S.; Tomita, J.; Nishimura, M.; Nishihira, J. Quercetin metabolism by fecal microbiota from healthy elderly human subjects. PLoS ONE 2017, 12, e0188271. [Google Scholar] [CrossRef]
- Jayachandran, M.; Xiao, J.; Xu, B. A critical review on health promoting benefits of edible mushrooms through gut microbiota. Int. J. Mol. Sci. 2017, 18, 1934. [Google Scholar] [CrossRef]
- Kang, N.J.; Lee, K.W.; Kim, B.H.; Bode, A.M.; Lee, H.-J.; Heo, Y.-S.; Boardman, L.; Limburg, P.; Lee, H.J.; Dong, Z. Coffee phenolic phytochemicals suppress colon cancer metastasis by targeting MEK and TOPK. Carcinogenesis 2011, 32, 921–928. [Google Scholar] [CrossRef]
- Jaquet, M.; Rochat, I.; Moulin, J.; Cavin, C.; Bibiloni, R. Impact of coffee consumption on the gut microbiota: A human volunteer study. Int. J. Food Microbiol. 2009, 130, 117–121. [Google Scholar] [CrossRef]
- Chen, C.; Ahn, E.H.; Kang, S.S.; Liu, X.; Alam, A.; Ye, K. Gut dysbiosis contributes to amyloid pathology, associated with C/EBPβ/AEP signaling activation in Alzheimer’s disease mouse model. Sci. Adv. 2020, 6, eaba0466. [Google Scholar] [CrossRef]
- Remely, M.; Ferk, F.; Sterneder, S.; Setayesh, T.; Roth, S.; Kepcija, T.; Noorizadeh, R.; Rebhan, I.; Greunz, M.; Beckmann, J. EGCG prevents high fat diet-induced changes in gut microbiota, decreases of DNA strand breaks, and changes in expression and DNA methylation of Dnmt1 and MLH1 in C57BL/6J male mice. Oxidative Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef]
- Ushiroda, C.; Naito, Y.; Takagi, T.; Uchiyama, K.; Mizushima, K.; Higashimura, Y.; Yasukawa, Z.; Okubo, T.; Inoue, R.; Honda, A. Green tea polyphenol (epigallocatechin-3-gallate) improves gut dysbiosis and serum bile acids dysregulation in high-fat diet-fed mice. J. Clin. Biochem. Nutr. 2019, 65, 34–46. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Q.; Ma, W.; Tian, F.; Shen, H.; Zhou, M. A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota. Food Funct. 2017, 8, 4644–4656. [Google Scholar] [CrossRef]
- Hesari, A.; Azizian, M.; Sheikhi, A.; Nesaei, A.; Sanaei, S.; Mahinparvar, N.; Derakhshani, M.; Hedayt, P.; Ghasemi, F.; Mirzaei, H. Chemopreventive and therapeutic potential of curcumin in esophageal cancer: Current and future status. Int. J. Cancer 2019, 144, 1215–1226. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its’ effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Nouri-Vaskeh, M.; Malek Mahdavi, A.; Afshan, H.; Alizadeh, L.; Zarei, M. Effect of curcumin supplementation on disease severity in patients with liver cirrhosis: A randomized controlled trial. Phytother. Res. 2020. [Google Scholar] [CrossRef]
- Di Meo, F.; Filosa, S.; Madonna, M.; Giello, G.; Di Pardo, A.; Maglione, V.; Baldi, A.; Crispi, S. Curcumin C3 complex®/Bioperine® has antineoplastic activity in mesothelioma: An in vitro and in vivo analysis. J. Exp. Clin. Cancer Res. 2019, 38, 360. [Google Scholar] [CrossRef]
- Mantzorou, M.; Pavlidou, E.; Vasios, G.; Tsagalioti, E.; Giaginis, C. Effects of curcumin consumption on human chronic diseases: A narrative review of the most recent clinical data. Phytother. Res. 2018, 32, 957–975. [Google Scholar] [CrossRef]
- Shabbir, U.; Rubab, M.; Tyagi, A.; Oh, D.-H. Curcumin and Its Derivatives as Theranostic Agents in Alzheimer’s Disease: The Implication of Nanotechnology. Int. J. Mol. Sci. 2021, 22, 196. [Google Scholar] [CrossRef]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef]
- Zam, W. Gut microbiota as a prospective therapeutic target for curcumin: A review of mutual influence. J. Nutr. Metab. 2018, 2018. [Google Scholar] [CrossRef]
- Di Meo, F.; Margarucci, S.; Galderisi, U.; Crispi, S.; Peluso, G. Curcumin, gut microbiota, and neuroprotection. Nutrients 2019, 11, 2426. [Google Scholar] [CrossRef]
- Scazzocchio, B.; Minghetti, L.; D’Archivio, M. Interaction between Gut Microbiota and Curcumin: A New Key of Understanding for the Health Effects of Curcumin. Nutrients 2020, 12, 2499. [Google Scholar] [CrossRef]
- Shen, L.; Ji, H.-F. Bidirectional interactions between dietary curcumin and gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 2896–2902. [Google Scholar] [CrossRef]
- Carmody, R.N.; Turnbaugh, P.J. Host-microbial interactions in the metabolism of therapeutic and diet-derived xenobiotics. J. Clin. Investig. 2014, 124, 4173–4181. [Google Scholar] [CrossRef]
- Tan, S.; Rupasinghe, T.W.; Tull, D.L.; Boughton, B.; Oliver, C.; McSweeny, C.; Gras, S.L.; Augustin, M.A. Degradation of curcuminoids by in vitro pure culture fermentation. J. Agric. Food Chem. 2014, 62, 11005–11015. [Google Scholar] [CrossRef]
- Jazayeri, S.D.; Mustafa, S.; Manap, M.; Ali, A.; Ismail, A.; Faujan, N.; Shaari, M. Survival of bifidobacteria and other selected intestinal bacteria in TPY medium supplemented with curcumin as assessed in vitro. Int. J. Probiotics Prebiotics 2009, 4, 15–22. [Google Scholar]
- Shen, L.; Liu, L.; Ji, H.-F. Regulative effects of curcumin spice administration on gut microbiota and its pharmacological implications. Food Nutr. Res. 2017, 61, 1361780. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Xiang, L.; Wang, Z.; Xiao, G.G.; Hu, J. Effect of curcumin on the diversity of gut microbiota in ovariectomized rats. Nutrients 2017, 9, 1146. [Google Scholar] [CrossRef]
- Al-Saud, N.B.S. Impact of curcumin treatment on diabetic albino rats. Saudi J. Biol. Sci. 2020, 27, 689–694. [Google Scholar] [CrossRef]
- Koboziev, I.; Scoggin, S.; Gong, X.; Mirzaei, P.; Zabet-Moghaddam, M.; Yosofvand, M.; Moussa, H.; Jones-Hall, Y.; Moustaid-Moussa, N. Effects of Curcumin in a Mouse Model of Very High Fat Diet-Induced Obesity. Biomolecules 2020, 10, 1368. [Google Scholar] [CrossRef]
- Wang, J.; Ghosh, S.S.; Ghosh, S. Curcumin improves intestinal barrier function: Modulation of intracellular signaling, and organization of tight junctions. Am. J. Physiol. Cell Physiol. 2017, 312, C438–C445. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Bie, J.; Wang, J.; Ghosh, S. Oral supplementation with non-absorbable antibiotics or curcumin attenuates western diet-induced atherosclerosis and glucose intolerance in LDLR−/− mice–role of intestinal permeability and macrophage activation. PLoS ONE 2014, 9, e108577. [Google Scholar] [CrossRef]
- Malinowski, B.; Wiciński, M.; Sokołowska, M.M.; Hill, N.A.; Szambelan, M. The Rundown of Dietary Supplements and Their Effects on Inflammatory Bowel Disease—A Review. Nutrients 2020, 12, 1423. [Google Scholar] [CrossRef]
- Friedland, R.P. Mechanisms of molecular mimicry involving the microbiota in neurodegeneration. J. Alzheimer’s Dis. 2015, 45, 349–362. [Google Scholar] [CrossRef]
- Peterson, C.T.; Vaughn, A.R.; Sharma, V.; Chopra, D.; Mills, P.J.; Peterson, S.N.; Sivamani, R.K. Effects of Turmeric and Curcumin Dietary Supplementation on Human Gut Microbiota: A double-Blind, Randomized, Placebo-Controlled Pilot Study; SAGE Publications Sage CA: Los Angeles, CA, USA, 2018. [Google Scholar]
- Wu, R.; Wang, L.; Yin, R.; Hudlikar, R.; Li, S.; Kuo, H.C.D.; Peter, R.; Sargsyan, D.; Guo, Y.; Liu, X. Epigenetics/epigenomics and prevention by curcumin of early stages of inflammatory-driven colon cancer. Mol. Carcinog. 2020, 59, 227–236. [Google Scholar] [CrossRef]
- Ohno, M.; Nishida, A.; Sugitani, Y.; Nishino, K.; Inatomi, O.; Sugimoto, M.; Kawahara, M.; Andoh, A. Nanoparticle curcumin ameliorates experimental colitis via modulation of gut microbiota and induction of regulatory T cells. PLoS ONE 2017, 12, e0185999. [Google Scholar] [CrossRef]
- Goulart, R.d.A.; Barbalho, S.M.; Lima, V.M.; Souza, G.A.d.; Matias, J.N.; Araújo, A.C.; Rubira, C.J.; Buchaim, R.L.; Buchaim, D.V.; Carvalho, A.C.A.d. Effects of the Use of Curcumin on Ulcerative Colitis and Crohn’s Disease: A Systematic Review. J. Med. Food 2020. [Google Scholar] [CrossRef]
- Yuan, T.; Yin, Z.; Yan, Z.; Hao, Q.; Zeng, J.; Li, L.; Zhao, J. Tetrahydrocurcumin ameliorates diabetes profiles of db/db mice by altering the composition of gut microbiota and up-regulating the expression of GLP-1 in the pancreas. Fitoterapia 2020, 146, 104665. [Google Scholar] [CrossRef]
- Ulusoy, H.G.; Sanlier, N. A minireview of quercetin: From its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food Sci. Nutr. 2020, 60, 3290–3303. [Google Scholar] [CrossRef]
- Guo, Y.; Bruno, R.S. Endogenous and exogenous mediators of quercetin bioavailability. J. Nutr. Biochem. 2015, 26, 201–210. [Google Scholar] [CrossRef]
- Khursheed, R.; Singh, S.K.; Wadhwa, S.; Gulati, M.; Awasthi, A. Enhancing the potential preclinical and clinical benefits of quercetin through novel drug delivery systems. Drug Discov. Today 2020, 25, 209–222. [Google Scholar] [CrossRef]
- Patel, R.V.; Mistry, B.M.; Shinde, S.K.; Syed, R.; Singh, V.; Shin, H.-S. Therapeutic potential of quercetin as a cardiovascular agent. Eur. J. Med. Chem. 2018, 155, 889–904. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Weldin, J.; Jack, R.; Dugaw, K.; Kapur, R.P. Quercetin, an over-the-counter supplement, causes neuroblastoma-like elevation of plasma homovanillic acid. Pediatric Dev. Pathol. 2003, 6, 547–551. [Google Scholar] [CrossRef]
- Bischoff, S.C. Quercetin: Potentials in the prevention and therapy of disease. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 733–740. [Google Scholar] [CrossRef]
- Moon, Y.J.; Wang, L.; DiCenzo, R.; Morris, M.E. Quercetin pharmacokinetics in humans. Biopharm. Drug Dispos. 2008, 29, 205–217. [Google Scholar] [CrossRef]
- Najmanová, I.; Pourová, J.; Vopršalová, M.; Pilařová, V.; Semecký, V.; Nováková, L.; Mladěnka, P. Flavonoid metabolite 3-(3-hydroxyphenyl) propionic acid formed by human microflora decreases arterial blood pressure in rats. Mol. Nutr. Food Res. 2016, 60, 981–991. [Google Scholar] [CrossRef]
- Di Pede, G.; Bresciani, L.; Calani, L.; Petrangolini, G.; Riva, A.; Allegrini, P.; Del Rio, D.; Mena, P. The Human Microbial Metabolism of Quercetin in Different Formulations: An In Vitro Evaluation. Foods 2020, 9, 1121. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, Z.; Zhang, N.; Liu, L.; Li, S.; Wei, H. In vitro catabolism of quercetin by human fecal bacteria and the antioxidant capacity of its catabolites. Food Nutr. Res. 2014, 58, 23406. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, X.; Li, S.; Zhang, N.; Wei, H. Isolation and identification of quercetin degrading bacteria from human fecal microbes. PLoS ONE 2014, 9, e90531. [Google Scholar] [CrossRef]
- Shi, T.; Bian, X.; Yao, Z.; Wang, Y.; Gao, W.; Guo, C. Quercetin improves gut dysbiosis in antibiotic-treated mice. Food Funct. 2020, 11, 8003–8013. [Google Scholar] [CrossRef]
- Sato, S.; Mukai, Y. Modulation of chronic inflammation by quercetin: The beneficial effects on obesity. J. Inflamm. Res. 2020, 13, 421. [Google Scholar] [CrossRef]
- Ju, S.; Ge, Y.; Li, P.; Tian, X.; Wang, H.; Zheng, X.; Ju, S. Dietary quercetin ameliorates experimental colitis in mouse by remodeling the function of colonic macrophages via a heme oxygenase-1-dependent pathway. Cell Cycle 2018, 17, 53–63. [Google Scholar] [CrossRef]
- Lin, R.; Piao, M.; Song, Y. Dietary quercetin increases colonic microbial diversity and attenuates colitis severity in citrobacter rodentium-infected mice. Front. Microbiol. 2019, 10, 1092. [Google Scholar] [CrossRef]
- Yang, D.K.; Kang, H.-S. Anti-diabetic effect of cotreatment with quercetin and resveratrol in streptozotocin-induced diabetic rats. Biomol. Ther. 2018, 26, 130. [Google Scholar] [CrossRef] [PubMed]
- Rezabakhsh, A.; Rahbarghazi, R.; Malekinejad, H.; Fathi, F.; Montaseri, A.; Garjani, A. Quercetin alleviates high glucose-induced damage on human umbilical vein endothelial cells by promoting autophagy. Phytomedicine 2019, 56, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, M.; Wang, J.; Guo, X.; Xiao, L.; Liu, P.; Liu, L.; Tang, Y.; Yao, P. Quercetin ameliorates autophagy in alcohol liver disease associated with lysosome through mTOR-TFEB pathway. J. Funct. Foods 2019, 52, 177–185. [Google Scholar] [CrossRef]
- Jahan, S.; Iftikhar, N.; Ullah, H.; Rukh, G.; Hussain, I. Alleviative effect of quercetin on rat testis against arsenic: A histological and biochemical study. Syst. Biol. Reprod. Med. 2015, 61, 89–95. [Google Scholar] [CrossRef]
- Khaki, A.; Khaki, A.; Nouri, M.; Ahmadi, A.H.R.; Rastgar, H.; Rezazadeh, S.; Fathi, A.F.; Ghanbari, M. Evaluation effects of Quercetin on liver apoptosis in streptozotocininduced diabetic rat. J. Med. Plants 2009, 8, 70–78. [Google Scholar]
- Porcu, E.P.; Cossu, M.; Rassu, G.; Giunchedi, P.; Cerri, G.; Pourová, J.; Najmanová, I.; Migkos, T.; Pilařová, V.; Nováková, L. Aqueous injection of quercetin: An approach for confirmation of its direct in vivo cardiovascular effects. Int. J. Pharm. 2018, 541, 224–233. [Google Scholar] [CrossRef]
- Li, G.; Shen, X.; Wei, Y.; Si, X.; Deng, X.; Wang, J. Quercetin reduces Streptococcus suis virulence by inhibiting suilysin activity and inflammation. Int. Immunopharmacol. 2019, 69, 71–78. [Google Scholar] [CrossRef]
- Zeng, H.; Guo, X.; Zhou, F.; Xiao, L.; Liu, J.; Jiang, C.; Xing, M.; Yao, P. Quercetin alleviates ethanol-induced liver steatosis associated with improvement of lipophagy. Food Chem. Toxicol. 2019, 125, 21–28. [Google Scholar] [CrossRef]
- Akinmoladun, A.C.; Oladejo, C.O.; Josiah, S.S.; Famusiwa, C.D.; Ojo, O.B.; Olaleye, M.T. Catechin, quercetin and taxifolin improve redox and biochemical imbalances in rotenone-induced hepatocellular dysfunction: Relevance for therapy in pesticide-induced liver toxicity? Pathophysiology 2018, 25, 365–371. [Google Scholar] [CrossRef]
- Daniel, O.O.; Adeoye, A.O.; Ojowu, J.; Olorunsogo, O.O. Inhibition of liver mitochondrial membrane permeability transition pore opening by quercetin and vitamin E in streptozotocin-induced diabetic rats. Biochem. Biophys. Res. Commun. 2018, 504, 460–469. [Google Scholar] [CrossRef]
- Lee, K.S.; Park, S.N. Cytoprotective effects and mechanisms of quercetin, quercitrin and avicularin isolated from Lespedeza cuneata G. Don against ROS-induced cellular damage. J. Ind. Eng. Chem. 2019, 71, 160–166. [Google Scholar] [CrossRef]
- Rastogi, S.; Haldar, C. Comparative effect of melatonin and quercetin in counteracting LPS induced oxidative stress in bone marrow mononuclear cells and spleen of Funambulus pennanti. Food Chem. Toxicol. 2018, 120, 243–252. [Google Scholar] [CrossRef]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Kılıç, C.S.; Sytar, O. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.; Wang, K.; Han, W.; Wang, X.; Gao, M.; Wang, Z.; Sun, Y.; Yan, H.; Zhang, H. Quercetin overcomes colon cancer cells resistance to chemotherapy by inhibiting solute carrier family 1, member 5 transporter. Eur. J. Pharmacol. 2020, 881, 173185. [Google Scholar] [CrossRef]
- Shang, H.S.; Lu, H.F.; Lee, C.H.; Chiang, H.S.; Chu, Y.L.; Chen, A.; Lin, Y.F.; Chung, J.G. Quercetin induced cell apoptosis and altered gene expression in AGS human gastric cancer cells. Environ. Toxicol. 2018, 33, 1168–1181. [Google Scholar] [CrossRef]
- Chen, Z.; Yuan, Q.; Xu, G.; Chen, H.; Lei, H.; Su, J. Effects of quercetin on proliferation and H2O2-induced apoptosis of intestinal porcine enterocyte cells. Molecules 2018, 23, 2012. [Google Scholar] [CrossRef]
- Forney, L.A.; Lenard, N.R.; Stewart, L.K.; Henagan, T.M. Dietary quercetin attenuates adipose tissue expansion and inflammation and alters adipocyte morphology in a tissue-specific manner. Int. J. Mol. Sci. 2018, 19, 895. [Google Scholar] [CrossRef]
- Cai, X.; Bao, L.; Ding, Y.; Dai, X.; Zhang, Z.; Li, Y. Quercetin alleviates cell apoptosis and inflammation via the ER stress pathway in vascular endothelial cells cultured in high concentrations of glucosamine. Mol. Med. Rep. 2017, 15, 825–832. [Google Scholar] [CrossRef]
- Isemura, M. Catechin in human health and disease. Molecules 2019, 24, 528. [Google Scholar] [CrossRef]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea polyphenols in promotion of human health. Nutrients 2019, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; van der Hooft, J.J.; Crozier, A. Human studies on the absorption, distribution, metabolism, and excretion of tea polyphenols. Am. J. Clin. Nutr. 2013, 98, 1619S–1630S. [Google Scholar] [CrossRef] [PubMed]
- Pastoriza, S.; Mesías, M.; Cabrera, C.; Rufián-Henares, J. Healthy properties of green and white teas: An update. Food Funct. 2017, 8, 2650–2662. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, N.; Arikawa, A.Y.; Chen, C. Inhibitory Effects of Green Tea Polyphenols on Microbial Metabolism of Aromatic Amino Acids in Humans Revealed by Metabolomic Analysis. Metabolites 2019, 9, 96. [Google Scholar] [CrossRef]
- Sánchez-Patán, F.; Chioua, M.; Garrido, I.; Cueva, C.; Samadi, A.; Marco-Contelles, J.; Moreno-Arribas, M.V.; Bartolomé, B.; Monagas, M. Synthesis, analytical features, and biological relevance of 5-(3′,4′-Dihydroxyphenyl)-γ-valerolactone, a microbial metabolite derived from the catabolism of dietary flavan-3-ols. J. Agric. Food Chem. 2011, 59, 7083–7091. [Google Scholar] [CrossRef]
- Dos Santos, A.N.; de, L. Nascimento, T.R.; Gondim, B.L.; Velo, M.M.; De A. Rêgo, R.I.; do C. Neto, J.R. Machado, J.R.; da Silva, M.V.; de Araújo, H.W.; Fonseca, M.G. Catechins as Model Bioactive Compounds for Biomedical Applications. Curr. Pharm. Des. 2020, 26, 4032–4047. [Google Scholar] [CrossRef]
- Al-Mahdi, Z.K.A.; Ewadh, R.M.; Hindi, N.K.K. Health Benefits of Aqueous Extract of Black and Green Tea Leaves. Bioact. Compd. 2020. [Google Scholar] [CrossRef]
- Bancirova, M. Comparison of the antioxidant capacity and the antimicrobial activity of black and green tea. Food Res. Int. 2010, 43, 1379–1382. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, X.; Sun, Y.; Hu, B.; Sun, Y.; Jabbar, S.; Zeng, X. Fermentation in vitro of EGCG, GCG and EGCG3" Me isolated from Oolong tea by human intestinal microbiota. Food Res. Int. 2013, 54, 1589–1595. [Google Scholar] [CrossRef]
- Liao, Z.-L.; Zeng, B.-H.; Wang, W.; Li, G.-H.; Wu, F.; Wang, L.; Zhong, Q.-P.; Wei, H.; Fang, X. Impact of the consumption of tea polyphenols on early atherosclerotic lesion formation and intestinal Bifidobacteria in high-fat-fed ApoE−/− mice. Front. Nutr. 2016, 3, 42. [Google Scholar] [CrossRef]
- Kim, H.S.; Moon, J.H.; Kim, Y.M.; Huh, J.Y. Epigallocatechin Exerts Anti-Obesity Effect in Brown Adipose Tissue. Chem. Biodivers. 2019, 16, e1900347. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Kawano, T.; Ukawa, Y.; Sagesaka, Y.M.; Fukuhara, I. Green tea beverages enriched with catechins with a galloyl moiety reduce body fat in moderately obese adults: A randomized double-blind placebo-controlled trial. Food Funct. 2016, 7, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Ding, S.; Li, F.; Zhang, C.; Sun-Waterhouse, D.; Chen, Y.; Li, D. Effects of (+)-catechin on the differentiation and lipid metabolism of 3T3-L1 adipocytes. J. Funct. Foods 2019, 62, 103558. [Google Scholar] [CrossRef]
- Tsou, L.K.; Yount, J.S.; Hang, H.C. Epigallocatechin-3-gallate inhibits bacterial virulence and invasion of host cells. Bioorganic Med. Chem. 2017, 25, 2883–2887. [Google Scholar] [CrossRef]
- Yang, J.; Tang, C.; Xiao, J.; Du, W.; Li, R. Influences of epigallocatechin gallate and citric acid on Escherichia coli O157: H7 toxin gene expression and virulence-associated stress response. Lett. Appl. Microbiol. 2018, 67, 435–441. [Google Scholar] [CrossRef]
- Xiong, L.-G.; Chen, Y.-J.; Tong, J.-W.; Huang, J.-A.; Li, J.; Gong, Y.-S.; Liu, Z.-H. Tea polyphenol epigallocatechin gallate inhibits Escherichia coli by increasing endogenous oxidative stress. Food Chem. 2017, 217, 196–204. [Google Scholar] [CrossRef]
- Toden, S.; Tran, H.-M.; Tovar-Camargo, O.A.; Okugawa, Y.; Goel, A. Epigallocatechin-3-gallate targets cancer stem-like cells and enhances 5-fluorouracil chemosensitivity in colorectal cancer. Oncotarget 2016, 7, 16158. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea and health: Studies in humans. Curr. Pharm. Des. 2013, 19, 6141–6147. [Google Scholar] [CrossRef]
- Sur, S.; Pal, D.; Mandal, S.; Roy, A.; Panda, C.K. Tea polyphenols epigallocatechin gallete and theaflavin restrict mouse liver carcinogenesis through modulation of self-renewal Wnt and hedgehog pathways. J. Nutr. Biochem. 2016, 27, 32–42. [Google Scholar] [CrossRef]
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef]
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, G.; Jiménez, M.; Capó, X.; Moranta, D.; Arnone, A.; Tenore, G.; Sureda, A.; Tejada, S. Microencapsulation as a tool to counteract the typical low bioavailability of polyphenols in the management of diabetes. Food Chem. Toxicol. 2020, 139, 111248. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Liu, X.; Zhang, C.; Zeng, X. Food macromolecule based nanodelivery systems for enhancing the bioavailability of polyphenols. J. Food Drug Anal. 2017, 25, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, G.; Franco, P.; De Marco, I.; Xiao, J.; Capanoglu, E. A review of microencapsulation methods for food antioxidants: Principles, advantages, drawbacks and applications. Food Chem. 2019, 272, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, G.; Arnone, A.; Ciampaglia, R.; Tenore, G.C.; Novellino, E. Fermentation of Foods and Beverages as a Tool for Increasing Availability of Bioactive Compounds. Focus on Short-Chain Fatty Acids. Foods 2020, 9, 999. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).