Long Chain Omega-3 Polyunsaturated Fatty Acids Improve Vascular Stiffness in Abdominal Aortic Aneurysm: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Vascular Stiffness Indices Are Elevated in Patients with Abdominal Aortic Aneurysm
3.2. AAA Is a Significant Independent Determinant of PWV
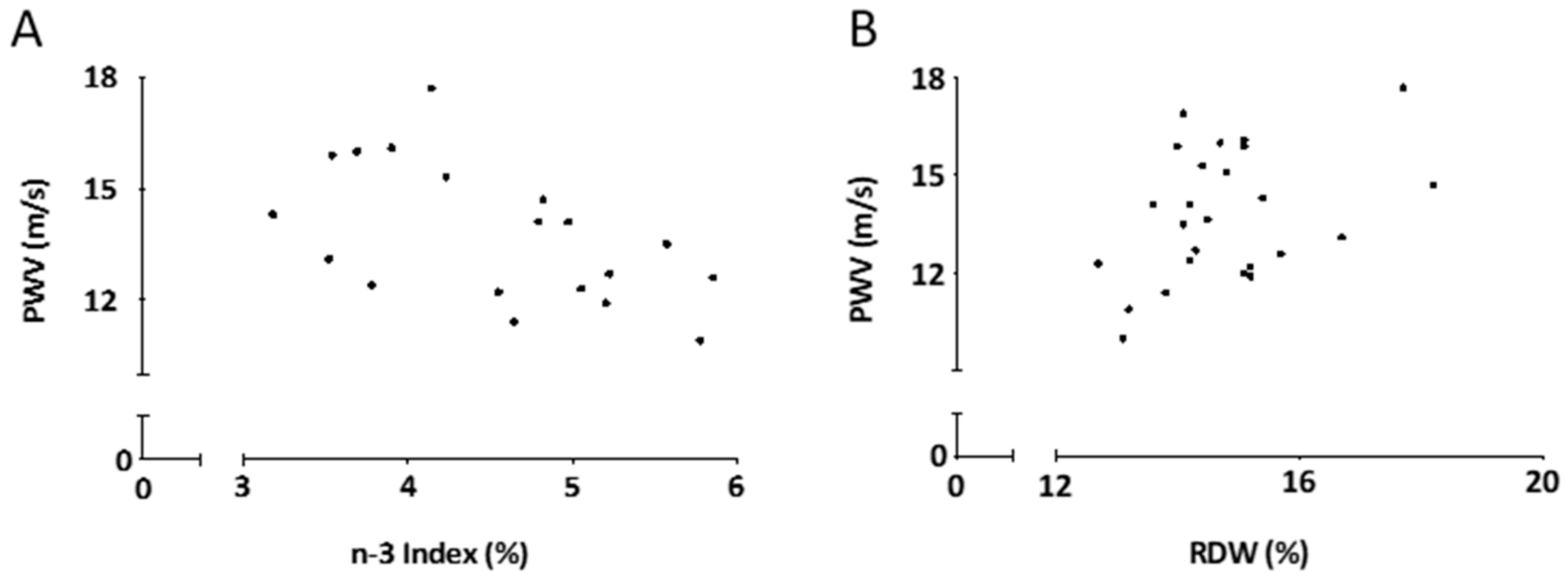
3.3. PWV Correlates with Omega-3 Index and Red Blood Cell Distribution Width (RDW) in Patients with AAA
3.4. Long-Chain Omega-3 PUFA Supplementation Improves Vascular Stiffness in Patients with AAA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raffort, J.; Lareyre, F.; Clément, M.; Hassen-Khodja, R.; Chinetti, G.; Mallat, Z. Monocytes and macrophages in abdominal aortic aneurysm. Nat. Rev. Cardiol. 2017, 14, 457–471. [Google Scholar] [CrossRef]
- Owens, D.K.; Davidson, K.W.; Krist, A.H.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Doubeni, C.A.; Epling, J.W.; Kubik, M.; Landefeld, C.S. Screening for abdominal aortic aneurysm: US Preventive Services Task Force recommendation statement. JAMA 2019, 322, 2211–2218. [Google Scholar] [CrossRef] [PubMed]
- Wales, K.M.; Kavazos, K.; Nataatmadja, M.; Brooks, P.R.; Williams, C.; Russell, F.D. N-3 PUFAs protect against aortic inflammation and oxidative stress in angiotensin II-infused apolipoprotein E-/- mice. PLoS ONE 2014, 9, e112816. [Google Scholar] [CrossRef] [PubMed]
- Kavazos, K.; Nataatmadja, M.; Wales, K.M.; Hartland, E.; Williams, C.; Russell, F.D. Dietary supplementation with omega-3 polyunsaturated fatty acids modulate matrix metalloproteinase immunoreactivity in a mouse model of pre-abdominal aortic aneurysm. Heart Lung Circ. 2015, 24, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Meital, L.T.; Windsor, M.T.; Maynard, A.E.; Schulze, K.; Magee, R.; O’Donnell, J.; Jha, P.; Meital, C.Y.; Perissiou, M.; Coverdale, S. Endotoxin tolerance in abdominal aortic aneurysm macrophages, in vitro: A case–control study. Antioxidants 2020, 9, 896. [Google Scholar] [CrossRef] [PubMed]
- Shirwany, N.A.; Zou, M.-h. Arterial stiffness: A brief review. Acta Pharmacol. Sin. 2010, 31, 1267–1276. [Google Scholar] [CrossRef]
- Lacolley, P.; Regnault, V.; Segers, P.; Laurent, S. Vascular smooth muscle cells and arterial stiffening: Relevance in development, aging, and disease. Physiol. Rev. 2017, 97, 1555–1617. [Google Scholar] [CrossRef]
- McManus, S.; Tejera, N.; Awwad, K.; Vauzour, D.; Rigby, N.; Fleming, I.; Cassidy, A.; Minihane, A.M. Differential effects of EPA versus DHA on postprandial vascular function and the plasma oxylipin profile in men. J. Lipid Res. 2016, 57, 1720–1727. [Google Scholar] [CrossRef]
- Perrault, R.; Omelchenko, A.; Taylor, C.G.; Zahradka, P. Establishing the interchangeability of arterial stiffness but not endothelial function parameters in healthy individuals. BMC Cardiovasc. Disord. 2019, 19, 190. [Google Scholar] [CrossRef]
- Pase, M.P.; Grima, N.A.; Sarris, J. The effects of dietary and nutrient interventions on arterial stiffness: A systematic review. Am. J. Clin. Nutr. 2011, 93, 446–454. [Google Scholar] [CrossRef]
- Butlin, M.; Qasem, A. Large artery stiffness assessment using SphygmoCor technology. Pulse 2016, 4, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Boutouyrie, P.; Bruno, R.-M. The clinical significance and application of vascular stiffness measurements. Am. J. Hypertens. 2019, 32, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Dobrin, P.B. Pathophysiology and pathogenesis of aortic aneurysms: Current concepts. Surg. Clin. N. Am. 1989, 69, 687–703. [Google Scholar] [CrossRef]
- Russo, L. Thoracic aortic compliance as a determinant of rupture of abdominal aortic aneurysms. Ann. N. Y. Acad. Sci. 2006, 1085, 363–366. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef]
- Kanamoto, R.; Otsuka, H.; Anegawa, T.; Takaseya, T.; Shintani, Y.; Tobinaga, S.; Onitsuka, S.; Ueno, M.; Hiromatsu, S.; Tanaka, H. P5601 High pulse wave velocity is associated with poor shrinkage of abdominal aortic aneurysm in endovascular aneurysm repair patients. Eur. Heart J. 2019, 40, ehz746.0545. [Google Scholar] [CrossRef]
- Wilkinson, I.B.; MacCallum, H.; Cockcroft, J.R.; Webb, D.J. Inhibition of basal nitric oxide synthesis increases aortic augmentation index and pulse wave velocity in vivo. Br. J. Clin. Pharmacol. 2002, 53, 189–192. [Google Scholar] [CrossRef]
- Buendía, P.; de Oca, A.M.; Madueño, J.A.; Merino, A.; Martín-Malo, A.; Aljama, P.; Ramírez, R.; Rodríguez, M.; Carracedo, J. Endothelial microparticles mediate inflammation-induced vascular calcification. FASEB J. 2015, 29, 173–181. [Google Scholar] [CrossRef]
- Jain, S.; Khera, R.; Corrales–Medina, V.F.; Townsend, R.R.; Chirinos, J.A. Inflammation and arterial stiffness in humans. Atherosclerosis 2014, 237, 381–390. [Google Scholar] [CrossRef]
- Watson, J.; Jones, H.E.; Banks, J.; Whiting, P.; Salisbury, C.; Hamilton, W. Use of multiple inflammatory marker tests in primary care: Using Clinical Practice Research Datalink to evaluate accuracy. Br. J. Gen. Pract. 2019, 69, e462–e469. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Sanchis-Gomar, F.; Picanza, A.; Lippi, G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit. Rev. Clin. Lab. Sci. 2015, 52, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Targher, G.; Montagnana, M.; Salvagno, G.L.; Zoppini, G.; Guidi, G.C. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch. Pathol. Lab. Med. 2009, 133, 628–632. [Google Scholar] [PubMed]
- Montagnana, M.; Cervellin, G.; Meschi, T.; Lippi, G. The role of red blood cell distribution width in cardiovascular and thrombotic disorders. Clin. Chem. Lab. Med. 2012, 50, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.V.; Mohanty, J.G.; Kanapuru, B.; Hesdorffer, C.; Ershler, W.B.; Rifkind, J.M. Association of the Red Cell Distribution Width with Red Blood Cell Deformability. Adv. Exp. Med. Biol. 2013, 765, 211–216. [Google Scholar] [PubMed]
- Meital, L.T.; Windsor, M.T.; Jewell, R.M.R.; Young, P.; Schulze, K.; Magee, R.; O’Donnell, J.; Jha, P.; Perissiou, M.; Golledge, J. n-3 PUFAs improve erythrocyte fatty acid profile in patients with small AAA: A randomized controlled trial. J. Lipid Res. 2019, 60, 1154–1163. [Google Scholar] [CrossRef]
- Meital, L.T.; Windsor, M.T.; Perissiou, M.; Schulze, K.; Magee, R.; Kuballa, A.; Golledge, J.; Bailey, T.G.; Askew, C.D.; Russell, F.D. Omega-3 fatty acids decrease oxidative stress and inflammation in macrophages from patients with small abdominal aortic aneurysm. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Del Gobbo, L.C.; Imamura, F.; Aslibekyan, S.; Marklund, M.; Virtanen, J.K.; Wennberg, M.; Yakoob, M.Y.; Chiuve, S.E.; Dela Cruz, L.; Frazier-Wood, A.C.; et al. ω-3 polyunsaturated fatty acid biomarkers and coronary heart disease: Pooling project of 19 cohort studies. JAMA Intern. Med. 2016, 176, 1155–1166. [Google Scholar] [CrossRef]
- Zheng, J.; Huang, T.; Yu, Y.; Hu, X.; Yang, B.; Li, D. Fish consumption and CHD mortality: An updated meta-analysis of seventeen cohort studies. Public Health Nutr. 2012, 15, 725–737. [Google Scholar] [CrossRef]
- Wu, S.; Ding, Y.; Wu, F.; Li, R.; Hou, J.; Mao, P. Omega-3 fatty acids intake and risks of dementia and Alzheimer’s disease: A meta-analysis. Neurosci. Biobehav. Rev. 2015, 48, 1–9. [Google Scholar] [CrossRef]
- Perissiou, M.; Bailey, T.G.; Windsor, M.; Greaves, K.; Nam, M.C.; Russell, F.D.; O’Donnell, J.; Magee, R.; Jha, P.; Schulze, K. Aortic and systemic arterial stiffness responses to acute exercise in patients with small abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 58, 708–718. [Google Scholar] [CrossRef]
- Durmus, I.; Kazaz, Z.; Altun, G.; Cansu, A. Augmentation index and aortic pulse wave velocity in patients with abdominal aortic aneurysms. Int. J. Clin. Exp. Med. 2014, 7, 421–425. [Google Scholar] [PubMed]
- Karthikesalingam, A.; Bahia, S.; Patterson, B.; Peach, G.; Vidal-Diez, A.; Ray, K.; Sharma, R.; Hinchliffe, R.; Holt, P.; Thompson, M. The shortfall in long-term survival of patients with repaired thoracic or abdominal aortic aneurysms: Retrospective case–control analysis of Hospital Episode Statistics. Eur. J. Vasc. Endovasc. Surg. 2013, 46, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Kaess, B.M.; Harris, W.S.; Lacey, S.; Larson, M.G.; Hamburg, N.M.; Vita, J.A.; Robins, S.J.; Benjamin, E.J.; Mitchell, G.F.; Vasan, R.S. The relation of red blood cell fatty acids with vascular stiffness, cardiac structure and left ventricular function: The Framingham Heart Study. Vasc. Med. 2015, 20, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Scoditti, E.; Carluccio, M.A.; De Caterina, R. Basic mechanisms behind the effects of n-3 fatty acids on cardiovascular disease. Prostaglandins Leukot. Essent. Fat. Acids 2008, 79, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Yates, C.M.; Calder, P.C.; Rainger, G.E. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol. Therapeut. 2014, 141, 272–282. [Google Scholar] [CrossRef]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Brevetti, G.; Silvestro, A.; Schiano, V.; Chiariello, M. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease: Additive value of flow-mediated dilation to ankle-brachial pressure index. Circulation 2003, 108, 2093–2098. [Google Scholar] [CrossRef]
- Tan, I.; Butlin, M.; Liu, Y.Y.; Ng, K.; Avolio, A.P. Heart rate dependence of aortic pulse wave velocity at different arterial pressures in rats. Hypertension 2012, 60, 528–533. [Google Scholar] [CrossRef]
- Magalhães, P.; Capingana, D.P.; Silva, A.B.; Ferreira, A.V.; de Sá Cunha, R.; Rodrigues, S.L.; Mill, J.G. Age-and gender-specific reference values of pulse wave velocity for African adults: Preliminary results. Age 2013, 35, 2345–2355. [Google Scholar] [CrossRef]
- Krantz, M.J.; Havranek, E.P.; Pereira, R.I.; Beaty, B.; Mehler, P.S.; Long, C.S. Effects of omega-3 fatty acids on arterial stiffness in patients with hypertension: A randomized pilot study. J. Negat. Results Biomed. 2015, 14, 1–6. [Google Scholar] [CrossRef]
- Monahan, K.D.; Feehan, R.P.; Blaha, C.; McLaughlin, D.J. Effect of omega-3 polyunsaturated fatty acid supplementation on central arterial stiffness and arterial wave reflections in young and older healthy adults. Physiol. Rep. 2015, 3, e12438. [Google Scholar] [CrossRef] [PubMed]
- Lindholt, J.S.; Kristensen, K.L.; Burillo, E.; Martinez-Lopez, D.; Calvo, C.; Ros, E.; Martín-Ventura, J.L.; Sala-Vila, A. Arachidonic acid, but not omega-3 index, relates to the prevalence and progression of abdominal aortic aneurysm in a population-based study of Danish men. J. Am. Heart Assoc. 2018, 7, e007790. [Google Scholar] [CrossRef] [PubMed]
- Bowen, K.J.; Harris, W.S.; Kris-Etherton, P.M. Omega-3 fatty acids and cardiovascular disease: Are there benefits? Curr. Treat. Options Cardio. Med. 2016, 18, 69. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Khatibzadeh, S.; Shi, P.; Fahimi, S.; Lim, S.; Andrews, K.G.; Engell, R.E.; Powles, J.; Ezzati, M.; Mozaffarian, D. Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: A systematic analysis including 266 country-specific nutrition surveys. BMJ 2015, 350, h1702. [Google Scholar] [CrossRef] [PubMed]
| Observational Study | Omega-3 Clinical Trial | |||
|---|---|---|---|---|
| Variable | Control Participants (n = 20) | AAA Patients (n = 30) | AAAn-3 Cohort (n = 15) | AAA Placebo Cohort (n = 15) |
| Age (years) | 73.2 ± 5.6 | 74.4 ± 5.3 | 73.6 ± 5.0 | 75.1 ± 5.7 |
| AAA size (mm) | 39.3 ± 5.3 | 39.3 ± 5.7 | 39.2 ± 5.0 | |
| Smoking: | ||||
| Never | 8 (40%) | 3 (10%) † | 1 (7%) | 2 (13%) |
| Past | 11 (55%) | 24 (80%) | 12 (80%) | 12 (80%) |
| Current | 1 (5%) | 3 (10%) | 2 (13%) | 1 (7%) |
| BMI (kg/m2) | 28.4 ± 5.3 | 29.5 ± 5.2 | 29.4 ± 4.1 | 29.0 ± 5.0 |
| SBP (mmHg) | 141 ± 17 | 140 ± 18 | 136 ± 16 | 144 ± 20 |
| DBP (mmHg) | 81 ± 7 | 79 ± 10 | 78 ± 8 | 80 ± 11 |
| Hypertension | 12 (60%) | 17 (57%) | 6 (40%) | 11 (73%) |
| Diabetes | 1 (5%) | 4 (13%) | 3 (20%) | 1 (7%) |
| Dyslipidemia | 15 (75%) | 19 (63%) | 7 (47%) | 12 (80%) |
| CHD | 6 (30%) | 9 (30%) | 5 (33%) | 4 (27%) |
| Anti-hypertensives | ||||
| Beta blockers | 3 (15%) | 7 (23%) | 4 (27%) | 3 (20%) |
| ARBs | 3 (15%) | 8 (27%) | 3 (20%) | 5 (33%) |
| ACE inhibitors | 7 (35%) | 4 (13%) | 3 (20%) | 1 (7%) |
| CCBs | 2 (10%) | 6 (20%) | 1 (7%) | 5 (33%) |
| Diuretics | 2 (10%) | 3 (10%) | 2 (13%) | 1 (7%) |
| Anti-platelet drugs | 3 (15%) | 18 (60%) † | 11 (73%) | 7 (47%) |
| NSAIDs | 1 (5%) | 3 (10%) | 1 (7%) | 2 (13%) |
| Statins | 14 (70%) | 21 (70%) | 8 (53%) ‡ | 13 (87%) |
| Baseline omega-3 Index | 4.53 ± 0.22 | 4.37 ± 0.20 | ||
| 12-Week omega-3 Index | 8.03 ± 0.20 | 4.26 ± 0.28 | ||
| Variable | Fish Oil Cohort | Placebo Cohort | ||||
|---|---|---|---|---|---|---|
| Baseline | Week 3 | Week 12 | Baseline | Week 3 | Week 12 | |
| PWV (ms−1) | 14.2 ± 0.6 | 13.8 ± 1.2 | 12.8 ± 0.7 * | 14.6 ± 0.6 | 14.7 ± 0.6 | 14.0 ± 0.5 |
| AIx75 (%) | 26.4 ± 3.0 | 24.0 ± 4.2 | 23.7 ± 2.5 | 28.3 ± 2.2 | 28.6 ± 2.6 | 28.9 ± 2.5 |
| RM (%) | 64.1 ± 2.2 | 61.5 ± 3.0 | 66.0 ± 3.3 | 64.7 ± 2.3 | 67.9 ± 3.3 | 67.8 ± 1.6 |
| HR (bpm) | 63 ± 3 | 59 ± 3 | 58 ± 3 ** | 66 ± 2 | 65 ± 2 | 66 ± 3 |
| cSBP (mmHg) | 126 ± 4 | 127 ± 5 | 124 ± 3 | 129 ± 4 | 129 ± 4 | 126 ± 3 |
| cDBP (mmHg) | 80 ± 3 | 79 ± 4 | 79 ± 3 | 81 ± 3 | 83 ± 3 | 79 ± 3 |
| cPP (mmHg) | 46 ± 3 | 52 ± 5 | 45 ± 2 | 49 ± 3 | 46 ± 3 | 47 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meital, L.T.; Schulze, K.; Magee, R.; O’Donnell, J.; Jha, P.; Meital, C.Y.; Donkin, R.; Bailey, T.G.; Askew, C.D.; Russell, F.D. Long Chain Omega-3 Polyunsaturated Fatty Acids Improve Vascular Stiffness in Abdominal Aortic Aneurysm: A Randomized Controlled Trial. Nutrients 2021, 13, 138. https://doi.org/10.3390/nu13010138
Meital LT, Schulze K, Magee R, O’Donnell J, Jha P, Meital CY, Donkin R, Bailey TG, Askew CD, Russell FD. Long Chain Omega-3 Polyunsaturated Fatty Acids Improve Vascular Stiffness in Abdominal Aortic Aneurysm: A Randomized Controlled Trial. Nutrients. 2021; 13(1):138. https://doi.org/10.3390/nu13010138
Chicago/Turabian StyleMeital, Lara T., Karl Schulze, Rebecca Magee, Jill O’Donnell, Pankaj Jha, Chaim Y. Meital, Rebecca Donkin, Tom G. Bailey, Christopher D. Askew, and Fraser D. Russell. 2021. "Long Chain Omega-3 Polyunsaturated Fatty Acids Improve Vascular Stiffness in Abdominal Aortic Aneurysm: A Randomized Controlled Trial" Nutrients 13, no. 1: 138. https://doi.org/10.3390/nu13010138
APA StyleMeital, L. T., Schulze, K., Magee, R., O’Donnell, J., Jha, P., Meital, C. Y., Donkin, R., Bailey, T. G., Askew, C. D., & Russell, F. D. (2021). Long Chain Omega-3 Polyunsaturated Fatty Acids Improve Vascular Stiffness in Abdominal Aortic Aneurysm: A Randomized Controlled Trial. Nutrients, 13(1), 138. https://doi.org/10.3390/nu13010138






