Protective Effects of Black Raspberry (Rubus occidentalis) Extract against Hypercholesterolemia and Hepatic Inflammation in Rats Fed High-Fat and High-Choline Diets
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of BR Extract
2.3. Determination of Total Phenolic Content (TPC)
2.4. HPLC-UV Analysis of Anthocyanins in BR Extract
2.5. Animals and Diets
2.6. Blood and Tissue Collection
2.7. Quantification of Choline, TMA, and TMAO
2.8. Serum Lipid Profile
2.9. Total RNA Extraction, cDNA Synthesis, and Real-Time Quantitative Polymerase Chain Reaction (qPCR)
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Chemical Properties of the BR Extract
3.2. Body Weights and Food and Water Intakes
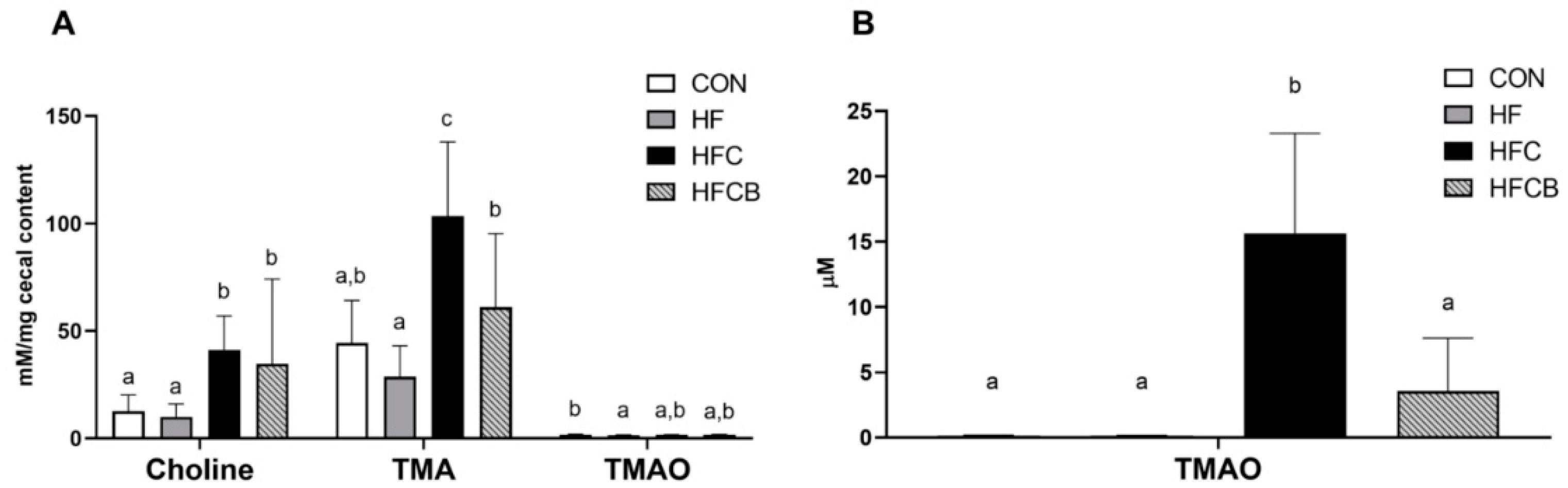
3.3. Serum TMAO Level and Cecal Choline, TMA, and TMAO Levels
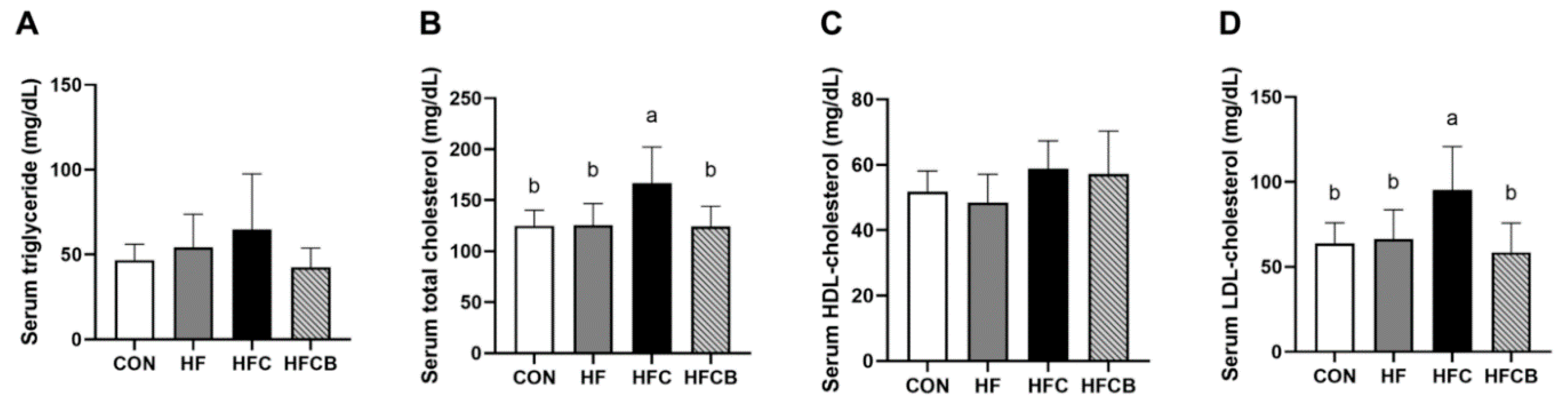
3.4. Serum Lipid Profile
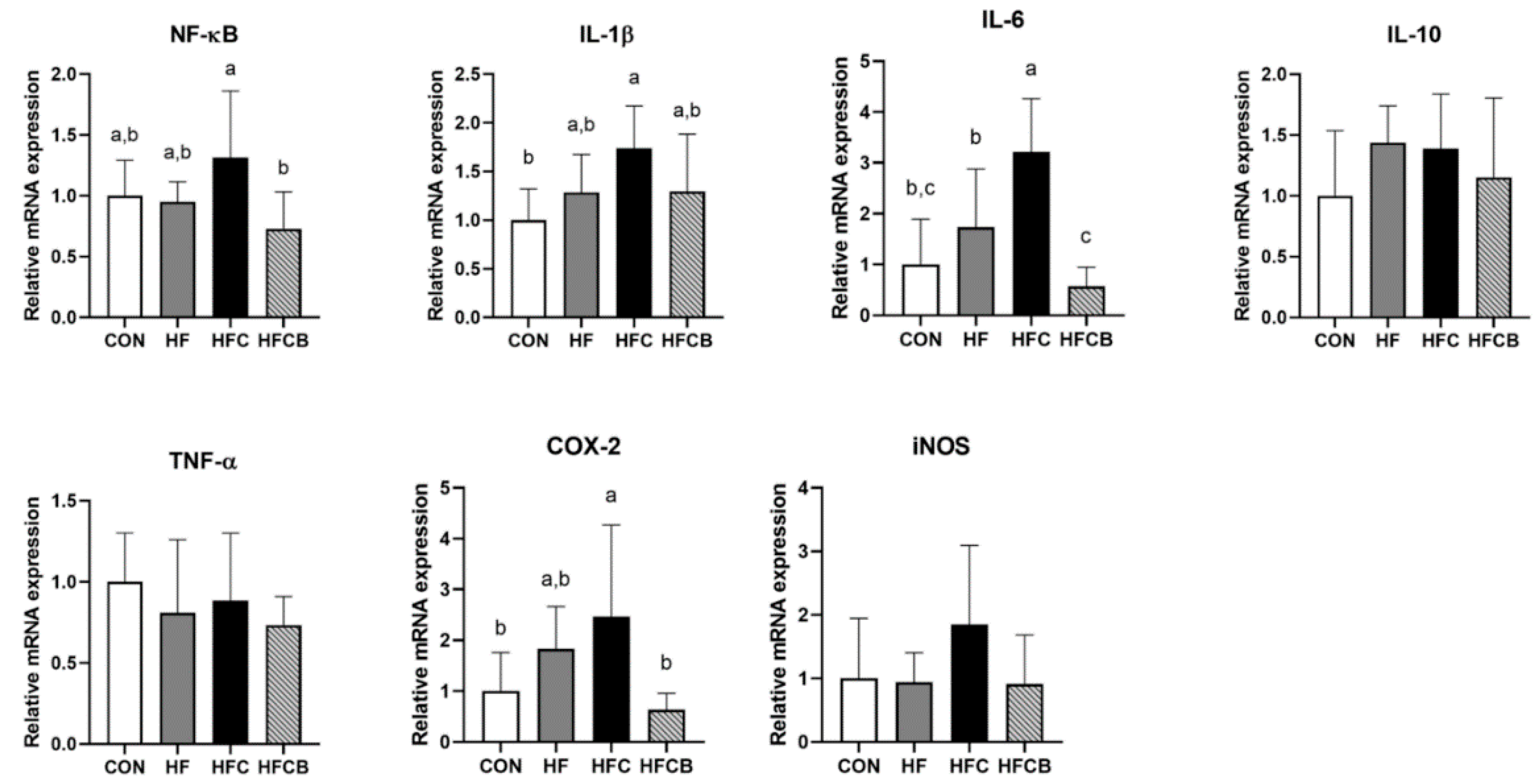
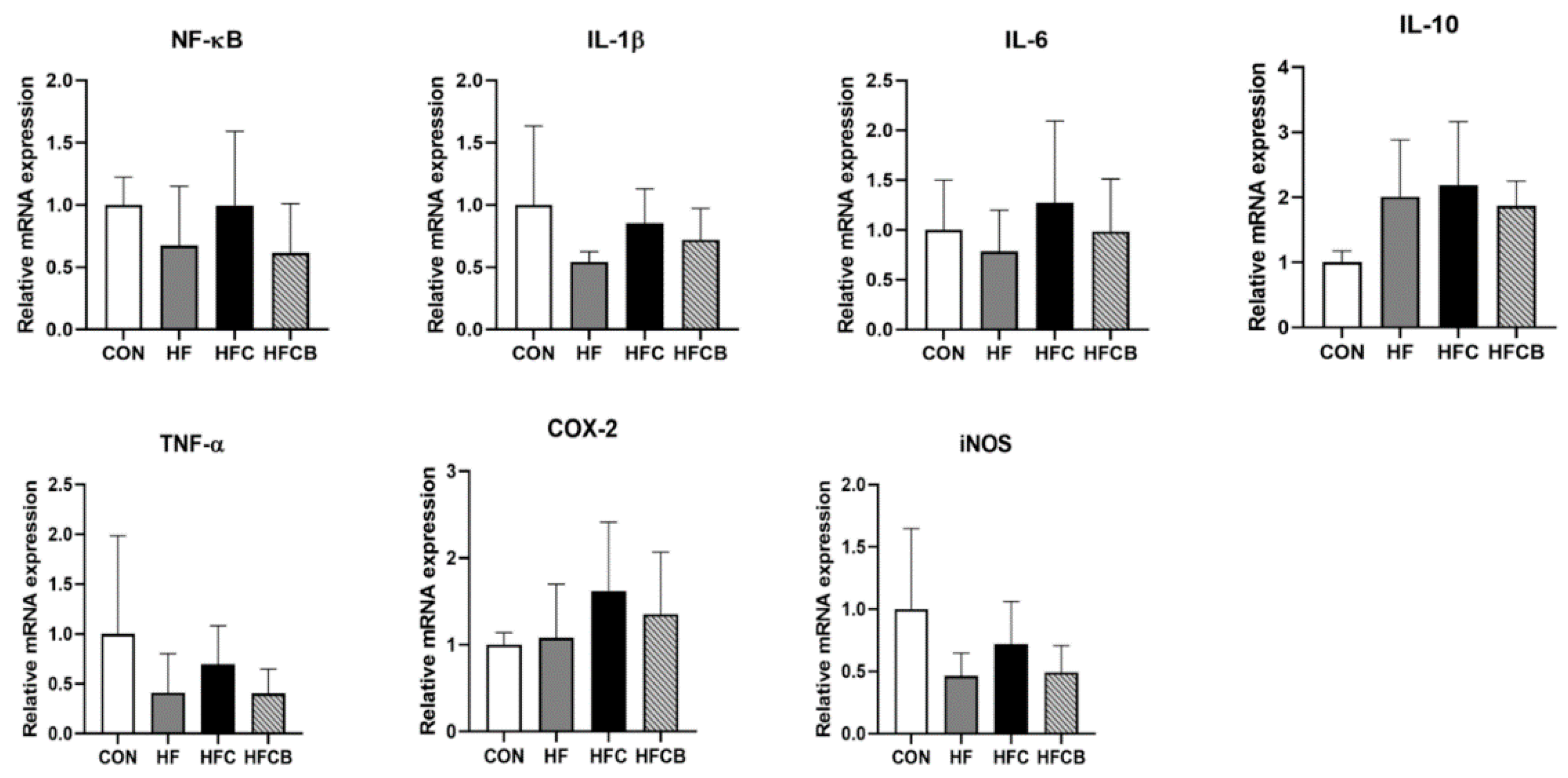
3.5. Relative mRNA Expression of Genes Involved in Inflammatory Response in the Liver and Adipose Tissue
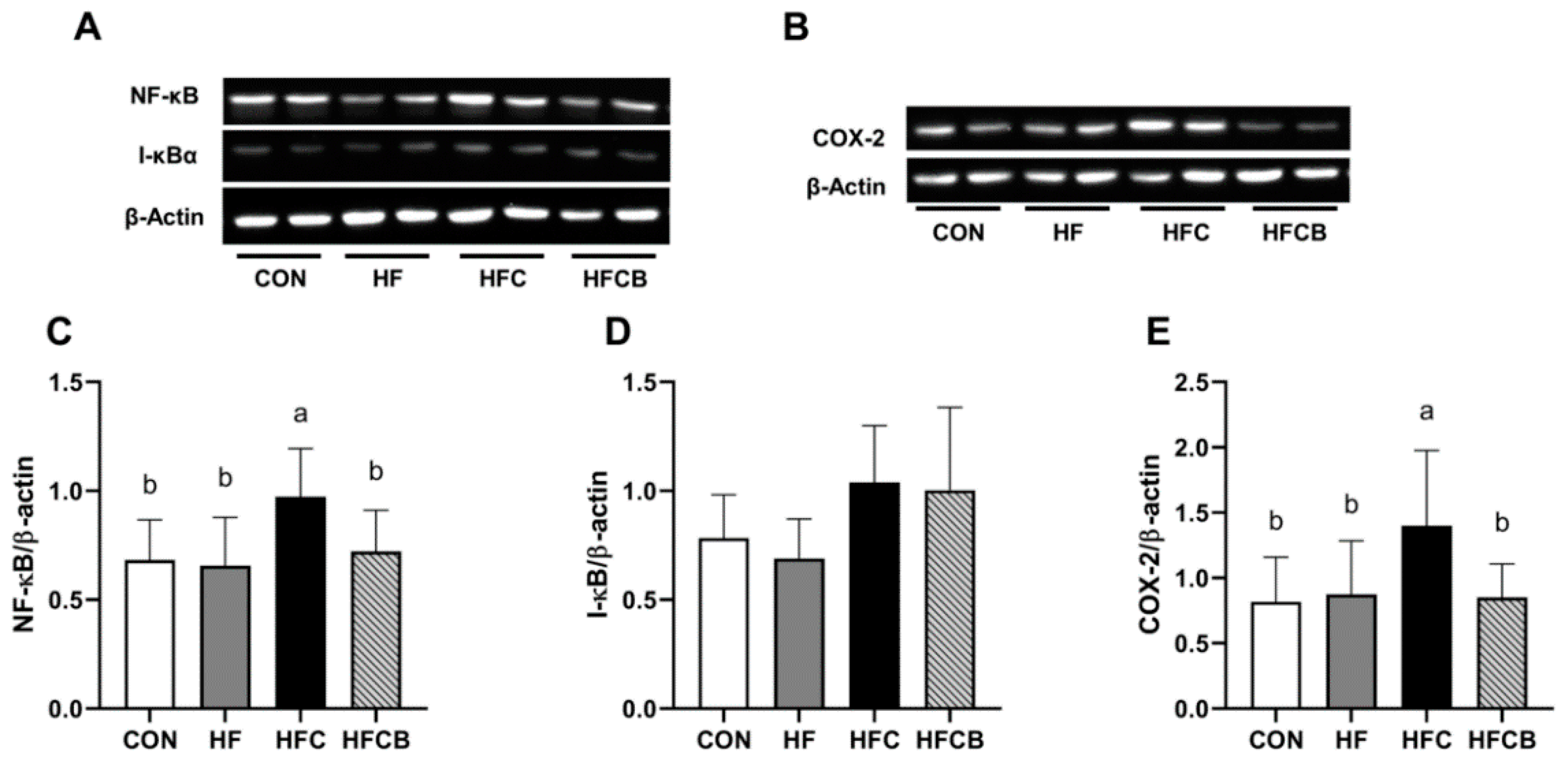
3.6. Protein Expression of NF-κB, I-κB, and COX-2 in the Liver
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zeisel, S.H.; Da Costa, K.A. Choline: An essential nutrient for public health. Nutr. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Warrier, M. Trimethylamine N-oxide, the microbiome, and heart and kidney disease. Annu. Rev. Nutr. 2017, 37, 157–181. [Google Scholar] [CrossRef] [PubMed]
- Seldin, M.M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. J. Am. Heart Assoc. 2016, 5, e002767. [Google Scholar] [CrossRef]
- Chen, M.L.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.D.; Zhang, Q.Y.; Mi, M.T. Resveratrol attenuates trimethylamine-N-oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. MBio 2016, 7, e02210–e02215. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xu, J.; Jiang, C.; Zhang, Y.; Xue, Y.; Li, Z.; Wang, J.; Xue, C.; Wang, Y. Fish oil ameliorates trimethylamine N-oxide-exacerbated glucose intolerance in high-fat diet-fed mice. Food Funct. 2015, 6, 1117–1125. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, W.H.; Buffa, J.A.; Fu, X.; Britt, E.B.; Koeth, R.A.; Levison, B.S.; Fan, Y.; Wu, Y.; Hazen, S.L. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur. Heart J. 2014, 35, 904–910. [Google Scholar] [CrossRef]
- Tang, W.H.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef]
- Jia, M.; Ren, D.; Nie, Y.; Yang, X. Beneficial effects of apple peel polyphenols on vascular endothelial dysfunction and liver injury in high choline-fed mice. Food Funct. 2017, 8, 1282–1292. [Google Scholar] [CrossRef]
- He, Z.; Lei, L.; Kwek, E.; Zhao, Y.; Liu, J.; Hao, W.; Zhu, H.; Liang, N.; Ma, K.Y.; Ho, H.M.; et al. Ginger attenuates trimethylamine-N-oxide (TMAO)-exacerbated disturbance in cholesterol metabolism and vascular inflammation. J. Funct. Foods 2019, 52, 25–33. [Google Scholar] [CrossRef]
- Ma, G.; Pan, B.; Chen, Y.; Guo, C.; Zhao, M.; Zheng, L.; Chen, B. Trimethylamine N-oxide in atherogenesis: Impairing endothelial self-repair capacity and enhancing monocyte adhesion. Biosci. Rep. 2017, 37, BSR20160244. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Liu, Y.; Zhao, Y.; Yang, X. Hepatotoxicity and endothelial dysfunction induced by high choline diet and the protective effects of phloretin in mice. Food Chem. Toxicol. 2016, 94, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Organ, C.L.; Otsuka, H.; Bhushan, S.; Wang, Z.; Bradley, J.; Trivedi, R.; Polhemus, D.J.; Tang, W.H.; Wu, Y.; Hazen, S.L.; et al. Choline diet and its gut microbe–derived metabolite, trimethylamine N-oxide, exacerbate pressure overload–induced heart failure. Circ. Heart Fail. 2016, 9, e002314. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Missailidis, C.; Hällqvist, J.; Qureshi, A.R.; Barany, P.; Heimbürger, O.; Lindholm, B.; Stenvinkel, P.; Bergman, P. Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS ONE 2016, 11, e0141738. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576. [Google Scholar] [CrossRef]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [CrossRef]
- Torre, L.C.; Barritt, B.H. Quantitative evaluation of Rubus fruit anthocyanin pigments. J. Food Sci. 1977, 42, 488–490. [Google Scholar] [CrossRef]
- Jung, H.; Kwak, H.K.; Hwang, K.T. Antioxidant and antiinflammatory activities of cyanidin-3-glucoside and cyanidin-3-rutinoside in hydrogen peroxide and lipopolysaccharide-treated RAW264.7 cells. Food Sci. Biotechnol. 2014, 23, 2053–2062. [Google Scholar] [CrossRef]
- Paudel, L.; Wyzgoski, F.J.; Scheerens, J.C.; Chanon, A.M.; Reese, R.N.; Smiljanic, D.; Wesdemiotis, C.; Blakeslee, J.J.; Riedl, K.M.; Rinaldi, P. Nonanthocyanin secondary metabolites of black raspberry (Rubus occidentalis L.) fruits: Identification by HPLC-DAD, NMR, HPLC-ESI-MS, and ESI-MS/MS analyses. J. Agric. Food Chem. 2013, 61, 12032–12043. [Google Scholar] [CrossRef]
- Kula, M.; Krauze-Baranowska, M. Rubus occidentalis: The black raspberry—Its potential in the prevention of cancer. Nutr. Cancer 2016, 68, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Bennett, B.J.; de Aguiar Vallim, T.Q.; Wang, Z.; Shih, D.M.; Meng, Y.; Gregory, J.; Allayee, H.; Lee, R.; Graham, M.; Crooke, R.; et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013, 17, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Shaddel, R.; Hesari, J.; Azadmard-Damirchi, S.; Hamishehkar, H.; Fathi-Achachlouei, B.; Huang, Q. Double emulsion followed by complex coacervation as a promising method for protection of black raspberry anthocyanins. Food Hydrocoll. 2018, 77, 803–816. [Google Scholar] [CrossRef]
- Jeong, J.H.; Jung, H.; Lee, S.R.; Lee, H.J.; Hwang, K.T.; Kim, T.Y. Anti-oxidant, anti-proliferative and anti-inflammatory activities of the extracts from black raspberry fruits and wine. Food Chem. 2010, 123, 338–344. [Google Scholar] [CrossRef]
- Seeram, N.P. Berry fruits: Compositional elements, biochemical activities, and the impact of their intake on human health, performance, and disease. J. Agric. Food Chem. 2008, 56, 627–629. [Google Scholar] [CrossRef]
- Wu, Q.; Li, S.; Li, X.; Sui, Y.; Yang, Y.; Dong, L.; Xie, B.; Sun, Z. Inhibition of advanced glycation endproduct formation by lotus seedpod oligomeric procyanidins through RAGE–MAPK signaling and NF-κB activation in high-fat-diet rats. J. Agric. Food Chem. 2015, 63, 6989–6998. [Google Scholar] [CrossRef]
- Xu, Z.J.; Fan, J.G.; Ding, X.D.; Qiao, L.; Wang, G.L. Characterization of high-fat, diet-induced, non-alcoholic steatohepatitis with fibrosis in rats. Dig. Dis. Sci. 2010, 55, 931–940. [Google Scholar] [CrossRef]
- Smaranda, C.; Emily, P.B. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc. Natl. Acad. Sci. USA 2012, 109, 21307–21312. [Google Scholar]
- Liang, X.; Zhang, Z.; Lv, Y.; Tong, L.; Liu, T.; Yi, H.; Zhou, X.; Yu, Z.; Tian, X.; Cui, Q.; et al. Reduction of intestinal trimethylamine by probiotics ameliorated lipid metabolic disorders associated with atherosclerosis. Nutrition 2020, 110941. [Google Scholar] [CrossRef]
- Qiu, L.; Yang, D.; Tao, X.; Yu, J.; Xiong, H.; Wei, H. Enterobacter aerogenes ZDY01 attenuates choline-induced trimethylamine N-oxide levels by remodeling gut microbiota in mice. J. Microbiol. Biotechnol. 2017, 27, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Tao, X.; Xiong, H.; Yu, J.; Wei, H. Lactobacillus plantarum ZDY04 exhibits a strain-specific property of lowering TMAO via the modulation of gut microbiota in mice. Food Funct. 2018, 9, 4299–4309. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liang, Q.; Balakrishnan, B.; Belobrajdic, D.P.; Feng, Q.-J.; Zhang, W. Role of Dietary Nutrients in the Modulation of Gut Microbiota: A Narrative Review. Nutrients 2020, 12, 381. [Google Scholar] [CrossRef] [PubMed]
- Loo, Y.T.; Howell, K.; Chan, M.; Zhang, P.; Ng, K. Modulation of the human gut microbiota by phenolics and phenolic fiber-rich foods. Compr. Rev. Food Sci. Food Saf. 2020, 1–31. [Google Scholar] [CrossRef]
- Wang, D.; Xia, M.; Gao, S.; Li, D.; Zhang, Y.; Jin, T.; Ling, W. Cyanidin-3-O-β-glucoside upregulates hepatic cholesterol 7α-hydroxylase expression and reduces hypercholesterolemia in mice. Mol. Nutr. Food Res. 2012, 56, 610–621. [Google Scholar] [CrossRef]
- Tornatore, L.; Thotakura, A.K.; Bennett, J.; Moretti, M.; Franzoso, G. The nuclear factor kappa B signaling pathway: Integrating metabolism with inflammation. Trends Cell Biol. 2012, 22, 557–566. [Google Scholar] [CrossRef]
- Wu, T.; Yin, J.; Zhang, G.; Long, H.; Zheng, X. Mulberry and cherry anthocyanin consumption prevents oxidative stress and inflammation in diet-induced obese mice. Mol. Nutr. Food Res. 2016, 60, 687–694. [Google Scholar] [CrossRef]
- Dragano, N.R.; Cintra, D.E.; Solon, C.; Morari, J.; Leite-Legatti, A.V.; Velloso, L.A.; Maróstica-Júnior, M.R. Freeze-dried jaboticaba peel powder improves insulin sensitivity in high-fat-fed mice. Br. J. Nutr. 2013, 110, 447–455. [Google Scholar] [CrossRef]
- Hasko, G.; Szabó, C.; Németh, Z.H.; Kvetan, V.; Pastores, S.M.; Vizi, E.S. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J. Immunol. 1996, 157, 4634–4640. [Google Scholar] [PubMed]
- Liu, D.; Ji, L.; Wang, Y.; Zheng, L. Cyclooxygenase-2 expression, prostacyclin production and endothelial protection of high-density lipoprotein. Cardiovasc. Haematol. Disord. Drug Targets 2012, 12, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Kowalski, G.M.; Leslie, S.J.; Risis, S.; Yang, C.; Lee-Young, R.S.; Babb, J.R.; Meikle, P.J.; Lancaster, G.I.; Henstridge, D.C.; et al. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia 2013, 56, 1638–1648. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef]
- Luedde, T.; Schwabe, R.F. NF-κB in the liver—linking injury, fibrosis and hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 108. [Google Scholar] [CrossRef]
- Bishayee, A.; Thoppil, R.J.; Mandal, A.; Darvesh, A.S.; Ohanyan, V.; Meszaros, J.G.; Háznagy-Radnai, E.; Hohmann, J.; Bhatia, D. Black currant phytoconstituents exert chemoprevention of diethylnitrosamine-initiated hepatocarcinogenesis by suppression of the inflammatory response. Mol. Carcinog. 2013, 52, 304–317. [Google Scholar] [CrossRef]
- Arjinajarn, P.; Chueakula, N.; Pongchaidecha, A.; Jaikumkao, K.; Chatsudthipong, V.; Mahatheeranont, S.; Norkaew, O.; Chattipakorn, N.; Lungkaphin, A. Anthocyanin-rich riceberry bran extract attenuates gentamicin-induced hepatotoxicity by reducing oxidative stress, inflammation and apoptosis in rats. Biomed. Pharmacother. 2017, 92, 412–420. [Google Scholar] [CrossRef]
| Gene | Sequence |
|---|---|
| GAPDH | Forward (5′-3′): ACCACAGTCCATGCCATCAC |
| Reverse (5′-3′): TCCACCACCCTGTTGCTGTA | |
| NF-κB | Forward (5′-3′): TGGACGATCTGTTTCCCCTC |
| Reverse (5′-3′): CCCTCGCACTTGTAACGGAA | |
| TNF-α | Forward (5′-3′): GTAGCCCACGTCGTAGCAAAC |
| Reverse (5′-3′): ACCACCAGTTGGTTGTCTTTGA | |
| IL-6 | Forward (5′-3′): TCCTACCCCAACTTCCAATGCTC |
| Reverse (5′-3′): TTGGATGGTCTTGGTCCTTAGCC | |
| IL-1β | Forward (5′-3′): GACTTCACCATGGAACCCGT |
| Reverse (5′-3′): CAGGGAGGGAAACACACGTT | |
| IL-10 | Forward (5′-3′): GCTAACGGGAGCAACTCCTT |
| Reverse (5′-3′): ATGTCCCCTATGGAAACAGCTT | |
| COX-2 | Forward (5′–3′): TGTATGCTACCATCTGGCTTCGG |
| Reverse (5′-3′): GTTTGGAACAGTCGCTCGTCATC | |
| iNOS | Forward (5′-3′): GCCATCCCGCTGCTCTAATA |
| Reverse (5′-3′): GTTGGGAGTGGACGAAGGTA |
| Group | ||||
|---|---|---|---|---|
| CON | HF | HFC | HFCB | |
| Initial body weight (g) | 135.3 ± 8.9 | 137.1 ± 6.1 | 137.5 ± 7.5 | 137.0 ± 7.9 |
| Final body weight (g) | 249.2 ± 25.5 a | 255.5 ± 19.3 a | 214.0 ± 22.1 b | 207.6 ± 22.7 b |
| Weight gain (g∙d−1) | 2.0 ± 0.3 a | 2.1 ± 0.3 a | 1.4 ± 0.4 b | 1.3 ± 0.4 b |
| Food intake (g∙d−1) * | 26.5 ± 2.3 a | 24.7 ± 4.3 a | 18.3 ± 2.3 b | 17.6 ± 2.7 b |
| Water intake (mL∙d−1) * | 40.2 ± 4.3 | 41.7 ± 4.0 | 43.4 ± 1.9 | 39.3 ± 8.3 |
| FER * | 0.15 ± 0.01 a,b | 0.17 ± 0.01 a | 0.15 ± 0.01 a,b | 0.14 ± 0.02 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, T.; Ryu, J.; Lee, K.; Park, S.Y.; Hwang, K.T. Protective Effects of Black Raspberry (Rubus occidentalis) Extract against Hypercholesterolemia and Hepatic Inflammation in Rats Fed High-Fat and High-Choline Diets. Nutrients 2020, 12, 2448. https://doi.org/10.3390/nu12082448
Lim T, Ryu J, Lee K, Park SY, Hwang KT. Protective Effects of Black Raspberry (Rubus occidentalis) Extract against Hypercholesterolemia and Hepatic Inflammation in Rats Fed High-Fat and High-Choline Diets. Nutrients. 2020; 12(8):2448. https://doi.org/10.3390/nu12082448
Chicago/Turabian StyleLim, Taehwan, Juhee Ryu, Kiuk Lee, Sun Young Park, and Keum Taek Hwang. 2020. "Protective Effects of Black Raspberry (Rubus occidentalis) Extract against Hypercholesterolemia and Hepatic Inflammation in Rats Fed High-Fat and High-Choline Diets" Nutrients 12, no. 8: 2448. https://doi.org/10.3390/nu12082448
APA StyleLim, T., Ryu, J., Lee, K., Park, S. Y., & Hwang, K. T. (2020). Protective Effects of Black Raspberry (Rubus occidentalis) Extract against Hypercholesterolemia and Hepatic Inflammation in Rats Fed High-Fat and High-Choline Diets. Nutrients, 12(8), 2448. https://doi.org/10.3390/nu12082448





