Low Serum Vitamin B12 Levels Are Associated with Adverse Lipid Profiles in Apparently Healthy Young Saudi Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Population, and Sample Size
2.2. Biochemical Assessment
2.2.1. Lipid Profile and Glucose
2.2.2. Vitamin B12
2.3. Dietary Assessment
2.4. Clinical Assessment
2.5. Other Risk Factors
Physical Activity Questionnaire
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics by Vitamin B12 Tertiles
3.2. Serum Vitamin B12 Levels and Lipid Profile
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zalaket, J.; Wehbe, T.; Jaoude, E.A. Vitamin B12 Deficiency in Diabetic Subjects Taking Metformin: A Cross Sectional Study in a Lebanese Cohort. J. Nutr. Intermed. Metab. 2018, 11, 9–13. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Al-Attas, O.S.; Alokail, M.S.; Alkharfy, K.M.; Yakout, S.M.; Aljohani, N.J.; Alfawaz, H.A.; Al-Ajlan, A.S.M.; Sheshah, E.S.; Al-Yousef, M.; et al. Lower Vitamin D Status is More Common Among Saudi Adults with Diabetes Mellitus Type 1 Than in Non-diabetics. BMC Public Health 2014, 14, 153. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin b6, Folate, Vitamin b12, Pantothenic Acid, Biotin, and Choline; National Academies Press: Washington, DC, USA, 1998.
- Saravanan, P.; Yajnik, C.S. Role of Maternal Vitamin B12 on the Metabolic Health of the Offspring: A Contributor to the Diabetes Epidemic? Br. J. Diabetes Vasc. Dis. 2010, 10, 109–114. [Google Scholar] [CrossRef]
- Rolfes, S.R.; Pinna, K.; Whitney, E. Understanding Normal and Clinical Nutrition, 9th ed.; Cengage Learning: Boston, MA, USA, 2012; pp. 321–365. ISBN1 084006845X. ISBN2 9780840068453. [Google Scholar]
- Palacios, G.; Sola, R.; Barrios, L.; Pietrzik, K.; Castillo, M.J.; González-Gross, M. Algorithm for the Early Diagnosis of Vitamin B12 Deficiency in Elderly People. Nutr. Hosp. 2013, 28, 1447–1452. [Google Scholar] [CrossRef] [PubMed]
- Mccombe, P.A.; Mcleod, J.G. The Peripheral Neuropathy of Vitamin B12 Deficiency. J. Neurol. Sci. 1984, 66, 117–126. [Google Scholar] [CrossRef]
- de Benoist, B. Conclusions of a WHO Technical Consultation on Folate and Vitamin B12 Deficiencies. Food Nutr. Bull. 2008, 29, S238–S244. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.M.; Molloy, A.M.; Ueland, P.M.; Fernandez-Ballart, J.D.; Schneede, J.; Arija, V.; Scott, J.M. Longitudinal Study of the Effect of Pregnancy on Maternal and Fetal Cobalamin Status in Healthy Women and Their Offspring. J. Nutr. 2007, 137, 1863–1867. [Google Scholar] [CrossRef]
- Bailey, R.L.; Carmel, R.; Green, R.; Pfeiffer, C.M.; Cogswell, M.E.; Osterloh, J.D.; Sempos, C.T.; Yetley, E.A. Monitoring of Vitamin B-12 Nutritional Status in the United States by Using Plasma Methylmalonic Acid and Serum Vitamin B-12. Am. J. Clin. Nutr. 2011, 94, 552–561. [Google Scholar] [CrossRef]
- Green, R.; Allen, L.H.; Bjørke-Monsen, A.-L.; Brito, A.; Guéant, J.-L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.-H.; et al. Vitamin B12 Deficiency. Nat. Rev. Dis. Primers 2017, 3, 17040. [Google Scholar] [CrossRef]
- Sukumar, N.; Adaikalakoteswari, A.; Venkataraman, H.; Maheswaran, H.; Saravana, P. Vitamin B12 Status in Women of Childbearing Age in the UK and Its Relationship with National Nutrient Intake Guidelines: Results From Two National Diet and Nutrition Surveys. BMJ Open 2016, 6, e011247. [Google Scholar] [CrossRef] [Green Version]
- Quay, T.A.; Schroder, T.H.; Jeruszka-Bielak, M.; Li, W.; Devlin, A.M.; Barr, S.I.; Lamers, Y. High Prevalence of Suboptimal Vitamin B12 Status in Young Adult Women of South Asian and European Ethnicity. Appl. Physiol. Nutr. Metab. 2015, 40, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, N.; Rafnsson, S.B.; Kandala, N.B.; Bhopal, R.; Yajnik, C.S.; Saravanan, P. Prevalence of Vitamin B-12 Insufficiency During Pregnancy and Its Effect on Offspring Birth Weight: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2016, 103, 1232–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Khateeb, M.; Khader, Y.; Batieha, A.; Jaddou, H.; Hyassat, D.; Belbisi, A.; Ajlouni, K. Vitamin B12 Deficiency in Jordan: A Population-Based Study. Ann. Nutr. Metab. 2014, 64, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, T.J.; Tourkmani, A.M.; Abdelhay, O.; Alkhashan, H.I.; Al-Asmari, A.K.; Bin Rsheed, A.M.; Abuhaimed, S.N.; Mohammed, N.; AlRasheed, A.N.; AlHarbi, N.G. The Association of Metformin Use with Vitamin B12 Deficiency and Peripheral Neuropathy in Saudi Individuals With Type 2 Diabetes Mellitus. PLoS ONE 2018, 13, e0204420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Sun, M.; Liu, B.; Du, Y.; Rong, S.; Xu, G.; Snetselaar, L.G.; Bao, W. Inverse Association Between Serum Vitamin B12 Concentration and Obesity Among Adults in the United States. Front. Endocrinol. 2019, 10, 414. [Google Scholar] [CrossRef] [Green Version]
- Knight, B.A.; Shields, B.M.; Brook, A.; Hill, A.; Bhat, D.S.; Hattersley, A.T.; Yajnik, C.S. Lower Circulating B12 is Associated with Higher Obesity and Insulin Resistance During Pregnancy in a Non-Diabetic White British Population. PLoS ONE 2015, 10, e0135268. [Google Scholar] [CrossRef] [Green Version]
- Krishnaveni, G.V.; Hill, J.C.; Veena, S.R.; Bhat, D.S.; Wills, A.K.; Karat, C.L.; Yajnik, C.S.; Fall, C.H. Low Plasma Vitamin B12 in Pregnancy is Associated with Gestational ‘Diabesity’ and Later Diabetes. Diabetologia 2009, 52, 2350–2358. [Google Scholar] [CrossRef] [Green Version]
- Saraswathy, K.N.; Joshi, S.; Yadav, S.; Garg, P.R. Metabolic Distress in Lipid and One Carbon Metabolic Pathway Through Low Vitamin B-12: A Population Based Study from North India. Lipids Health Dis. 2018, 17, 96. [Google Scholar] [CrossRef] [Green Version]
- Boachie, J.; Adaikalakoteswari, A.; Samavat, J.; Saravanan, P. Low Vitamin B12 and Lipid Metabolism: Evidence from Pre-Clinical and Clinical Studies. Nutrients 2020, 12, 1925. [Google Scholar] [CrossRef]
- Mahalle, N.; Kulkarni, M.V.; Garg, M.K.; Naik, S.S. Vitamin B12 Deficiency and Hyperhomocysteinemia as Correlates of Cardiovascular Risk Factors in Indian Subjects with Coronary Artery Disease. J. Cardiolol. 2013, 61, 289–294. [Google Scholar] [CrossRef] [Green Version]
- Adaikalakoteswari, A.; Finer, S.; Voyias, P.D.; McCarthy, C.M.; Vatish, M.; Moore, J.; Smart-Halajko, M.; Bawazeer, N.; Al-Daghri, N.M.; McTernan, P.G.; et al. Vitamin B12 Insufficiency Induces Cholesterol Biosynthesis by Limiting S-Adenosylmethionine and Modulating the Methylation of SREBF1 and LDLR Genes. Clin. Epigenetics 2015, 7, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukumar, N.; Venkataraman, H.; Wilson, S.; Goljan, I.; Selvamoni, S.; Patel, V.; Saravanan, P. Vitamin B12 Status Among Pregnant Women in the UK and Its Association with Obesity and Gestational Diabetes. Nutrients 2016, 8, 768. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.P.; Christian, P.; Schulze, K.J.; Arguello, M.; LeClerq, S.C.; Khatry, S.K.; West, K.P., Jr. Low Maternal Vitamin B-12 Status is Associated with Offspring Insulin Resistance Regardless of Antenatal Micronutrient Supplementation in Rural Nepal. J. Nutr. 2011, 141, 1912–1917. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L.B.; Stover, P.J.; McNulty, H.; Fenech, M.F.; Gregory, J.F., 3rd; Mills, J.L.; Pfeiffer, C.M.; Fazili, Z.; Zhang, M.; Ueland, P.M.; et al. Biomarkers of Nutrition for Development-Folate Review. J. Nutr. 2015, 145, 1636S–1680S. [Google Scholar] [CrossRef] [Green Version]
- Keser, I.; Ilich, J.Z.; Vrkić, N.; Giljević, Z.; Colić Barić, I. Folic Acid and Vitamin B(12) Supplementation Lowers Plasma Homocysteine but has no Effect on Serum Bone Turnover Markers in Elderly Women: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutr. Res. 2013, 33, 211–219. [Google Scholar] [CrossRef]
- Obeid, R.; Herrmann, W. Homocysteine and Lipids: S-Adenosyl Methionine as a Key Intermediate. FEBS Lett. 2009, 583, 1215–1225. [Google Scholar] [CrossRef] [Green Version]
- Rafnsson, S.B.; Saravanan, P.; Bhopal, R.S.; Yajnik, C.S. Is a Low Blood Level of Vitamin B12 a Cardiovascular and Diabetes Risk Factor? A Systematic Review of Cohort Studies. Eur. J. Nutr. 2011, 50, 97–106. [Google Scholar] [CrossRef]
- Kumar, K.A.; Lalitha, A.; Pavithra, D.; Padmavathi, I.J.; Ganeshan, M.; Rao, K.R.; Venu, L.; Balakrishna, N.; Shanker, N.H.; Reddy, S.U.; et al. Maternal Dietary Folate and/or Vitamin B12 Restrictions Alter Body Composition (Adiposity) and Lipid Metabolism in Wistar Rat Offspring. J. Nutr. Biochem. 2013, 24, 25–31. [Google Scholar] [CrossRef]
- Al-Rubeaan, K.; Bawazeer, N.; Al Farsi, Y.; Youssef, A.M.; Al-Yahya, A.A.; AlQumaidi, H.; Al-Malki, B.M.; Naji, K.A.; Al-Shehri, K.; Al Rumaih, F.I. Prevalence of Metabolic Syndrome in Saudi Arabia—A Cross Sectional Study. BMC Endocr. Disord. 2018, 18, 16. [Google Scholar] [CrossRef]
- Al-Rifai, R.H.; Majeed, M.I.; Tariq, M.R.; Irfan, A. Type 2 Diabetes and Pre-Diabetes Mellitus: A Systematic Review and Meta-Analysis of Prevalence Studies in Women of Childbearing Age in the Middle East and North Africa, 2000-2018. Syst. Rev. 2019, 8, 268. [Google Scholar] [CrossRef]
- Adaikalakoteswari, A.; Jayashri, R.; Sukumar, N.; Venkataraman, H.; Pradeepa, R.; Gokulakrishnan, K.; Anjana, R.M.; McTernan, P.G.; Tripathi, G.; Patel, V.; et al. Vitamin B12 Deficiency is Associated with Adverse Lipid Profile in Europeans and Indians With Type 2 Diabetes. Cardiovasc. Diabetol. 2014, 13, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and Management of the Metabolic Syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saely, C.H.; Koch, L.; Schmid, F.; Marte, T.; Aczel, S.; Langer, P.; Hoefle, G.; Drexel, H. Adult Treatment Panel III 2001 but not International Diabetes Federation 2005 Criteria of the Metabolic Syndrome Predict Clinical Cardiovascular Events in Subjects Who Underwent Coronary Angiography. Diabetes Care 2006, 29, 901–907. [Google Scholar] [CrossRef] [Green Version]
- Jellinger, P.S.; Handelsman, Y.; Rosenblit, P.D.; Bloomgarden, Z.T.; Fonseca, V.A.; Garber, A.J.; Grunberger, G.; Guerin, C.K.; Bell, D.S.H.; Mechanick, J.I.; et al. American Association of Clinical Endocrinologists and American College of Endocrinology Guidelines for Management of Dyslipidemia and Prevention of Cardiovascular Disease. Endocr. Pract. 2017, 23, 1–87. [Google Scholar] [CrossRef]
- Stone, N.J.; Robinson, J.J.; Lichtenstein, A.H.; Merz, C.N.B.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2889–2934. [Google Scholar] [CrossRef] [Green Version]
- Genuth, S.; Alberti, K.G.; Bennett, P.; Buse, J.; Defronzo, R.; Kahn, R.; Kitzmiller, J.; Knowler, W.C.; Lebovitz, H.; Lernmark, A.; et al. Follow-Up Report on the Diagnosis of Diabetes Mellitus. Diabetes Care 2003, 26, 3160–3168. [Google Scholar] [CrossRef] [Green Version]
- Hunt, A.; Harrington, D.; Robinson, S. Vitamin B12 Deficiency. BMJ 2014, 349, 5226. [Google Scholar] [CrossRef]
- Alkhalaf, M.; Edwards, C.; Combet, E. Validation of a Food Frequency Questionnaire Specific for Salt Intake in Saudi Arabian Adults Using Urinary Biomarker and Repeated Multiple Pass 24-Hour Dietary Recall. Proc. Nutr. Soc. 2015, 74, E337. [Google Scholar] [CrossRef] [Green Version]
- Roe, M.; Pinchen, H.; Church, S.; Finglas, P. McCance and Widdowson’s The Composition of Foods Seventh Summary Edition and Updated Composition of Foods Integrated Dataset. Nutr. Bull. 2015, 40, 36–39. [Google Scholar] [CrossRef]
- Mearns, G.J.; Rush, E.C. Screening for Inadequate Dietary Vitamin B-12 Intake in South Asian Women Using a Nutrient-Specific, Semi-Quantitative Food Frequency Questionnaire. Asia Pac. J. Clin. Nutr. 2017, 26, 1119. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A. Clinical Evaluation of the Obese Patient. Best Pract. Res. Clin. Endocrinol. Metab. 1999, 13, 71–92. [Google Scholar] [CrossRef] [PubMed]
- World Health Organizaion. Obesity and Overweight. World Health Organization, 2018. Available online: http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 15 January 2020).
- Al-Musharaf, S.; Fouda, M.A.; Turkestani, I.Z.; Al-Ajlan, A.; Sabico, S.; Alnaami, A.M.; Wani, K.; Hussain, S.D.; Alraqebah, B.; Al-Serehi, A.; et al. Vitamin D Deficiency Prevalence and Predictors in Early Pregnancy Among Arab Women. Nutrients 2018, 10, 489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkahtani, S.A. Convergent Validity: Agreement Between Accelerometry and the Global Physical Activity Questionnaire in College-Age Saudi Men. BMC Res. Notes 2016, 9, 436. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Global Physical Activity Questionnaire (GPAQ) Analysis Guide: World Health Organization. Available online: http://www.who.int/chp/steps/resources/GPAQ_Analysis_Guide.pdf (accessed on 12 May 2020).
- Vogiatzoglou, A.; Smith, A.D.; Nurk, E.; Berstad, P.; Drevon, C.A.; Ueland, P.M.; Vollset, S.E.; Tell, G.S.; Refsum, H. Dietary Sources of Vitamin B-12 and Their Association with Plasma Vitamin B-12 Concentrations in the General Population: The Hordaland Homocysteine Study. Am. J. Clin. Nutr. 2009, 89, 1078–1087. [Google Scholar] [CrossRef]
- Aroda, V.R.; Edelstein, S.L.; Goldberg, R.B.; Knowler, W.C.; Marcovina, S.M.; Orchard, T.J.; Bray, G.A.; Schade, D.S.; Temprosa, M.G.; White, N.H.; et al. Long-term Metformin Use and Vitamin B12 Deficiency in the Diabetes Prevention Program Outcomes Study. J. Clin. Endocrinol. Metab. 2016, 101, 1754–1761. [Google Scholar] [CrossRef]
- Ingelsson, E.; Schaefer, E.J.; Contois, J.H.; McNamara, J.R.; Sullivan, L.; Keyes, M.J.; Pencina, M.J.; Schoonmaker, C.; Wilson, P.W.; D’Agostino, R.B.; et al. Clinical Utility of Different Lipid Measures for Prediction of Coronary Heart Disease in Men and Women. JAMA 2007, 298, 776–785. [Google Scholar] [CrossRef] [Green Version]
- Russo, G.T.; Giandalia, A.; Romeo, E.L.; Marotta, M.; Alibrandi, A.; De Francesco, C.; Horvath, K.V.; Asztalos, B.F.; Cucinotta, D. Lipid and non-lipid cardiovascular risk factors in postmenopausal type 2 diabetic women with and without coronary heart disease. J. Endocrinol. Investig. 2014, 37, 261–268. [Google Scholar] [CrossRef]
- Kim, H.-N.; Eun, Y.-M.; Song, S.-W. Serum Folate and Vitamin B 12 Levels are not Associated with the Incidence Risk of Atherosclerotic Events Over 12 Years: The Korean Genome and Epidemiology Study. Nutr. Res. 2019, 63, 34–41. [Google Scholar] [CrossRef]
- Gorban de Lapertosa, S.; Alvariñas, J.; Elgart, J.F.; Salzberg, S.; Gagliardino, J.J.; EduGest Group. The Triad Macrosomia, Obesity, and Hypertriglyceridemia in Gestational Diabetes. Diabetes Metab. Res. Rev. 2020, 36, e03302. [Google Scholar] [CrossRef] [Green Version]
- Strain, J.J.; Dowey, L.; Ward, M.; Pentieva, K.; McNulty, H. B-Vitamins, Homocysteine Metabolism and CVD. Proc. Nutr. Soc. 2004, 63, 597–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, I.H. Metabolic Programming of Offspring by Vitamin B12/folate Imbalance During Pregnancy. Diabetologia 2008, 51, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Brindle, N.P.; Zammit, V.A.; Pogson, C.I. Regulation of Carnitine Palmitoyltransferase Activity by malonyl-CoA in Mitochondria from Sheep Liver, a Tissue With a Low Capacity for Fatty Acid Synthesis. Biochem. J. 1985, 232, 177–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmel, R. Biomarkers of Cobalamin (Vitamin B-12) Status in the Epidemiologic Setting: A Critical Overview of Context, Applications, and Performance Characteristics of Cobalamin, Methylmalonic Acid, and Holotranscobalamin II. Am. J. Clin. Nutr. 2011, 94, 348S–358S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
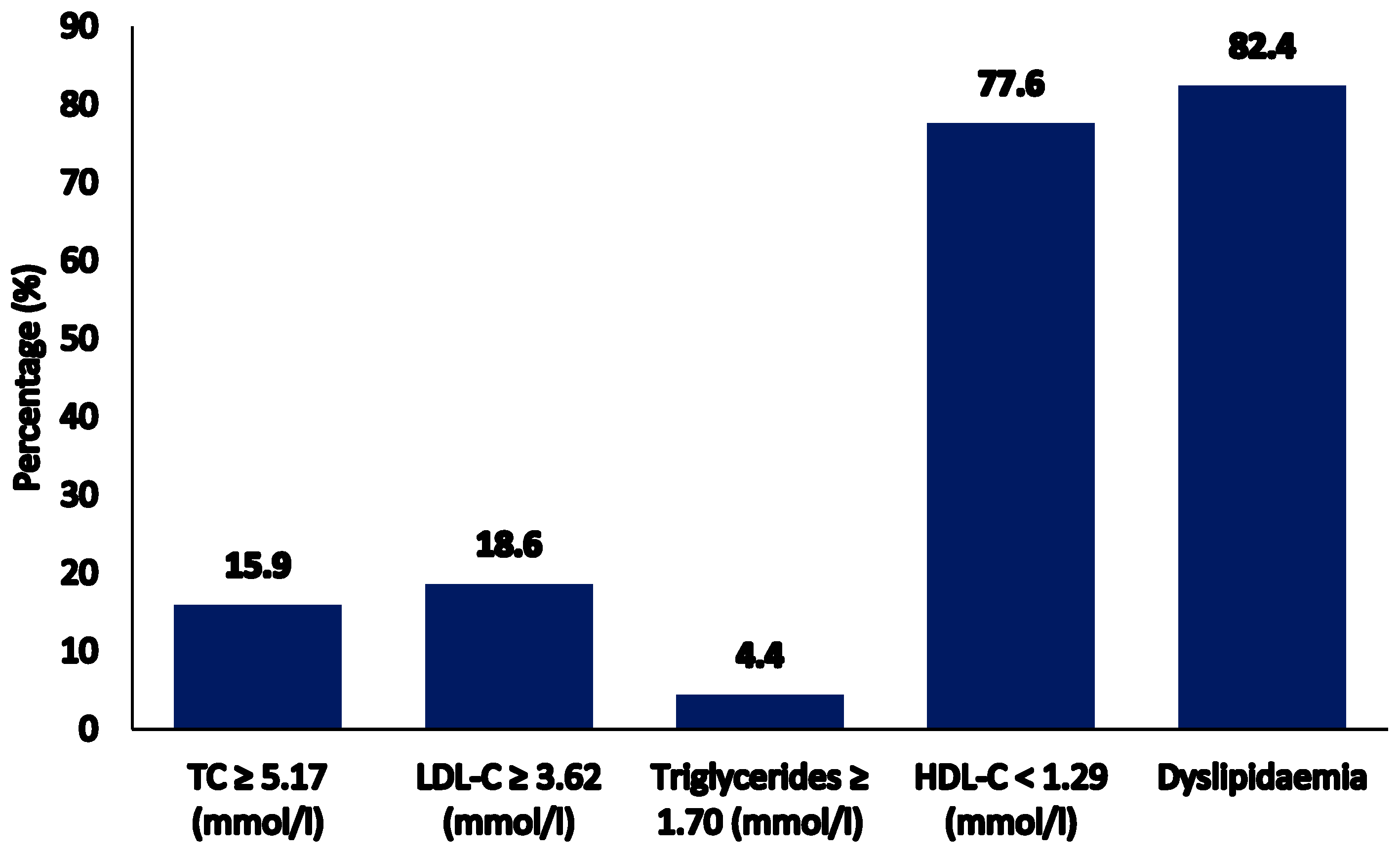
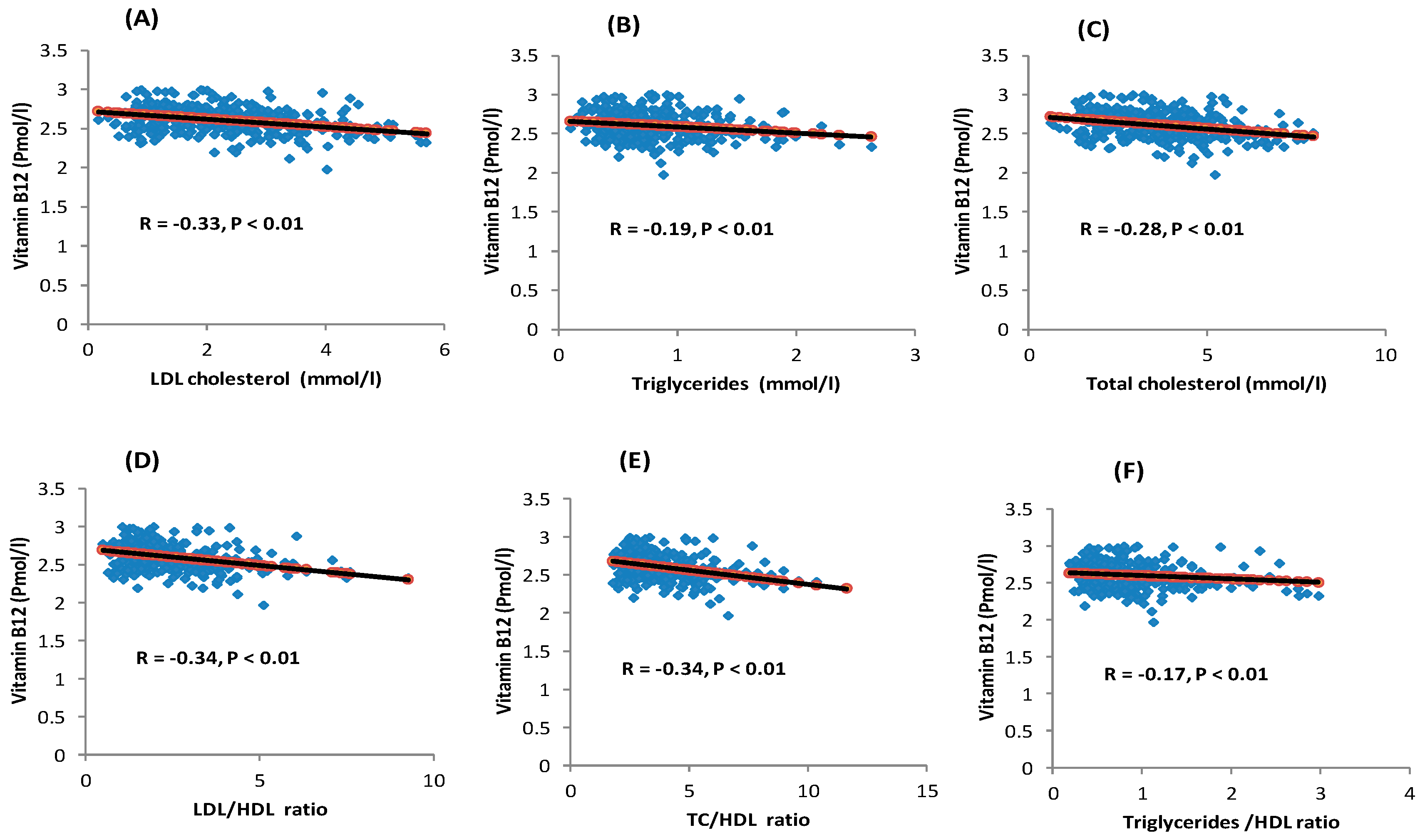
| Parameters | Total | Tertile 1 | Tertile 2 | Tertile 3 | p-Value |
|---|---|---|---|---|---|
| N | 341 | 114 | 113 | 114 | |
| Age (years) | 20.7 ± 1.5 | 20.7 ± 1.6 | 20.9 ± 1.8 | 20.7 ± 1.2 | 0.40 |
| BMI (kg/m2) | 23.6 ± 5.2 | 23.9 ± 5.6 | 23.5 ± 4.7 | 23.7 ± 5.4 | 0.85 |
| Waist circumference (cm) | 71.1 ± 10.4 | 72.1 ± 11.0 | 69.7 ± 9.3 | 71.4 ± 10.7 | 0.22 |
| Hip circumference (cm) | 99.5 ± 11.7 | 100.1 ± 10.7 | 98.7 ± 13.1 | 99.5 ± 11.1 | 0.66 |
| Waist/hip ratio | 0.72 ± 0.06 | 0.72 ± 0.07 | 0.70 ± 0.05 A | 0.72 ± 0.06 | 0.03 |
| Fat (%) | 36.9 ± 8.2 | 37.1 ± 8.1 | 36.8 ± 8.0 | 37.3 ± 8.1 | 0.91 |
| Family history of dyslipidemia | 8 (2.3) | 2 (1.8) | 5 (4.4) | 1 (0.9) | 0.19 |
| Family history of hyperlipidemia | 129 (37.8) | 42 (36.8) | 50 (44.2) | 37 (32.5) | 0.19 |
| Family history of heart disease | 104 (30.6) | 39 (34.2) | 35 (31.0) | 30 (26.5) | 0.55 |
| Income level (<10,000 SAR) | 70 (20.5) | 26 (22.8) | 24 (21.2) | 20 (17.5) | 0.60 |
| Vitamin B12 intake (mcg/day) | 6.9 (4.4–10.8) | 6.3 (3.5–10.1) | 6.8 (4.9–9.9) | 8.3 (4.8–11.6) A | 0.02 |
| Adequate vitamin B12 (≥2.4 mcg/day) | 323 (94.7) | 102 (89.5) | 109 (96.5) | 112 (98.2) A | 0.01 |
| Median B12 levels | 398.9 (305.8–534.6) | 269.1 (243.0–305.8) | 398.9 (361.0–448.3) A | 596.6 (534.6–683.7) AB | <0.001 |
| GPAQ score (MET-minute/week) # | 504.0 (160.0–1240.0) | 600.0 (200.0–1620.0) | 560.0 (160.0–1200.0) | 400.0 (180.0–940.0) | 0.18 |
| Biochemical characteristics | |||||
| Fasting glucose (mmol/L) | 4.6 ± 1.0 | 4.6 ± 1.0 | 4.6 ± 1.1 | 4.7 ± 0.9 | 0.77 |
| TC (mmol/L) | 3.8 ± 1.4 | 4.3 ± 1.6 | 3.8 ± 1.4 A | 3.3 ± 1.2 A | <0.001 |
| HDL-C (mmol/L) | 1.0 ± 0.4 | 1.0 ± 0.3 | 1.0 ± 0.4 | 1.1 ± 0.4 | 0.34 |
| LDL-Cl (mmol/L) | 2.4 ± 1.2 | 2.8 ± 1.3 | 2.3 ± 1.0 A | 2.0 ± 0.9 A | <0.001 |
| TG (mmol/L) | 0.8 ± 0.4 | 0.9 ± 0.5 | 0.7 ± 0.4 A | 0.7 ± 0.4 A | <0.001 |
| TC/HDL ratio | 3.9 ± 1.6 | 4.6 ± 2.0 | 3.8 ± 1.4 A | 3.3 ± 1.0 A | <0.001 |
| TG/HDL ratio | 0.9 ± 0.6 | 1.0 ± 0.6 | 0.8 ± 0.8 | 0.7 ± 0.4 A | <0.01 |
| LDL-C/HDL-C | 2.4 ± 1.4 | 3.0 ± 1.7 A | 2.4 ± 1.2 A | 2.0 ± 0.9 | <0.001 |
| Parameters | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| B ± SE/OR | B (S)/95%CI | p-Value | B ± SE | B (S)/95%CI | p-Value | |
| Total cholesterol (mmol/L) | −0.39 ± 0.08 | −0.27 | <0.0001 | −0.38 ± 0.07 | −0.26 | <0.0001 |
| Triglycerides (mmol/L) | −0.07 ± 0.02 | −0.17 | <0.01 | −0.07 ± 0.02 | −0.16 | <0.01 |
| LDL-C (mmol/L) | −0.35 ± 0.06 | −0.30 | <0.0001 | −0.34 ± 0.06 | −0.30 | <0.0001 |
| HDL-C (mmol/L) | 0.01 ± 0.02 | 0.04 | 0.51 | 0.01 ± 0.02 | 0.04 | 0.48 |
| LDL/HDL ratio | −0.41 ± 0.07 | −0.30 | <0.0001 | −0.41 ± 0.07 | −0.30 | <0.0001 |
| TC/HDL ratio | −0.48 ± 0.09 | −0.30 | <0.0001 | −0.47 ± 0.09 | −0.29 | <0.0001 |
| Triglyceride/HDL ratio | −0.09 ± 0.04 | −0.14 | 0.02 | −0.08 ± 0.04 | −0.13 | 0.02 |
| Dyslipidemia * | 0.75 | 0.56–1.00 | 0.05 | 0.74 | 0.55–1.01 | 0.06 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Musharaf, S.; Aljuraiban, G.S.; Danish Hussain, S.; Alnaami, A.M.; Saravanan, P.; Al-Daghri, N. Low Serum Vitamin B12 Levels Are Associated with Adverse Lipid Profiles in Apparently Healthy Young Saudi Women. Nutrients 2020, 12, 2395. https://doi.org/10.3390/nu12082395
Al-Musharaf S, Aljuraiban GS, Danish Hussain S, Alnaami AM, Saravanan P, Al-Daghri N. Low Serum Vitamin B12 Levels Are Associated with Adverse Lipid Profiles in Apparently Healthy Young Saudi Women. Nutrients. 2020; 12(8):2395. https://doi.org/10.3390/nu12082395
Chicago/Turabian StyleAl-Musharaf, Sara, Ghadeer S. Aljuraiban, Syed Danish Hussain, Abdullah M. Alnaami, Ponnusamy Saravanan, and Nasser Al-Daghri. 2020. "Low Serum Vitamin B12 Levels Are Associated with Adverse Lipid Profiles in Apparently Healthy Young Saudi Women" Nutrients 12, no. 8: 2395. https://doi.org/10.3390/nu12082395
APA StyleAl-Musharaf, S., Aljuraiban, G. S., Danish Hussain, S., Alnaami, A. M., Saravanan, P., & Al-Daghri, N. (2020). Low Serum Vitamin B12 Levels Are Associated with Adverse Lipid Profiles in Apparently Healthy Young Saudi Women. Nutrients, 12(8), 2395. https://doi.org/10.3390/nu12082395







