Gut Microbiota, Probiotics and Psychological States and Behaviors after Bariatric Surgery—A Systematic Review of Their Interrelation
Abstract
1. Introduction
- The GI microbial diversity will remain stable for richness and biodiversity (alpha diversity), but community structure will be dissimilar after BS compared to pre-surgery.
- Shifts in the abundance of specific microbial taxa will occur after BS, and the specificity of these changes in humans will be identified via comparison of different BS techniques to sham operations in vertebrate studies.
- The abundance of specific GI microbial taxa will shift after BS, and these changes will be associated with psychological and behavioral factors.
- The use of probiotics will influence outcomes after BS, including quality of life and psychological states.
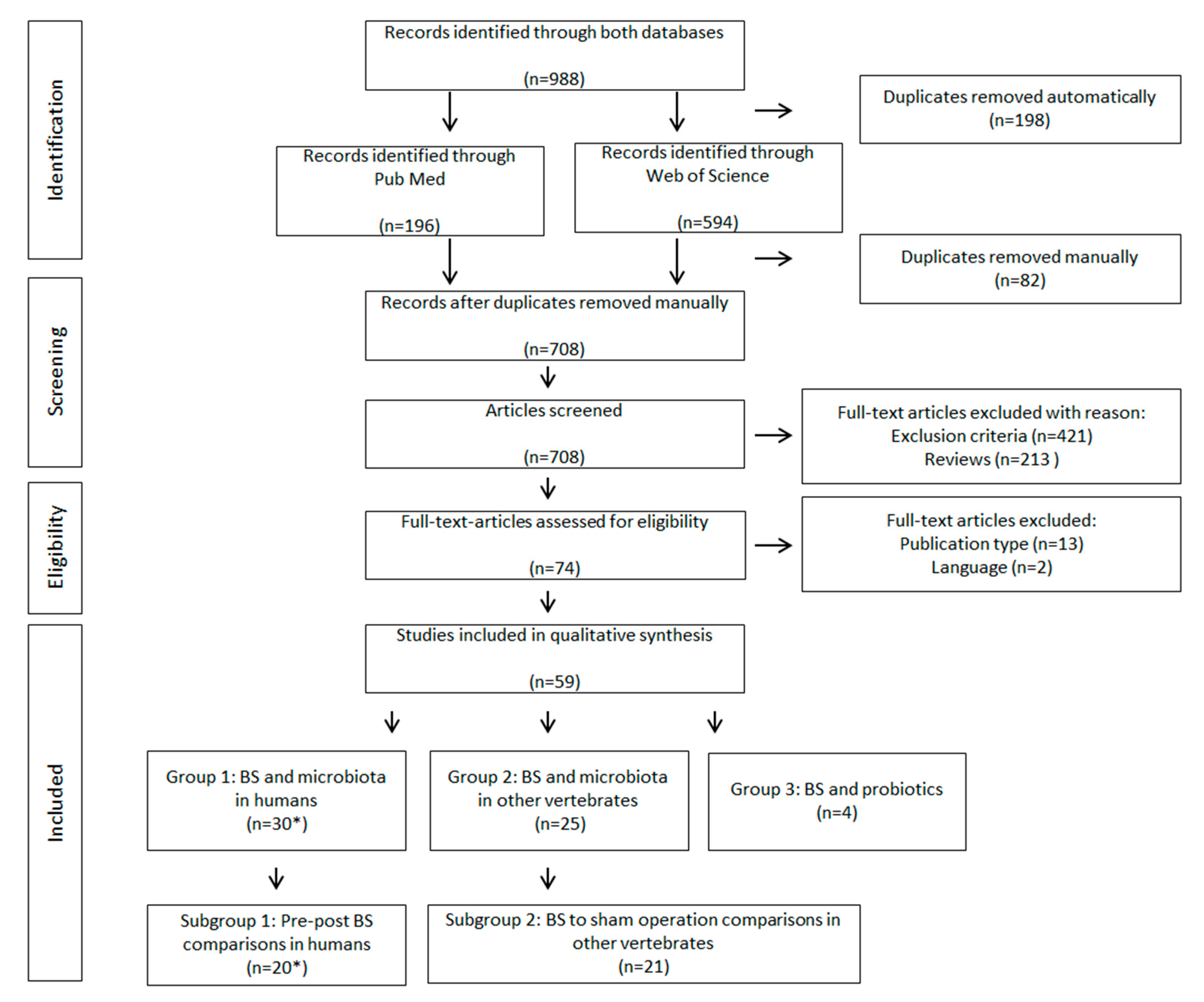
2. Materials and Methods
2.1. Literature Information Sources and Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection, Data Collection and Organisation
- Group 1—BS and microbiota in humans
- Group 2—BS and microbiota in other vertebrates
- Group 3—BS and probiotics
- Subgroup 1—Pre-post BS comparisons in human
- Subgroup 2—BS to sham operation comparisons in other vertebrates
2.4. Data Items and Statistics
2.5. Risk of Bias
3. Results
3.1. Summary of Study Characteristics
3.2. Summary of Study Outcomes
3.3. Overview of Microbiota Changes Following BS in Humans (Group 1) and Other Vertebrates (Group 2)
3.4. Subgroup Analysis
3.4.1. Subgroup 1 (Pre-Post BS Comparisons in Humans)
3.4.2. Subgroup 2 (BS to Sham Operation Comparisons in Other Vertebrates)
3.4.3. Similarities and Differences Identified between Subgroup 1 and Subgroup 2
3.5. Influence of GI Microbiota and BS on Psychological and Behavioural Outcomes
3.6. Influence of Probiotics on BS Outcomes (Group 3)
3.7. Risk of Bias
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AGB | Adjustable gastric banding |
| BIB | Bilio–intestinal bypass |
| BL | Blind loop |
| BMI | Body mass index |
| BPD/DS | Bilopancreal diversion with duodenal switch |
| BS | Bariatric surgery |
| CNS | Central nervous system |
| DES | Duodenal endoluminal barrier sleeve |
| DJB | Duodenal–jejunal bypass (B-DJB: with biliopancreatic limb, J-DJB: with jejunectomy) |
| DS | Duodenal switch |
| GG | Glandular gastrectomy |
| GI | Gastrointestinal |
| IQR | Interquartile range |
| IT | Ileal interposition |
| LSG | Laparoscopic sleeve gastrectomy |
| LGB | Laparoscopic gastric bypass |
| N.R. | Not reported |
| QoL | Quality of life |
| RYGB | Roux-en-Y gastric bypass |
| SCFA | Short chain fatty acids |
| VBG | Vertical banded gastroplasty |
References
- Seganfredo, F.B.; Blume, C.A.; Moehlecke, M.; Giongo, A.; Casagrande, D.S.; Spolidoro, J.V.N.; Padoin, A.V.; Schaan, B.D.; Mottin, C.C. Weight-loss interventions and gut microbiota changes in overweight and obese patients: A systematic review. Obes. Rev. 2017, 18, 832–851. [Google Scholar] [CrossRef] [PubMed]
- Catoi, A.F.; Vodnar, D.C.; Corina, A.; Nikolic, D.; Citarrela, R.; Petez-Martinez, P.; Rizzo, M. Gut microbiota, obesity and bariatric surgery: Current knowledge and future perspectives. Curr. Pharm. Des. 2019, 25, 2038–2050. [Google Scholar] [CrossRef] [PubMed]
- Angrisani, L.; Santonicola, A.; Iovino, P.; Formisano, G.; Buchwald, H.; Scopinaro, N. Bariatric Surgery Worldwide 2013. Obes. Surg. 2015, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The Human Microbiota in Health and Disease. Engineering 2017, 3, 71–82. [Google Scholar] [CrossRef]
- Murugesan, S.; Nirmalkar, K.; Hoyo-Vadillo, C.; Garcia-Espitia, M.; Ramirez-Sanchez, D.; Garcia-Mena, J. Gut microbiome production of short-chain fatty acids and obesity in children. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 621–625. [Google Scholar] [CrossRef]
- Mack, I.; Penders, J.; Cook, J.; Dugmore, J.; Mazurak, N.; Enck, P. Is the impact of starvation on the gut microbiota specific or unspecific to anorexia nervosa? A narrative review based on a systematic literature search. Curr. Neuropharmacol. 2018, 16, 1131–1149. [Google Scholar] [CrossRef]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Ley, R.E.; Hamady, M.; Lozupone, C.; Turnbaugh, P.; Ramey, R.R.; Bircher, J.S.; Schlegel, M.L.; Tucker, T.A.; Schrenzel, M.D.; Knight, R.; et al. Evolution of mammals and their gut microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef]
- Jost, L. Partitioning diversity into independent alpha and beta components. Ecology 2007, 88, 2427–2439. [Google Scholar] [CrossRef] [PubMed]
- Tuomisto, H. A diversity of beta diversities: Straightening up a concept gone awry. Part 1. Defining beta diversity as a function of alpha and gamma diversity. Ecography 2010, 33, 2–22. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.T.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N.R.F.; Zaparolli, M.R.; Cruz, M.R.R.; Schieferdecker, M.E.M.; Campos, A.C.L. Postoperative changes in intestinal microbiota and use of probiotics in roux-en-y gastric bypass and sleeve vertical gastrectomy: An integrative review. Arq. Bras. Cir. Dig. 2018, 31, e1400. [Google Scholar] [CrossRef]
- Davies, N.K.; O’Sullivan, J.M.; Plank, L.D.; Murphy, R. Altered gut microbiome after bariatric surgery and its association with metabolic benefits: A systematic review. Surg. Obes. Relat. Dis. 2019, 15, 656–665. [Google Scholar] [CrossRef]
- Shen, N.; Caixàs, A.; Ahlers, M.; Patel, K.; Gao, Z.; Dutia, R.; Blaser, M.J.; Clemente, J.C.; Laferrere, B. Longitudinal changes of microbiome composition and microbial metabolomics after surgical weight loss in individuals with obesity. Surg. Obes. Relat. Dis. 2019. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, Z.-P.; Liu, C.-Q.; Qi, L.; Sheng, Y.; Zou, D.-J. Modulation of the gut microbiome: A systematic review of the effect of bariatric surgery. Eur. J. Endocrinol. 2018, 178, 43–56. [Google Scholar] [CrossRef]
- Morales-Marroquin, E.; Hanson, B.; Greathouse, L.; de la Cruz-Munoz, N.; Messiah, S.E. Comparison of methodological approaches to human gut microbiota changes in response to metabolic and bariatric surgery: A systematic review. Obes. Rev. 2020, 1–15. [Google Scholar] [CrossRef]
- Huang, R.; Wang, K.; Hu, J. Effect of probiotics on depression: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2016, 8, 483. [Google Scholar] [CrossRef]
- Clapp, M.; Aurora, N.; Herrera, L.; Bhatia, M.; Wilen, E.; Wakefield, S. Gut microbiota’s effect on mental health: The gut-brain axis. Clin. Pract. 2017, 7, 987. [Google Scholar] [CrossRef]
- Ganci, M.; Suleyman, E.; Butt, H.; Ball, M. The role of the brain-gut-microbiota axis in psychology: The importance of considering gut microbiota in the development, perpetuation, and treatment of psychological disorders. Brain. Behav. 2019, 9, e01408. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C. Mental health: Thinking from the gut. Nature 2015, 518, S12–S15. [Google Scholar] [CrossRef] [PubMed]
- Neff, A.S. Technical and theoretic limitations of the experimental evidence supporting a gut bacterial etiology in mental illness. Clin. Ther. 2020. [Google Scholar] [CrossRef] [PubMed]
- Mazloom, K.; Siddiqi, I.; Covasa, M. Probiotics: How Effective Are They in the Fight against Obesity? Nutrients 2019, 11, 258. [Google Scholar] [CrossRef] [PubMed]
- Takada, M.; Nishida, K.; Kataoka-Kato, A.; Gondo, Y.; Ishikawa, H.; Suda, K.; Kawai, M.; Hoshi, R.; Watanabe, O.; Igarashi, T.; et al. Probiotic Lactobacillus casei strain Shirota relieves stress-associated symptoms by modulating the gut-brain interaction in human and animal models. Neurogastroenterol. Motil. 2016, 28, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.A.; Jazayeri, S.; Khosravi-Darani, K.; Solati, Z.; Mohammadpour, N.; Asemi, Z.; Adab, Z.; Djalali, M.; Tehrani-Doost, M.; Hosseini, M.; et al. The effects of probiotics on mental health and hypothalamic-pituitary-adrenal axis: A randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutr. Neurosci. 2016, 19, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Bested, A.C.; Beaulne, T.M.; Katzman, M.A.; Iorio, C.; Berardi, J.M.; Logan, A.C. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut. Pathog. 2009, 1, 6. [Google Scholar] [CrossRef]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain. Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef]
- Messaoudi, M.; Violle, N.; Bisson, J.-F.; Desor, D.; Javelot, H.; Rougeot, C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut. Microbes. 2011, 2, 256–261. [Google Scholar] [CrossRef]
- Kato-Kataoka, A.; Nishida, K.; Takada, M.; Suda, K.; Kawai, M.; Shimizu, K.; Kushiro, A.; Hoshi, R.; Watanabe, O.; Igarashi, T.; et al. Fermented milk containing Lactobacillus casei strain Shirota prevents the onset of physical symptoms in medical students under academic examination stress. Benef. Microbes. 2016, 7, 153–156. [Google Scholar] [CrossRef]
- Langkamp-Henken, B.; Rowe, C.C.; Ford, A.L.; Christman, M.C.; Nieves, C.J.; Khouri, L.; Specht, G.J.; Girard, S.-A.; Spaiser, S.J.; Dahl, W.J. Bifidobacterium bifidum R0071 results in a greater proportion of healthy days and a lower percentage of academically stressed students reporting a day of cold/flu: A randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2015, 113, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Diop, L.; Guillou, S.; Durand, H. Probiotic food supplement reduces stress-induced gastrointestinal symptoms in volunteers: A double-blind, placebo-controlled, randomized trial. Nutr. Res. 2008, 28, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-B.; Xin, S.-S.; Ding, L.-N.; Ding, W.-Y.; Hou, Y.-L.; Lui, C.-Q.; Zhang, X.-D. The Potential Role of Probiotics in Controlling Overweight/Obesity and Associated Metabolic Parameters in Adults: A Systematic Review and Meta-Analysis. Evid. Based. Complement. Alternat. Med. 2019, 2019, 3862971. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 16. [Google Scholar] [CrossRef]
- Rooney, A.A.; Boyles, A.L.; Wolfe, M.S.; Bucher, J.R.; Thayer, K.A. Systematic review and evidence integration for literature-based environmental health science assessments. Environ. Health Perspect. 2014, 122, 711–718. [Google Scholar] [CrossRef]
- Campisciano, G.; Palmisano, S.; Cason, C.; Guiricin, M.; Silvestri, M.; Guerra, M.; Macor, D.; De Manzini, N.; Croce, L.S.; Comar, M. Gut microbiota characterisation in obese patients before and after bariatric surgery. Benef. Microbes. 2018, 9, 367–373. [Google Scholar] [CrossRef]
- Campisciano, G.; Cason, C.; Palmisano, S.; Guiricin, M.; Rizzardi, A.; Croce, L.S.; De Manzini, N.; Comar, M. Bariatric surgery drives major rearrangements of the intestinal microbiota including the biofilm composition. Front. Biosci. (Elite Ed.) 2018, 10, 495–505. [Google Scholar]
- Aron-Wisnewsky, J.; Prifti, E.; Belda, E.; Ichou, F.; Kayser, B.D.; Doa, M.C.; Verger, E.O.; Hedjazi, L.; Bouillot, J.-L.; Chevallier, J.-M.; et al. Major microbiota dysbiosis in severe obesity: Fate after bariatric surgery. Gut 2019, 68, 70–82. [Google Scholar] [CrossRef]
- Chen, H.; Qian, L.; Lv, Q.; Yu, J.; Wu, W.; Qian, H. Change in gut microbiota is correlated with alterations in the surface molecule expression of monocytes after Roux-en-Y gastric bypass surgery in obese type 2 diabetic patients. Am. J. Transl. Res. 2017, 9, 1243–1254. [Google Scholar]
- Cortez, R.V.; Petry, T.; Caravatto, P.; Pessoa, R.; Sanabani, S.S.; Martinez, M.B.; Sarian, T.; Salles, J.E.; Cohen, R.; Taddei, C.R. Shifts in intestinal microbiota after duodenal exclusion favor glycemic control and weight loss: A randomized controlled trial. Surg. Obes. Relat. Dis. 2018, 14, 1748–1754. [Google Scholar] [CrossRef] [PubMed]
- Damms-Machado, A.; Mitra, S.; Schollenberger, A.E.; Kramer, K.M.; Meile, T.; Königsrainer, A.; Huson, D.H.; Bischoff, S.C. Effects of surgical and dietary weight loss therapy for obesity on gut microbiota composition and nutrient absorption. BioMed Res. Int. 2015, 2015, 806248. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Dallio, M.; Tolone, S.; Gravina, A.G.; Patrone, V.; Romano, M.; Tuccillo, C.; Mozzillo, A.L.; Amoroso, V.; Misso, G.; et al. Gastrointestinal Hormones, Intestinal Microbiota and Metabolic Homeostasis in Obese Patients: Effect of Bariatric Surgery. Vivo 2016, 30, 321–330. [Google Scholar]
- Furet, J.-P.; Kong, L.-C.; Tap, J.; Poitou, C.; Basdevant, A.; Bouillot, J.-L.; Mariat, D.; Corthier, G.; Dore, J.; Henegar, C.; et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: Links with metabolic and low-grade inflammation markers. Diabetes 2010, 59, 3049–3057. [Google Scholar] [CrossRef]
- Graessler, J.; Qin, Y.; Zhong, H.; Zhang, J.; Licinio, J.; Wong, M.-L.; Xu, A.; Chavakis, T.; Bornstein, A.B.; Ehrhart-Bornstein, M.; et al. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: Correlation with inflammatory and metabolic parameters. Pharm. J. 2013, 13, 514–522. [Google Scholar] [CrossRef]
- Kellerer, T.; Brandl, B.; Büttner, J.; Lagkouvardos, I.; Hauner, H.; Skurk, T. Impact of Laparoscopic Sleeve Gastrectomy on Gut Permeability in Morbidly Obese Subjects. Obes. Surg. 2019, 29, 2132–2143. [Google Scholar] [CrossRef]
- Kong, L.-C.; Tap, J.; Aron-Wisnewsky, J.; Pelloux, V.; Basdevant, A.; Bouillot, J.-L.; Zucker, J.-D.; Dore, J.; Clement, K. Gut microbiota after gastric bypass in human obesity: Increased richness and associations of bacterial genera with adipose tissue genes. Am. J. Clin. Nutr. 2013, 98, 16–24. [Google Scholar] [CrossRef]
- Lee, C.J.; Florea, L.; Sears, C.L. Changes in Gut Microbiome after Bariatric Surgery Versus Medical Weight Loss in a Pilot Randomized Trial. Obes. Surg. 2019, 29, 3239–3245. [Google Scholar] [CrossRef]
- Lin, B.Y.; Lin, W.D.; Huang, C.K.; Hsin, M.C.; Lin, W.Y.; Pryor, A.D. Changes of gut microbiota between different weight reduction programs. Surg. Obes. Relat. Dis. 2019. [Google Scholar] [CrossRef]
- Liu, R.; Hong, J.; Xu, X.; Feng, Q.; Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X.; et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 2017, 23, 859–868. [Google Scholar] [CrossRef]
- Medina, D.A.; Pedreros, J.P.; Turiel, D.; Quezada, N.; Pimentel, F.; Escalona, A.; Garrido, D. Distinct patterns in the gut microbiota after surgical or medical therapy in obese patients. Peer J. 2017, 5, e3443. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.; Tsai, P.; Jüllig, M.; Liu, A.; Plank, L.; Booth, M. Differential Changes in Gut Microbiota After Gastric Bypass and Sleeve Gastrectomy Bariatric Surgery Vary According to Diabetes Remission. Obes. Surg. 2017, 27, 917–925. [Google Scholar] [CrossRef]
- Paganelli, F.L.; Luyer, M.; Hazelbag, C.M. Roux-Y Gastric Bypass and Sleeve Gastrectomy directly change gut microbiota composition independent of surgery type. Sci. Rep. 2019, 9, 10979. [Google Scholar] [CrossRef]
- Pajecki, D.; Oliveira, L.C.; Sabino, E.C.; de Souza-Basqueira, M.; Dantas, A.C.B.; Nunes, G.C.; de Cleva, R.; Santo, M.A. Changes in the intestinal microbiota of superobese patients after bariatric surgery. Clinics 2019, 74, e1198. [Google Scholar] [CrossRef] [PubMed]
- Palleja, A.; Kashani, A.; Allin, K.H.; Nielsen, T.; Zhang, C.; Li, Y.; Brach, T.; Liang, S.; Feng, Q.; Jorgensen, N.B.; et al. Roux-en-Y gastric bypass surgery of morbidly obese patients induces swift and persistent changes of the individual gut microbiota. Genome Med. 2016, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, S.; Campisciano, G.; Silverstri, M.; Guerra, M.; Giuricin, M.; Casagranda, B.; Comar, M.; de Manzini, N. Changes in Gut Microbiota Composition after Bariatric Surgery: A New Balance to Decode. J. Gastrointest. Surg. 2019. [Google Scholar] [CrossRef]
- Patrone, V.; Vajana, E.; Minuti, A.; Callegari, M.L.; Federico, A.; Loguercio, C.; Dallio, M.; Tolone, S.; Docimo, L.; Morelli, L. Postoperative Changes in Fecal Bacterial Communities and Fermentation Products in Obese Patients Undergoing Bilio-Intestinal Bypass. Front. Microbiol. 2016, 7, 200. [Google Scholar] [CrossRef]
- Sanmiguel, C.P.; Jacobs, J.; Gupta, A.; Ju, T.; Stains, J.; Coveleskie, K.; Lagishetty, V.; Balioukova, A.; Chen, Y.; Dutson, E.; et al. Surgically Induced Changes in Gut Microbiome and Hedonic Eating as Related to Weight Loss: Preliminary Findings in Obese Women Undergoing Bariatric Surgery. Psychosom. Med. 2017, 79, 880–887. [Google Scholar] [CrossRef]
- Wang, F.G.; Ba, R.X.; Yan, W.M.; Yan, M.; Dong, L.Y.; Song, M.M. Differential composition of gut microbiota among healthy volunteers, morbidly obese patients and post bariatric surgery patients. Exp. Ther. Med. 2019, 17, 2268–2278. [Google Scholar] [CrossRef]
- Huang, X.; Weng, P.; Zhang, H.; Lu, Y. Remodeling intestinal flora with sleeve gastrectomy in diabetic rats. J. Diabetes Res. 2014, 2014, 196312. [Google Scholar] [CrossRef]
- Jahansouz, C.; Staley, C.; Bernlohr, D.A.; Sadowsky, M.J.; Khoruts, A.; Ikramuddin, S. Sleeve gastrectomy drives persistent shifts in the gut microbiome. Surg. Obes. Relat. Dis. 2017, 13, 916–924. [Google Scholar] [CrossRef]
- Li, J.V.; Reshat, R.; Wu, Q.; Ashrafian, H.; Bueter, M.; le Roux, C.W.; Darzi, A.; Athanasiou, T.; Marchesi, J.R.; Nicholson, J.K.; et al. Experimental bariatric surgery in rats generates a cytotoxic chemical environment in the gut contents. Front. Microbiol. 2011, 2, 183. [Google Scholar] [CrossRef]
- Li, S.; Vinci, A.; Behnsen, J.; Cheng, C.; Jellbauer, S.; Raffatellu, M.; Sousa, K.M.; Edwards, R.; Nguyen, N.T.; Stamos, M.J.; et al. Bariatric surgery attenuates colitis in an obese murine model. Surg. Obes. Relat. Dis. 2017, 13, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Liou, A.P.; Paziuk, M.; Luevano, J.-M.; Machineni, S.; Turnbaugh, P.J.; Kaplan, L.M. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci. Transl. Med. 2013, 5, 178ra41. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, H.; Wang, X.; Yu, X.; Hu, C.; Zhang, X. The family Coriobacteriaceae is a potential contributor to the beneficial effects of Roux-en-Y gastric bypass on type 2 diabetes. Surg. Obes. Relat. Dis. 2018, 14, 584–593. [Google Scholar] [CrossRef]
- Mukorako, P.; Lopez, C.; Baraboi, E.D.; Roy, M.C.; Plamondon, J.; Lemoine, N.; Biertho, L.; Varin, T.V.; Marette, A.; Richard, D. Alterations of Gut Microbiota After Biliopancreatic Diversion with Duodenal Switch in Wistar Rats. Obes. Surg. 2019, 29, 2831–2842. [Google Scholar] [CrossRef] [PubMed]
- Schippers, E.; Willis, S.; Ruckdeschel, G.; Schumpelick, V. Small intestinal myoelectrical activity and bacterial flora after Roux-en-Y reconstruction. Br. J. Surg. 1996, 83, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Ding, R.; Xu, B.; Hua, R.; Shen, Q.; He, K.; Yao, Q. Alterations of Gut Microbiota After Roux-en-Y Gastric Bypass and Sleeve Gastrectomy in Sprague-Dawley Rats. Obes. Surg. 2017, 27, 295–302. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Q.; Bai, J.; Zhoa, N.; Wang, Y.; Zhou, R.; Kong, W.; Zeng, T.; Toa, K.; Wang, G.; et al. Upregulation of Intestinal NLRP6 Inflammasomes After Roux-en-Y Gastric Bypass Promotes Gut Immune Homeostasis. Obes. Surg. 2019, 30, 327–335. [Google Scholar] [CrossRef]
- Chen, J.C.; Lee, W.J.; Tsou, J.J.; Liu, T.P.; Tsai, P.L. Effect of probiotics on postoperative quality of gastric bypass surgeries: A prospective randomized trial. Surg. Obes. Relat. Dis. 2016, 12, 57–61. [Google Scholar] [CrossRef]
- Kazzi, F.; Daher, N.; Kray, G.; Zimmermann, G.; Scharf, K. The Effect of Bacillus Coagulans and Galactomannans on the Quality of Life of Patients Undergoing Laparoscopic Sleeve Gastrectomy: A Randomized Controlled Clinical Trial. Hell. J. Surg. 2018, 90, 121–126. [Google Scholar] [CrossRef]
- Sherf-Dagan, S.; Zelber-Sagi, S.; Zilberman-Schapira, G.; Webb, M.; Buch, A.; Keidar, A.; Raziel, A.; Sakran, N.; Goitein, D.; Goldenberg, N.; et al. Probiotics administration following sleeve gastrectomy surgery: A randomized double-blind trial. Int. J. Obes. (Lond) 2018, 42, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Woodard, G.A.; Encarnacion, B.; Downey, J.R.; Peraza, J.; Chong, K.; Hernandez-Boussard, T.; Morton, J.M. Probiotics improve outcomes after Roux-en-Y gastric bypass surgery: A prospective randomized trial. J. Gastrointest. Surg. 2009, 13, 1198–1204. [Google Scholar] [CrossRef]
- Bjørneklett, A.; Viddal, K.O.; Midtvedt, T.; Nygaard, K. Intestinal and Gastric Bypass. Scand. J. Gastroenterol. 1981, 16, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Fouladi, F.; Brooks, A.E.; Fodor, A.A.; Carroll, I.M.; Bulik-Sullivan, E.C.; Tsilimigras, M.C.B.; Sioda, M.; Steffen, K.J. The role of the gut microbiota in sustained weight loss following roux-en-y gastric bypass surgery. Obes. Surg. 2019, 29, 1259–1267. [Google Scholar] [CrossRef]
- Gutierrez-Repiso, C.; Moreno-Indias, I.; de Hollanda, A.; Martin-Nunez, G.M.; Vidal, J.; Tinahones, F.J. Gut microbiota specific signatures are related to the successful rate of bariatric surgery. Am. J. Transl. Res. 2019, 11, 942–952. [Google Scholar]
- Rosina, M.; Micheletto, G.; Vita, P.M.; Restelli, A.; Caspani, P.; Ferla, G.; Doldi, S.B. Intestinal Microflora Settlement in Patients with Jejunoileal Bypass for Morbid Obesity. Obes. Surg. 1993, 3, 239–245. [Google Scholar] [CrossRef]
- Tremaroli, V.; Karlsson, F.; Werling, M.; Stahlman, M.; Kovatcheva-Datchary, P.; Olbers, T.; Fändriks, L.; le Roux, C.W.; Nielsen, J.; Bäckhed, F. Roux-en-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. Cell. Metab. 2015, 22, 228–238. [Google Scholar] [CrossRef]
- Zhang, H.; DiBaise, J.K.; Zuccolo, A.; Kudrna, D.; Braidorri, M.; Yu, Y.; Parameswaran, P.; Crowell, M.D.; Wing, R.; Rittmann, B.E.; et al. Human gut microbiota in obesity and after gastric bypass. Proc. Natl. Acad. Sci. USA 2009, 106, 2365–2370. [Google Scholar] [CrossRef]
- Alvarez, R.; Lewis, A.G.; Toure, M.H.; Seeley, R.J. A comparison of rodent models of vertical sleeve gastrectomy. Surg. Obes. Relat. Dis. 2018, 14, 1471–1479. [Google Scholar] [CrossRef]
- Basso, N.; Soricelli, E.; Castagneto-Gissey, L.; Casella, G.; Albanese, D.; Fava, F.; Donati, C.; Tuohy, K.; Angelini, G.; La Neve, F.; et al. Insulin Resistance, Microbiota, and Fat Distribution Changes by a New Model of Vertical Sleeve Gastrectomy in Obese Rats. Diabetes 2016, 65, 2990–3001. [Google Scholar] [CrossRef] [PubMed]
- Bastos, E.L.S.; Liberatore, A.M.A.; Tedesco, R.C.; Koh, I.H.J. Gut Microbiota Imbalance Can Be Associated with Non-malabsorptive Small Bowel Shortening Regardless of Blind Loop. Obes. Surg. 2019, 29, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Cummings, B.P.; Bettaieb, A.; Graham, J.L.; Kim, J.; Ma, F.; Shibata, N.; Stanhope, K.L.; Guilivi, C.; Hansen, F.; Jelsing, J.; et al. Bile-acid-mediated decrease in endoplasmic reticulum stress: A potential contributor to the metabolic benefits of ileal interposition surgery in UCD-T2DM rats. Dis. Model. Mech. 2013, 6, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Duboc, H.; Nguyen, C.C.; Cavin, J.-B.; Ribeiro-Parenti, L.; Jarry, A.-C.; Rainteau, D.; Humbert, L.; Coffin, B.; Le Gall, M.; Bado, A.; et al. Roux-en-Y Gastric-Bypass and sleeve gastrectomy induces specific shifts of the gut microbiota without altering the metabolism of bile acids in the intestinal lumen. Int. J. Obes. (Lond) 2019, 43, 428–431. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, C.-Q.; Shan, C.-X.; Chen, Y.; Li, H.-H.; Huang, Z.-P.; Zou, D.-J. Gut microbiota after Roux-en-Y gastric bypass and sleeve gastrectomy in a diabetic rat model: Increased diversity and associations of discriminant genera with metabolic changes. Diabetes Metab. Res. Rev. 2017, 33. [Google Scholar] [CrossRef]
- Kim, T.; Holleman, C.L.; Ptacek, T.; Morrow, C.D.; Habegger, K.M. Duodenal endoluminal barrier sleeve alters gut microbiota of ZDF rats. Int. J. Obes. (Lond.) 2017, 41, 381–389. [Google Scholar] [CrossRef]
- Miyachi, T.; Nagao, M.; Shibata, C.; Kitahara, Y.; Tanaka, N.; Watanabe, K.; Tsuchiya, T.; Motoi, F.; Naitoh, T.; Unno, M. Biliopancreatic limb plays an important role in metabolic improvement after duodenal-jejunal bypass in a rat model of diabetes. Surgery 2016, 159, 1360–1371. [Google Scholar] [CrossRef]
- Osto, M.; Abegg, K.; Bueter, M.; le Roux, C.W.; Cani, P.D.; Lutz, T.A. Roux-en-Y gastric bypass surgery in rats alters gut microbiota profile along the intestine. Physiol. Behav. 2013, 119, 92–96. [Google Scholar] [CrossRef]
- Yang, P.-J.; Yang, W.-S.; Nien, H.-C.; Chen, C.-N.; Lee, P.-H.; Yu, L.C.-H.; Lin, M.-T. Duodenojejunal Bypass Leads to Altered Gut Microbiota and Strengthened Epithelial Barriers in Rats. Obes. Surg. 2016, 26, 1576–1583. [Google Scholar] [CrossRef]
- Huh, Y.J.; Seo, J.Y.; Nam, J.; Yang, J.; McDowell, A.; Kim, Y.K.; Lee, J.H. Bariatric/Metabolic Surgery Induces Noticeable Changes of Microbiota and Their Secreting Extracellular Vesicle Composition in the Gut. Obes. Surg. 2019, 29, 2470–2484. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, Q.; Huang, Z.; Song, A.; Peng, Y.; Hou, S.; Guo, S.; Zhu, W.; Yan, S.; Lin, Z.; et al. Gastric Bypass Surgery Reverses Diabetic Phenotypes in Bdnf-Deficient Mice. Am. J. Pathol. 2016, 186, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Shen, Q.; Hua, R.; Evers, S.S.; He, K.; Yao, Q. Effects of sleeve gastrectomy on the composition and diurnal oscillation of gut microbiota related to the metabolic improvements. Surg. Obes. Relat. Dis. 2018, 14, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Zhong, M.; Liu, T.; Han, H.; Zhang, G.; Liu, S.; Wei, M.; Wu, Q.; Hu, S. Duodenal-Jejunal Bypass Preferentially Elevates Serum Taurine-Conjugated Bile Acids and Alters Gut Microbiota in a Diabetic Rat Model. Obes. Surg. 2016, 26, 1890–1899. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, Z.E.; DiBaise, J.K.; Isern, N.G.; Hoyt, D.W.; Marcus, A.K.; Kang, D.-W.; Crowell, M.D.; Rittmann, B.E.; Krajmalnik-Brown, R. Distinctive microbiomes and metabolites linked with weight loss after gastric bypass, but not gastric banding. ISME J. 2017, 11, 2047–2058. [Google Scholar] [CrossRef] [PubMed]
- Kashihara, H.; Shimada, M.; Yoshikawa, K.; Higashijima, J.; Nakao, T.; Nishi, M.; Tatasu, C. Duodenal-jejunal bypass changes the composition of the gut microbiota. Surg. Today 2017, 47, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.S.; Spakowicz, D.J.; Hong, B.-Y.; Petersen, L.M.; Demkowicz, P.; Chen, L.; Leopold, S.R.; Hanson, B.M.; Agresta, H.O.; Gerstein, M.; et al. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nat. Commun. 2019, 10, 5029. [Google Scholar] [CrossRef]
- Teng, F.; Darveekaran Nair, S.S.; Zhu, P.; Li, S.; Huang, S.; Li, X.; Xu, J.; Yang, F. Impact of DNA extraction method and targeted 16S-rRNA hypervariable region on oral microbiota profiling. Sci. Rep. 2018, 8, 16321. [Google Scholar] [CrossRef]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Tito, R.Y.; Joossens, M.; Raes, J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 2016, 65, 57–62. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- da la Cuesta-Zuluaga, J.; Corrales-Agudelo, V.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Body size phenotypes comprehensively assess cardiometabolic risk and refine the association between obesity and gut microbiota. Int. J. Obes. (Lond.) 2018, 42, 424–432. [Google Scholar] [CrossRef]
- Louis, S.; Tappu, R.M.; Damms-Machado, A.; Huson, D.H.; Bischoff, S.C. Characterization of the gut microbial community of obese patients following a weight-loss intervention using whole metagenome shotgun sequencing. PLoS ONE 2016, 11, e0149564. [Google Scholar] [CrossRef] [PubMed]
- Mack, I.; Cuntz, U.; Grämer, C.; Niedermaier, S.; Pohl, C.; Schwiertz, A.; Zimmermann, K.; Zipfel, S.; Enck, P.; Penders, J. Weight gain in anorexia nervosa does not ameliorate the faecal microbiota, branched chain fatty acid profiles, and gastrointestinal complaints. Sci. Rep. 2016, 6, 26752. [Google Scholar] [CrossRef] [PubMed]
- Santacruz, A.; Collado, M.C.; Garcia-Valdes, L.; Segura, M.T.; Martin-Lagos, J.A.; Anjos, T.; Marti-Romero, M.; Lopez, R.M.; Florido, J.; Campoy, C.; et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br. J. Nutr. 2010, 104, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Cobb, L.A.; Thomas, G.I.; Dillard, D.H.; Merendino, K.A.; Bruce, R.A. An evaluation of internal-mammary-artery ligation by a double-blind technic. N. Engl. J. Med. 1959, 260, 1115–1118. [Google Scholar] [CrossRef]
- Moseley, J.B.; O’Malley, K.; Petersen, N.J.; Menke, T.J.; Brody, B.A.; Kuykendall, D.H.; Hollingsworth, J.C.; Ashton, C.M.; Wray, N.P. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N. Engl. J. Med. 2002, 347, 81–88. [Google Scholar] [CrossRef]
- Beard, D.J.; Rees, J.L.; Cook, J.A.; Rombach, I.; Cooper, C.; Merritt, N.; Shirkey, B.A.; Donovan, J.L.; Gwilym, S.; Savulescu, J.; et al. Arthroscopic subacromial decompression for subacromial shoulder pain (CSAW): A multicentre, pragmatic, parallel group, placebo-controlled, three-group, randomised surgical trial. Lancet 2018, 391, 329–338. [Google Scholar] [CrossRef]
- Mack, I.; Ölschläger, S.; Sauer, H.; von Feilitzsch, M.; Weimer, K.; Junne, F.; Peeraully, R.; Enck, P.; Zipfel, S.; Teufel, M. Does laparoscopic sleeve gastrectomy improve depression, stress and eating behaviour? A 4-year follow-up study. Obes. Surg. 2016, 26, 2967–2973. [Google Scholar] [CrossRef]
- White, M.A.; Kalarchian, M.A.; Levine, M.D.; Masheb, R.M.; Marcus, M.D.; Grilo, C.M. Prognostic significance of depressive symptoms on weight loss and psychosocial outcomes following gastric bypass surgery: A prospective 24-month follow-up study. Obes. Surg. 2015, 25, 1909–1916. [Google Scholar] [CrossRef]
- Kelly, J.R.; Clarke, G.; Cryan, J.F.; Dinan, T.G. Brain-gut-microbiota axis: Challenges for translation in psychiatry. Ann. Epidemiol. 2016, 26, 366–372. [Google Scholar] [CrossRef]
- Wang, H.; Braun, C.; Murphy, E.F.; Enck, P. Bifidobacterium longum 1714TM strain modulated brain activity of healthy volunteers during social stress. Am. J. Gastroenterol. 2019, 114, 1152–1162. [Google Scholar] [CrossRef]
- Yang, X.; Yu, D.; Li, H.; Du, J. Probiotics modulate the microbiota-gut-brain axis and improve memory deficits in aged SAMP8 mice. Acta Pharm. Sin. B 2020, 10, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Nagabhushanam, K.; Arumugam, S.; Majeed, S.; Ali, F. Bacillus coagulans MTCC 5856 for the management of major depression with irritable bowel syndrome: A randomised, double-blind, placebo controlled, multi-centre pilot clinical study. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.J.; Sexton, D.J. Control Measures to Prevent Surgical Site Infection Following Gastrointestinal Procedure in Adults. In UpToDate; Post, T.W., Ed.; UpToDate: Waltham, MA, USA, 2019; Available online: www.uptodate.com (accessed on 19 March 2020).
- Surgical Site Infections: Prevention and Treatment. National Institute for Health and Care Excellence (NICE) Website. April 2019. Available online: https://www.nice.org.uk/guidance/ng125 (accessed on 17 March 2020).


| Follow up after Surgery 1 (Months) | Median | IQR | Minimum | Maximum |
|---|---|---|---|---|
| All (n = 30) | 6.0 | [0.5–24.0] | 0.5 | 112.8 |
| BS and microbiota in humans (n = 26) | 6.0 | [5.3–12.0] | 1.0 | 112.8 |
| BS and probiotics in humans (n = 4) | 4.5 | [2.4–6.0] | 0.5 | 6.0 |
| Sample size | ||||
| All (n = 33) | 24.0 | [14.0–45.0] | 6.0 | 267.0 |
| BS and microbiota in humans (n = 29) | 21.0 | [13.0–43.0] | 6.0 | 267.0 |
| BS and probiotics in humans (n = 4) | 52.0 | [43.0–70.0] | 40.0 | 100.0 |
| Age of Study Population 2 (Years) | ||||
| All (n = 27) | 43.5 | [39.0–47.8] | 23.5 | 51.5 |
| BS and microbiota in humans (n = 23) | 43.5 | [39.0–47.8] | 23.5 | 51.5 |
| BS and probiotics in humans (n = 4) | 43.4 | [40.2–45.7] | 35.1 | 48.0 |
| Sexes of Study Population 3 | Both Sexes | Only Females | Only Males | |
| All (n = 33) | n = 26 | n = 7 | n = 0 | |
| BS and microbiota in humans (n = 29) | n = 22 | n = 7 | n = 0 | |
| BS and probiotics in humans (n = 4) | n = 4 | n = 0 | n = 0 | |
| BMI: Categorization 4,5 | Overweight (25–< 29.9) | Obesity Class 1 (30–< 34.9) | Obesity Class 2 (35–< 39.9) | Obesity Class 3 (≥ 40) |
| All (n = 30) | n = 6 | n = 9 | n = 13 | n = 23 |
| BS and microbiota in humans (n = 26) | n = 6 | n = 9 | n = 13 | n = 19 |
| BS and probiotics in humans (n = 4) | n = 0 | n = 0 | n = 0 | n = 4 |
| Diabetes Status 6 | Only Patients with Diabetes | Patients with and without Diabetes | No Patients with Diabetes | |
| All (n = 24) | n = 4 | n = 15 | n = 5 | |
| BS and microbiota in humans (n = 21) | n = 4 | n = 12 | n = 5 | |
| BS and probiotics in humans (n = 3) | n = 0 | n = 3 | n = 0 | |
| Type of Surgery 7 | RYGB | LSG | AGB | Other 8 |
| All (n = 33) | n = 20 | n = 12 | n = 3 | n = 8 |
| BS and microbiota in humans (n = 29) | n = 18 | n = 10 | n = 3 | n = 7 |
| BS and probiotics in humans (n = 4) | n = 2 | n = 2 | n = 0 | n = 1 |
| Study Length 1 (Weeks) | Median | IQR | Minimum | Maximum |
|---|---|---|---|---|
| BS and microbiota in other vertebrates (n = 23) | 9.0 | [5.3–12.0] | 2.0 | 24.0 |
| Sample size 2 | ||||
| BS and microbiota in other vertebrates (n = 21) | 21.0 | [17.5–30.5] | 6.0 | 100.0 |
| Ages of Animals 3 (Weeks) | ||||
| BS and microbiota in other vertebrates (n = 17) | 8.0 | [6.0–10.0] | 4.0 | 80.0 |
| Sexes of Animals 4 | Both Sexes | Only Females | Only Males | |
| BS and microbiota in other vertebrates (n = 24) | n = 1 | n = 1 | n = 22 | |
| Species of Animal | Rats | Mice | Dogs | |
| BS and microbiota in other vertebrates (n = 25) | n = 19 | n = 5 | n = 1 | |
| Diabetes Status | Only Animals with Diabetes | Animals with and without Diabetes | No Specific Information/Test | |
| BS and microbiota in other vertebrates (n = 25) | n = 6 | n = 4 | n = 15 | |
| Type of Surgery 5 | RYGB | LSG | DJB | Other 6 |
| BS and microbiota in other vertebrates (n = 25) | n = 11 | n = 8 | n = 6 | n = 7 |
| Subgroup 1: Pre-Post BS Comparisons in Humans | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author (Year) | Surgery Type | Alpha D. Richness | Alpha D. Biodiversity | Community Structure | Firmic. | Bactero. | Actinob. | Proteob. | Verruco. | |||||||||
| Campisciano (2017/18) | Bypass/LSG | ↔ | N.R. | N.R. | ↑ | ↓ | ↓ | ↔ | ↓ | ↑ | ↓ | ↔ | ||||||
| Chen (2017) | RYGB | N.R. | N.R. | N.R. | ↔ | ↑ | ↔ | ↔ | ↔ | |||||||||
| Cortez (2018) | DJB | ↑ | ↑ | Dis | ↓ | ↑ | ↔ | ↔ | ↑ | |||||||||
| Damms-Machado (2014) | LSG | N.R. | N.R. | Dis | ↓ | ↑ | ↔ | ↔ | ↔ | |||||||||
| Graessler (2013) | RYGB | N.R. | N.R. | N.R. | ↓ | ↓ | ↓ | ↑ | ↑ | |||||||||
| Kellerer (2019) | LSG | ↑ | ↑ | Sim | ↔ | ↔ | ↔ | ↔ | ↔ | |||||||||
| Kong (2013) | RYGB | ↑ | N.R. | N.R. | N.R. | N.R. | N.R. | N.R. | N.R. | |||||||||
| Lee (2019) | RYGB/AGB | N.R. | N.R. | Sim | ↔ | ↔ | ↑ | ↔ | ↑ | ↔ | ||||||||
| Lin (2019) | LSG | ↑ | N.R. | N.R. | ↔ | ↔ | ↔ | ↔ | ↔ | |||||||||
| Liu R.X (2017) | LSG | ↑ | N.R. | Dis | N.R. | N.R. | N.R. | N.R. | N.R. | |||||||||
| Medina (2017) | RYGB/LSG | N.R. | N.R. | Dis | ↔ | ↑ | ↔ | ↓ | ↑ | ↔ | ↑ | ↔ | ||||||
| Murphy (2017) | RYGB/LSG | ↑ | ↔ | ↑ | ↔ | N.R. | ↑ | ↔ | ↓ | ↑ | ↑ | ↔ | ↔ | ↔ | ||||
| Paganelli (2019) | RYGB/LSG | N.R. | ↔ | Dis | ↔ | ↔ | ↓ | ↑ | ↔ | |||||||||
| Pajecki (2019) | RYGB | ↔ | ↔ | Dis * | ↔ | ↔ | ↔ | ↓ | ↔ | |||||||||
| Palleja (2016) | RYGB | ↑ | ↑ | Dis | ↔ | ↔ | ↔ | ↑ | ↔ | |||||||||
| Palmisano (2019) | RYGB/LSG | ↔ | ↔ | Dis | ↔ | ↔ | ↔ | ↑ | ↔ | ↔ | ||||||||
| Patrone (2016) | BIB | ↓ | ↓ | Dis | ↔ | ↔ | ↔ | ↑ | ↔ | |||||||||
| Sanmiguel (2017) | LSG | ↔ | ↔ | Dis | ↓ | ↔ | ↔ | ↔ | ↔ | |||||||||
| Wang (2019) | RYGB/LSG | ↑ | ↑ | Dis | ↔ | ↔ | ↔ | ↔ | ↔ | |||||||||
| Subgroup 2: BS to sham operation comparisons in other vertebrates | ||||||||||||||||||
| Author (Year) | Surgery Type | Alpha D.Richness | Alpha D.Biodiversity | Community Structure | Firmic. | Bactero. | Actinob. | Proteob. | Verruco. | |||||||||
| Alvarez (2018) | LSG1/LSG2 | ↔ | ↔ | N.R. | ↔ | ↔ | ↔ | ↑ | ↔ | ↔ | ||||||||
| Basso (2016) | GG | ↔ | ↑ | Dis | ↔ | ↔ | ↔ | ↔ | ↔ | |||||||||
| Cummings (2013) | IT | N.R. | N.R. | N.R. | ↔ | ↔ | ↔ | ↔ | ↔ | |||||||||
| Duboc (2017) | RYGB/LSG | ↔ | ↔ | Dis | ↔ | ↔ | ↔ | ↔ | ↔ | |||||||||
| Guo (2016) | RYGB/LSG | ↔ | ↑ | Dis | ↑ | ↓ | ↔ | ↑ | ↔ | ↑ | ↑ | ↔ | ↔ | |||||
| Huang (2014) | LSG | N.R. | N.R. | N.R. | ↔ | ↔ | ↔ | ↔ | ↔ | |||||||||
| Huh (2019) | RYGB/LSG | ↑ | N.R. | Dis | ↓ | ↔ | ↔ | ↑ | ↔ | |||||||||
| Jahansouz (2017) | LSG (A/B) | ↔ | ↔ | Sim | ↓ | ↑ | ↔ | ↓ | ↔ | ↔ | ||||||||
| Jiang (2016) | DJB | N.R. | ↓ | Dis | ↑ | ↓ | ↓ | ↓ | ↑ | |||||||||
| Kashihara (2015) | DJB | N.R. | N.R. | N.R. | ↔ | ↔ | ↔ | ↔ | ↔ | |||||||||
| Kim (2017) | DES | N.R. | N.R. | Sim | ↔ | ↓ | ↔ | ↔ | ↑ | |||||||||
| Li J.V. (2011) | RYGB | N.R. | N.R. | N.R. | ↑ | ↔ | ↔ | ↑ | ↔ | |||||||||
| Li S. (2017) | DJB/LSG | N.R. | N.R. | N.R. | N.R. | N.R. | N.R. | N.R. | N.R. | |||||||||
| Liou (2013) | RYGB | N.R. | N.R. | Dis | ↓ | ↑ | ↔ | ↑ | ↑ | |||||||||
| Liu (2018) | RYGB | N.R. | ↔ | Dis | ↓ | ↑ | ↔ | ↔ | ↔ | |||||||||
| Miyachi (2017) | B-DJB/J-DJB | N.R. | N.R. | N.R. | ↔ | ↔ | ↔ | ↔ | ↔ | |||||||||
| Mukorako (2019) | BPD/DS/DS/LSG | ↔ | ↓ | ↔ | ↓ | ↓ | ↔ | Dis | ↔ | ↔ | ↔ | ↔ | ↔ | |||||
| Shao (2017) | RYGB/LSG | N.R. | ↓ | Dis | Sim | ↔ | ↔ | ↔ | ↑ | ↔ | ↔ | |||||||
| Shao (2018) | LSG | ↑ | ↔ | Dis | ↓ | ↑ | ↔ | ↔ | ↑ | |||||||||
| Wang (2019) | RYGB | N.R. | N.R. | N.R. | ↓ | ↔ | ↔ | ↑ | ↔ | |||||||||
| Zhang (2015) | DJB | ↔ | N.R. | N.R. | ↑ | ↓ | ↔ | ↑ | ↔ | |||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cook, J.; Lehne, C.; Weiland, A.; Archid, R.; Ritze, Y.; Bauer, K.; Zipfel, S.; Penders, J.; Enck, P.; Mack, I. Gut Microbiota, Probiotics and Psychological States and Behaviors after Bariatric Surgery—A Systematic Review of Their Interrelation. Nutrients 2020, 12, 2396. https://doi.org/10.3390/nu12082396
Cook J, Lehne C, Weiland A, Archid R, Ritze Y, Bauer K, Zipfel S, Penders J, Enck P, Mack I. Gut Microbiota, Probiotics and Psychological States and Behaviors after Bariatric Surgery—A Systematic Review of Their Interrelation. Nutrients. 2020; 12(8):2396. https://doi.org/10.3390/nu12082396
Chicago/Turabian StyleCook, Jessica, Christine Lehne, Alisa Weiland, Rami Archid, Yvonne Ritze, Kerstin Bauer, Stephan Zipfel, John Penders, Paul Enck, and Isabelle Mack. 2020. "Gut Microbiota, Probiotics and Psychological States and Behaviors after Bariatric Surgery—A Systematic Review of Their Interrelation" Nutrients 12, no. 8: 2396. https://doi.org/10.3390/nu12082396
APA StyleCook, J., Lehne, C., Weiland, A., Archid, R., Ritze, Y., Bauer, K., Zipfel, S., Penders, J., Enck, P., & Mack, I. (2020). Gut Microbiota, Probiotics and Psychological States and Behaviors after Bariatric Surgery—A Systematic Review of Their Interrelation. Nutrients, 12(8), 2396. https://doi.org/10.3390/nu12082396








