Low Vitamin C Status in Patients with Cancer Is Associated with Patient and Tumor Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Ethics and Patient Consent
2.2. Eligibility Criteria
2.3. Study Populations
2.4. Blood Sample Collection and Processing
2.5. Ascorbate Measurement in Plasma
2.6. Health Data and Dietary Intake Assessment
2.7. Statistical Analyses
3. Results
3.1. Characteristics of the Two Cohorts of Patients with Cancer
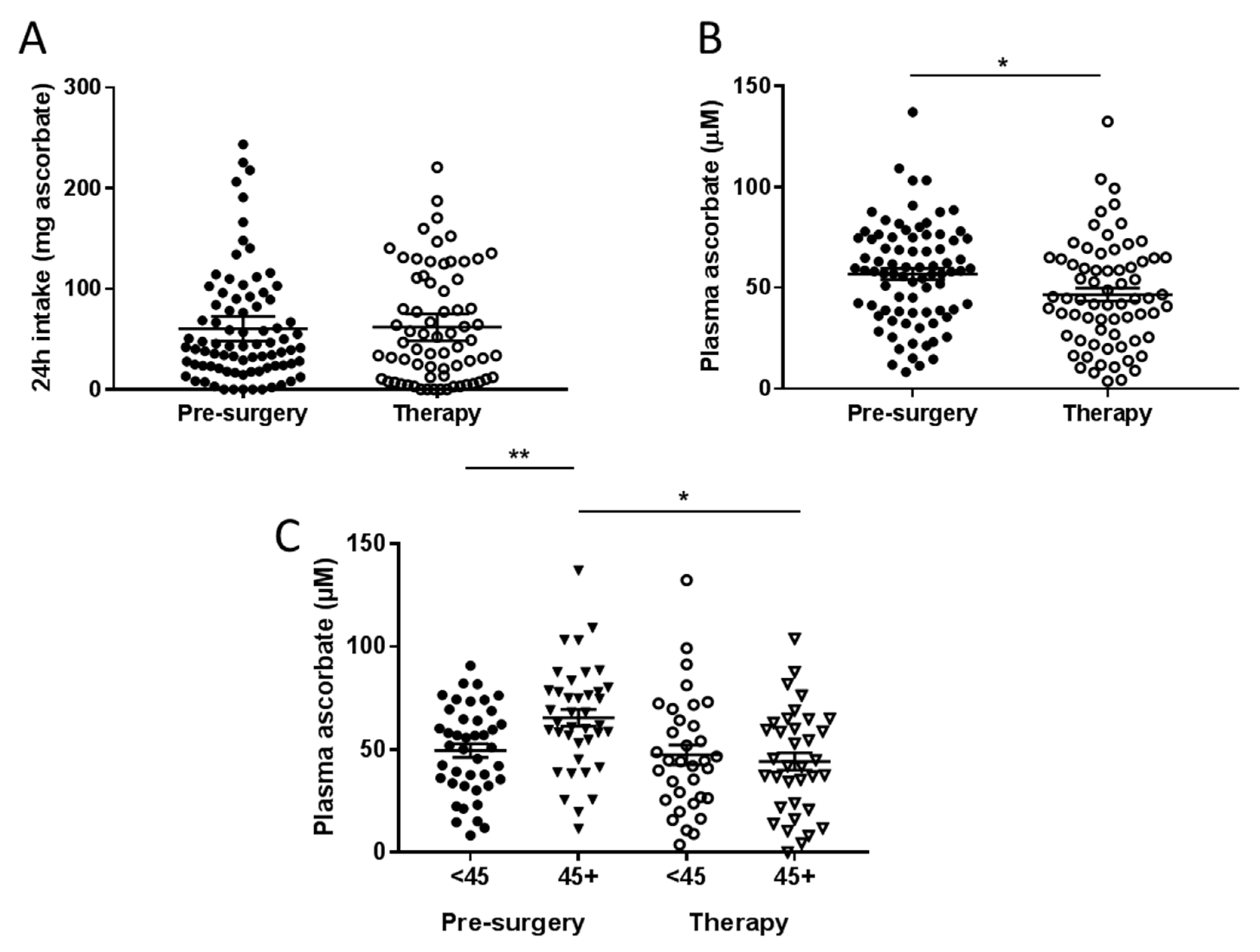
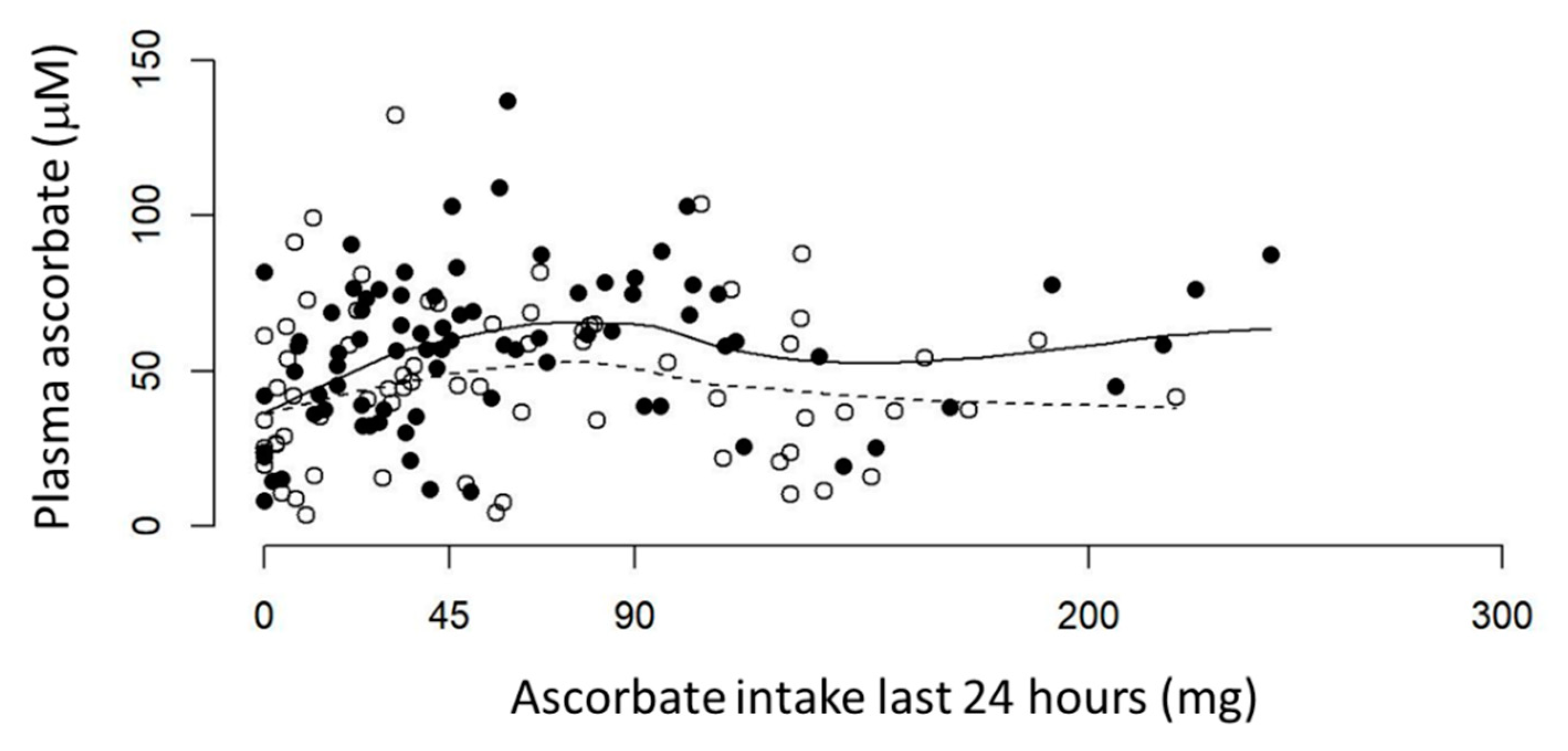
3.2. Ascorbate Intake versus Plasma Ascorbate Status of Fasting Patients with Cancer
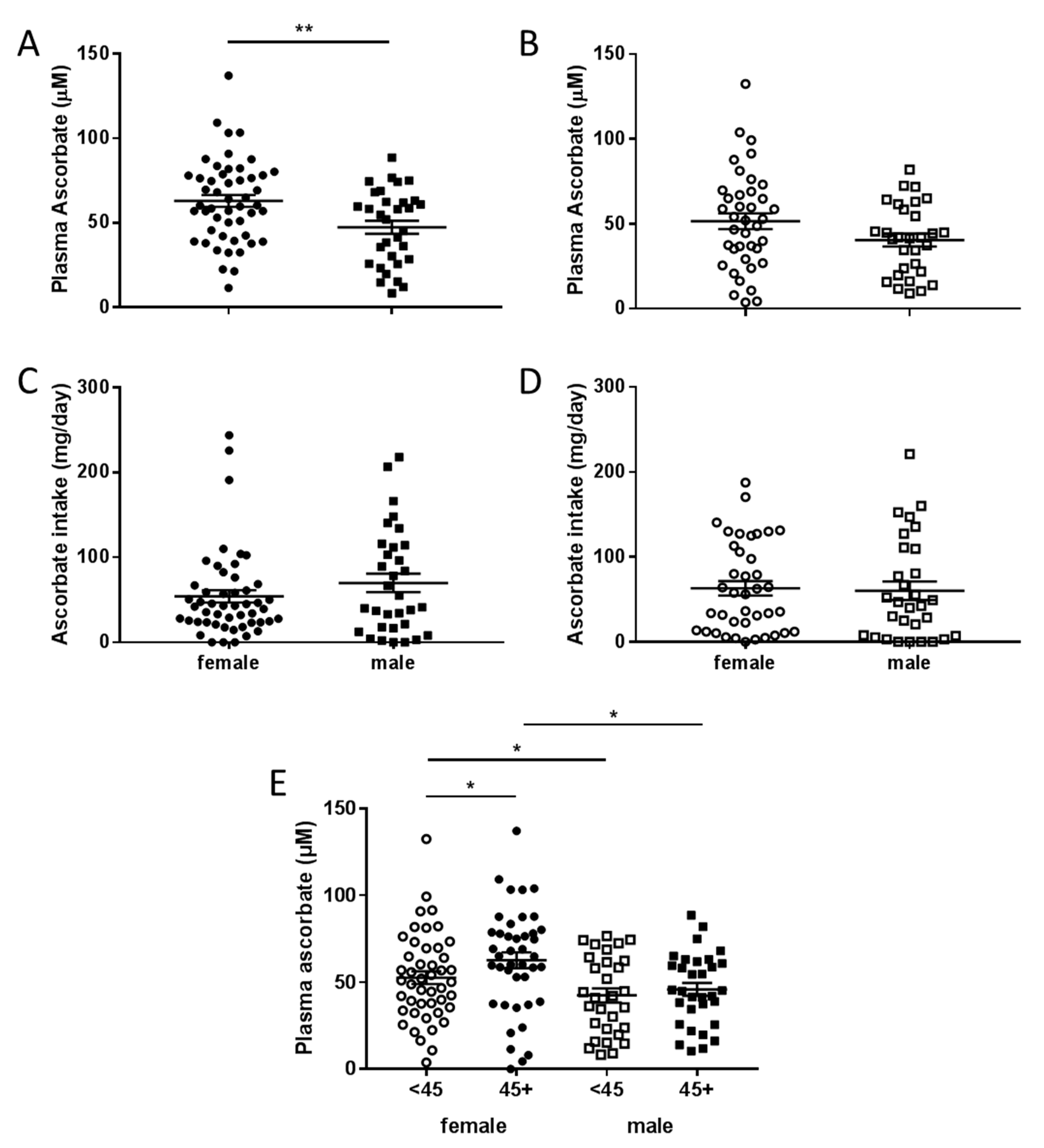
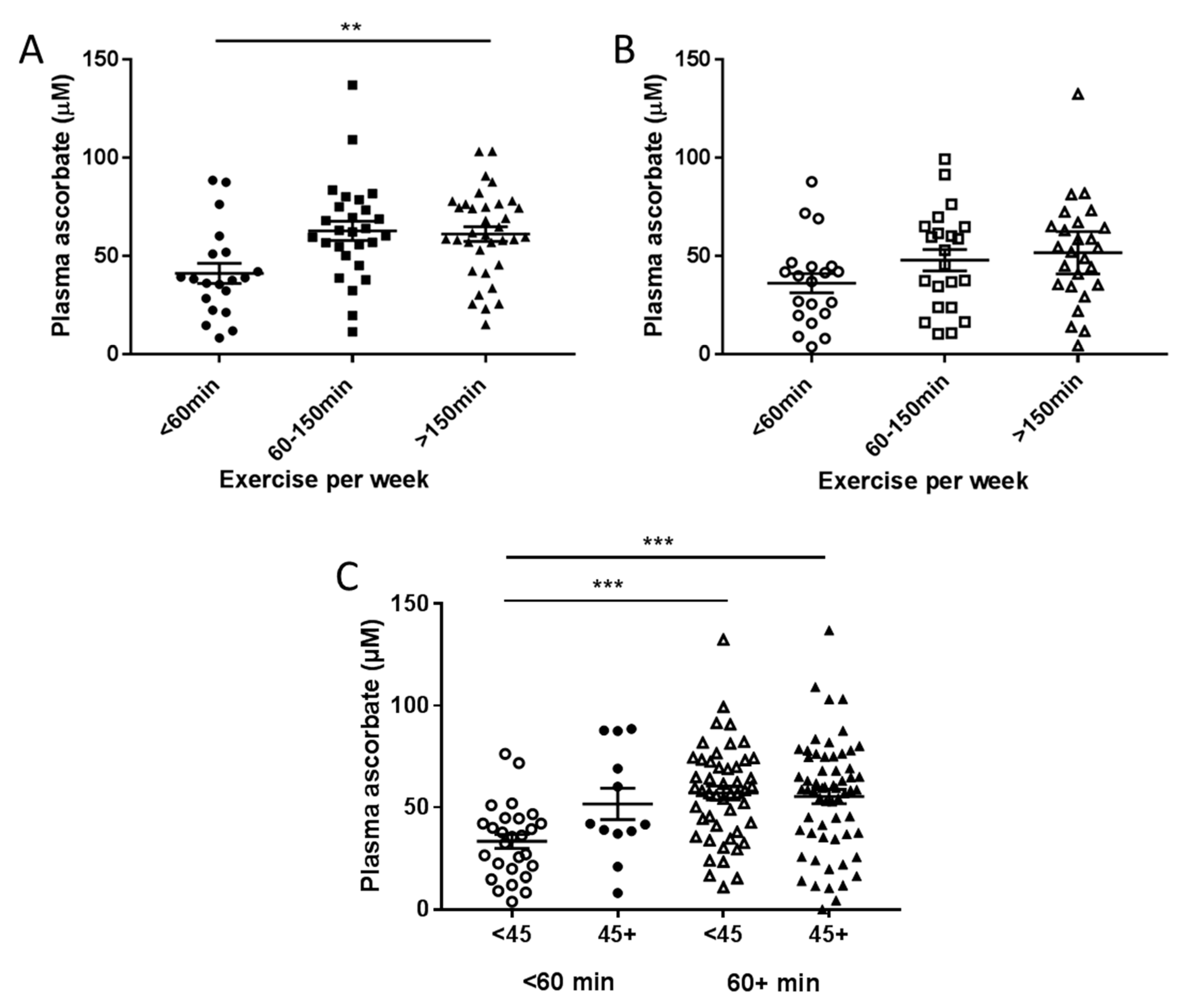
3.3. Associations between Plasma Concentrations of Ascorbate and Patient Characteristics
3.4. Modeling of Associations with Plasma Ascorbate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wilson, M.K.; Baguley, B.C.; Wall, C.; Jameson, M.B.; Findlay, M.P. Review of high-dose intravenous vitamin C as an anticancer agent. Asia Pac. J. Clin. Oncol. 2014, 10, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Ngo, B.; Van Riper, J.M.; Cantley, L.C.; Yun, J. Targeting cancer vulnerabilities with high-dose vitamin C. Nat. Rev. Cancer 2019, 19, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Sun, A.Y.; Chen, Q.; Espey, M.G.; Drisko, J.; Levine, M. Vitamin C: Intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS ONE 2010, 5, e11414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dachs, G.U.; Munn, D.G.; Carr, A.C.; Vissers, M.C.; Robinson, B.A. Consumption of vitamin C is below recommended daily intake in many cancer patients and healthy volunteers in Christchurch. N. Z. Med. J. 2014, 127, 73–76. [Google Scholar] [PubMed]
- van Gorkom, G.N.Y.; Lookermans, E.L.; Van Elssen, C.H.M.J.; Bos, G.M.J. The Effect of Vitamin C (Ascorbic Acid) in the Treatment of Patients with Cancer: A Systematic Review. Nutrients 2019, 11, 977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef] [Green Version]
- Nutrient Reference Values for Australia and New Zealand. Available online: https://www.nrv.gov.au/nutrients/vitamin-c (accessed on 2 July 2020).
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press: Washington, DC, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK225480/ (accessed on 2 July 2020).
- German Nutrition Society (DGE): New Reference Values for Vitamin C Intake. Ann. Nutr. Metab. 2015, 67, 13–20. [CrossRef]
- Smirnoff, N. Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free Radic. Biol. Med. 2018, 122, 116–129. [Google Scholar] [CrossRef]
- Vissers, M.C.; Kuiper, C.; Dachs, G.U. Regulation of the 2-oxoglutarate-dependent dioxygenases and implications for cancer. Biochem. Soc. Trans. 2014, 42, 945–951. [Google Scholar] [CrossRef]
- Kuiper, C.; Molenaar, I.G.; Dachs, G.U.; Currie, M.J.; Sykes, P.H.; Vissers, M.C. Low ascorbate levels are associated with increased hypoxia-inducible factor-1 activity and an aggressive tumor phenotype in endometrial cancer. Cancer Res. 2010, 70, 5749–5758. [Google Scholar] [CrossRef] [Green Version]
- Kuiper, C.; Dachs, G.U.; Munn, D.; Currie, M.J.; Robinson, B.A.; Pearson, J.F.; Vissers, M.C. Increased tumor ascorbate is associated with extended disease-free survival and decreased hypoxia-inducible factor-1 activation in human colorectal cancer. Front. Oncol. 2014, 4, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jóźwiak, P.; Ciesielski, P.; Zaczek, A.; Lipińska, A.; Pomorski, L.; Wieczorek, M.; Bryś, M.; Forma, E.; Krześlak, A. Expression of hypoxia inducible factor 1α and 2α and its association with vitamin C level in thyroid lesions. J. Biomed. Sci. 2017, 24, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wohlrab, C.; Vissers, M.C.M.; Phillips, E.; Morrin, H.; Robinson, B.A.; Dachs, G.U. The association between ascorbate and the hypoxia-inducible factors in human renal cell carcinoma requires a functional von Hippel-Lindau protein. Front. Oncol. 2018, 8, 574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, E.J.; Dachs, G.U.; Morrin, H.R.; Davey, V.C.; Robinson, B.A.; Vissers, M.C.M. Activation of the hypoxia pathway in breast cancer tissue and patient survival are inversely associated with tumor ascorbate levels. BMC Cancer 2019, 19, 307. [Google Scholar] [CrossRef] [PubMed]
- Marcus, S.L.; Dutcher, J.P.; Paietta, E.; Ciobanu, N.; Strauman, J.; Wiernik, P.H.; Hutner, S.H.; Frank, O.; Baker, H. Severe hypovitaminosis C occurring as the result of adoptive immunotherapy with high-dose interleukin 2 and lymphokine-activated killer cells. Cancer Res. 1987, 47, 4208–4212. [Google Scholar] [PubMed]
- Khanzode, S.S.; Khanzode, S.D.; Dakhale, G.N. Serum and plasma concentration of oxidant and antioxidants in patients of Helicobacter pylori gastritis and its correlation with gastric cancer. Cancer Lett. 2003, 195, 27–31. [Google Scholar] [CrossRef]
- Marakala, V.; Malathi, M.; Shivashankara, A.R. Lipid peroxidation and antioxidant vitamin status in oral cavity and oropharyngeal cancer patients. Asian Pac. J. Cancer Prev. 2012, 13, 5763–5765. [Google Scholar] [CrossRef] [Green Version]
- Mayland, C.; Allen, K.R.; Degg, T.J.; Bennet, M. Micronutrient concentrations in patients with malignant disease: Effect of the inflammatory response. Ann. Clin. Biochem. 2004, 41 Pt 2, 138–141. [Google Scholar] [CrossRef]
- Gackowski, D.; Kowalewski, J.; Siomek, A.; Olinski, R. Oxidative DNA damage and antioxidant vitamin level: Comparison among lung cancer patients, healthy smokers and nonsmokers. Int. J. Cancer 2005, 114, 153–156. [Google Scholar] [CrossRef]
- Mayland, C.R.; Bennett, M.I.; Allan, K. Vitamin C deficiency in cancer patients. Palliat. Med. 2005, 19, 17–20. [Google Scholar] [CrossRef]
- Levine, M.; Wang, Y.; Rumsey, S.C. Analysis of ascorbic acid and dehydroascorbic acid in biological samples. Methods Enzymol. 1999, 299, 65–76. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Tveden-Nyborg, P. The Pharmacokinetics of Vitamin C. Nutrients 2019, 11, 2412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, J.F.; Pullar, J.M.; Wilson, R.; Spittlehouse, J.K.; Vissers, M.C.M.; Skidmore, P.M.L.; Willis, J.; Cameron, V.A.; Carr, A.C. Vitamin C status correlates with markers of metabolic and cognitive health in 50-year-olds: Findings of the CHALICE cohort study. Nutrients 2017, 9, 831. [Google Scholar] [CrossRef] [PubMed]
- Eating and Activity Guidelines. Available online: https://www.health.govt.nz/our-work/eating-and-activity-guidelines (accessed on 2 July 2020).
- Food Data Central, U.S. Department of Agriculture. Available online: https://fdc.nal.usda.gov (accessed on 2 July 2020).
- Carr, A.C.; Bozonet, S.M.; Vissers, M.C. A randomised cross-over pharmacokinetic bioavailability study of synthetic versus kiwifruit-derived vitamin C. Nutrients 2013, 5, 4451–4461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, M.; Padayatty, S.J.; Espey, M.G. Vitamin C: A concentration-function approach yields pharmacology and therapeutic discoveries. Adv. Nutr. 2011, 2, 78–88. [Google Scholar] [CrossRef] [Green Version]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: A systematic review and dose-response meta-analysis of prospective studies. Am. J. Clin. Nutr. 2018, 108, 1069–1091. [Google Scholar] [CrossRef]
- Ang, A.; Pullar, J.M.; Currie, M.J.; Vissers, M.C.M. Vitamin C and immune cell function in inflammation and cancer. Biochem. Soc. Trans. 2018, 46, 1147–1159. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Robitaille, L.; Eintracht, S.; Hoffer, L.J. Vitamin C provision improves mood in acutely hospitalized patients. Nutrition 2011, 27, 530–533. [Google Scholar] [CrossRef]
- Nechuta, S.; Lu, W.; Chen, Z.; Zheng, Y.; Gu, K.; Cai, H.; Zheng, W.; Shu, X.O. Vitamin supplement use during breast cancer treatment and survival: A prospective cohort study. Cancer Epidemiol. Biomarkers Prev. 2011, 20, 262–271. [Google Scholar] [CrossRef] [Green Version]
- Poole, E.M.; Shu, X.; Caan, B.J.; Flatt, S.W.; Holmes, M.D.; Lu, W.; Kwan, M.L.; Nechuta, S.J.; Pierce, J.P.; Chen, W.Y. Postdiagnosis supplement use and breast cancer prognosis in the After Breast Cancer Pooling Project. Breast Cancer Res. Treat 2013, 139, 529–537. [Google Scholar] [CrossRef] [Green Version]
- Huijskens, M.J.; Wodzig, W.K.; Walczak, M.; Germeraad, W.T.; Bos, G.M. Ascorbic acid serum levels are reduced in patients with hematological malignancies. Results Immunol. 2016, 6, 8–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gacitúa, T.A.; Sotomayor, C.G.; Groothof, D.; Eisenga, M.F.; Pol, R.A.; Borst, M.H.; Gans, R.O.B.; Berger, S.P.; Rodrigo, R.; Navis, G.J.; et al. Plasma Vitamin C and cancer mortality in kidney transplant recipients. J. Clin. Med. 2019, 8, 2064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackenzie, K.A.; Miller, A.P.; Hock, B.D.; Gardner, J.; Simcock, J.W.; Roake, J.A.; Dachs, G.U.; Robinson, B.A.; Currie, M.J. Angiogenesis and host immune response contribute to the aggressive character of non-melanoma skin cancers in renal transplant recipients. Histopathology 2011, 58, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Yokoyama, T.; Yoshida, H.; Kim, H.; Shimada, H.; Yoshida, Y.; Iwasa, H.; Shimizu, Y.; Kondo, Y.; Handa, S.; et al. A significant relationship between plasma vitamin C concentration and physical performance among Japanese elderly women. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 295–301. [Google Scholar] [CrossRef]
- McCall, S.J.; Clark, A.B.; Luben, R.N.; Wareham, N.J.; Khaw, K.T.; Myint, P.K. Plasma Vitamin C levels: Risk factors for deficiency and association with self-reported functional health in the European Prospective Investigation into Cancer-Norfolk. Nutrients 2019, 11, 1552. [Google Scholar] [CrossRef] [Green Version]
- Wilson, R.; Willis, J.; Gearry, R.; Skidmore, P.; Fleming, E.; Frampton, C.; Carr, A. Inadequate vitamin C status in prediabetes and type 2 diabetes mellitus: Associations with glycaemic control, obesity, and smoking. Nutrients 2017, 9, 997. [Google Scholar] [CrossRef]
- Johnston, C.S.; Barkyoumb, G.M.; Schumacher, S.S. Vitamin C supplementation slightly improves physical activity levels and reduces cold incidence in men with marginal vitamin C status: A randomized controlled trial. Nutrients 2014, 6, 2572–2583. [Google Scholar] [CrossRef]
- Levine, M.; Wang, Y.; Padayatty, S.J.; Morrow, J. A new recommended dietary allowance of vitamin C for healthy young women. Proc. Natl. Acad. Sci. USA 2001, 98, 9842–9846. [Google Scholar] [CrossRef] [Green Version]
- Carr, A.C.; Frei, B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am. J. Clin. Nutr. 1999, 69, 1086–1107. [Google Scholar] [CrossRef] [Green Version]
- Kapil, U.; Singh, P.; Bahadur, S.; Shukla, N.K.; Dwivedi, S.; Pathak, P.; Singh, R. Association of vitamin A, vitamin C and zinc with laryngeal cancer. Indian J. Cancer 2003, 40, 67–70. [Google Scholar]
| Pre-Surgical Cohort n = 81 (%) | Therapy Cohort n = 69 (%) | Effect Size [95% CI] | p Value | ||
|---|---|---|---|---|---|
| Age (years) | Mean (±SD) | 63.88 (±12.08) | 58.71 (±13.76) | 5.17 [0.95,9.38] | 0.017 |
| Gender | Female | 49 (60) | 39 (57) | ||
| Male | 32 (40) | 30 (43) | 1.18 [0.61,2.26] | 0.740 | |
| Ethnicity | European | 72 (89) | 63 (91) | ||
| Māori/Pacifica | 9 (11) | 6 (9) | 0.76 [0.26,2.26] | 0.786 | |
| BMI (kg/m2) | Mean (±SD) | 30.50 (±7.17) | 28.62 (±7.13) | 1.88 [−0.45,4.20] | 0.112 |
| Smoking | never | 42 (52) | 35 (51) | ||
| ex | 35 (43) | 25 (36) | 0.86 [0.43,1.69] | 0.730 | |
| current | 4 (5) | 9 (13) | 2.70 [0.77,9.52] | 0.140 | |
| Exercise (min/week) | >150 | 35 (43) | 26 (38) | ||
| 60–150 | 27 (33) | 22 (32) | 1.10 [0.51,2.34] | 0.848 | |
| <60 | 19 (23) | 20 (29) | 1.42 [0.63,3.18] | 0.418 | |
| Stage | TNM 1–3 | 67 (83) | 30 (43) | ||
| TNM 4, recurrence | 12 (15) | 39 (57) | 7.26 [3.34,15.79] | 1.30 × 10−7 | |
| Ascorbate intake (mg/day) | <45 | 42 (52) | 34 (49) | ||
| 45–90 | 19 (23) | 15 (22) | 0.98 [0.43,2.20] | 1.000 | |
| ≥90 | 20 (25) | 20 (29) | 1.24 [0.57,2.66] | 0.696 | |
| Supplement | No | 66 (81) | 56 (81) | ||
| Yes | 15 (19) | 13 (19) | 1.02 [0.45,2.33] | 1.000 |
| Plasma Ascorbate | Pre-Surgical Cohort | Therapy Cohort | OR [95% CI] | p-Value |
|---|---|---|---|---|
| n = 81 (%) | n = 69 (%) | |||
| >50 μM | 53 (65.4) | 29 (42.0) | 1 | |
| 23–50 μM | 20 (24.7) | 26 (37.7) | 2.38 [1.14, 4.97] | 0.026 |
| <23 μM | 8 (9.9) | 14 (20.3) | 3.20 [1.20, 8.52] | 0.027 |
| Estimate | 95% CI | p-Value | ||
|---|---|---|---|---|
| Age | 50–70 | 3.39 | [−7.41,14.19] | 0.415 |
| 70+ | −3.21 | [−15.49,9.07] | ||
| Gender | Male | −13.97 | [−22.14,−5.79] | 0.001 |
| BMI | 30–40 | 2.63 | [−6.68,11.93] | 0.719 |
| <18.5 or >40 | −3.57 | [−18.71,11.56] | ||
| Smoking | ex | −5.69 | [−14.46,3.07] | 0.272 |
| current | −9.90 | [−25.16,5.37] | ||
| Ethnicity | Māori/Pacifica | 3.19 | [−10.73,17.11] | 0.651 |
| Exercise | 60–150 | −1.09 | [−10.37,8.20] | 0.001 |
| <60 | −18.24 | [−28.15,−8.32] | ||
| Ascorbate Intake | 45–90 | 9.76 | [−1.96,21.47] | 0.049 |
| <45 | −3.10 | [−13.01,6.81] | ||
| Supplementation | yes | 12.32 | [1.79,22.86] | 0.022 |
| Tumor Stage | TNM 1–3 | −7.16 | [−18.29,3.97] | 0.084 |
| TNM 4, recurrent | −10.42 | [−19.83,−1.00] | ||
| Cohort | Treatment | −10.43 | [−18.64,−2.22] | 0.013 |
| Predictor | Level | Effect | 95% CI | p-Value |
|---|---|---|---|---|
| Gender | Male | −12.31 | [−19.73,−4.89] | 0.0013 |
| Ascorbate Intake | >45 mg | 29.74 | [12.47,47.01] | 0.0009 |
| Exercise | >60 min | 22.08 | [11.40,32.77] | 0.0001 |
| Ascorbate Intake × Exercise | >45 mg and >60 min | −19.93 | [−37.62,−2.25] | 0.0275 |
| Cohort | Therapy | 0.02 | [−10.24,10.28] | 0.9968 |
| Ascorbate Intake × Cohort | >45 mg and Therapy | −20.83 | [−35.47,−6.18] | 0.0056 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
White, R.; Nonis, M.; Pearson, J.F.; Burgess, E.; Morrin, H.R.; Pullar, J.M.; Spencer, E.; Vissers, M.C.M.; Robinson, B.A.; Dachs, G.U. Low Vitamin C Status in Patients with Cancer Is Associated with Patient and Tumor Characteristics. Nutrients 2020, 12, 2338. https://doi.org/10.3390/nu12082338
White R, Nonis M, Pearson JF, Burgess E, Morrin HR, Pullar JM, Spencer E, Vissers MCM, Robinson BA, Dachs GU. Low Vitamin C Status in Patients with Cancer Is Associated with Patient and Tumor Characteristics. Nutrients. 2020; 12(8):2338. https://doi.org/10.3390/nu12082338
Chicago/Turabian StyleWhite, Rebecca, Maria Nonis, John F. Pearson, Eleanor Burgess, Helen R. Morrin, Juliet M. Pullar, Emma Spencer, Margreet C. M. Vissers, Bridget A. Robinson, and Gabi U. Dachs. 2020. "Low Vitamin C Status in Patients with Cancer Is Associated with Patient and Tumor Characteristics" Nutrients 12, no. 8: 2338. https://doi.org/10.3390/nu12082338
APA StyleWhite, R., Nonis, M., Pearson, J. F., Burgess, E., Morrin, H. R., Pullar, J. M., Spencer, E., Vissers, M. C. M., Robinson, B. A., & Dachs, G. U. (2020). Low Vitamin C Status in Patients with Cancer Is Associated with Patient and Tumor Characteristics. Nutrients, 12(8), 2338. https://doi.org/10.3390/nu12082338







