Urinary Metabolomic Profile of Preterm Infants Receiving Human Milk with Either Bovine or Donkey Milk-Based Fortifiers
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Trial and Intervention
2.2. Study Population
- ▪ Bovine—Human Milk Fortifier (B-HMF) Group (n = 27) receiving ADJ fortification with commercial multi-component fortifier (FM85 Nestlè) and protein concentrate (Protifar Nutricia), derived from bovine milk, for a minimum of 21 days;
- ▪ Donkey—Human Milk Fortifier (D-HMF) Group (n = 27) receiving ADJ fortification with multi-component fortifier and protein concentrate derived from donkey milk, not commercially available, and prepared according to current EU legislation on foods for special medical purposes, for a minimum of 21 days.
2.3. Urine Sample Collection
2.4. Sample Preparation
2.5. H NMR Measurements and Data Processing
2.6. Statistical Analyses
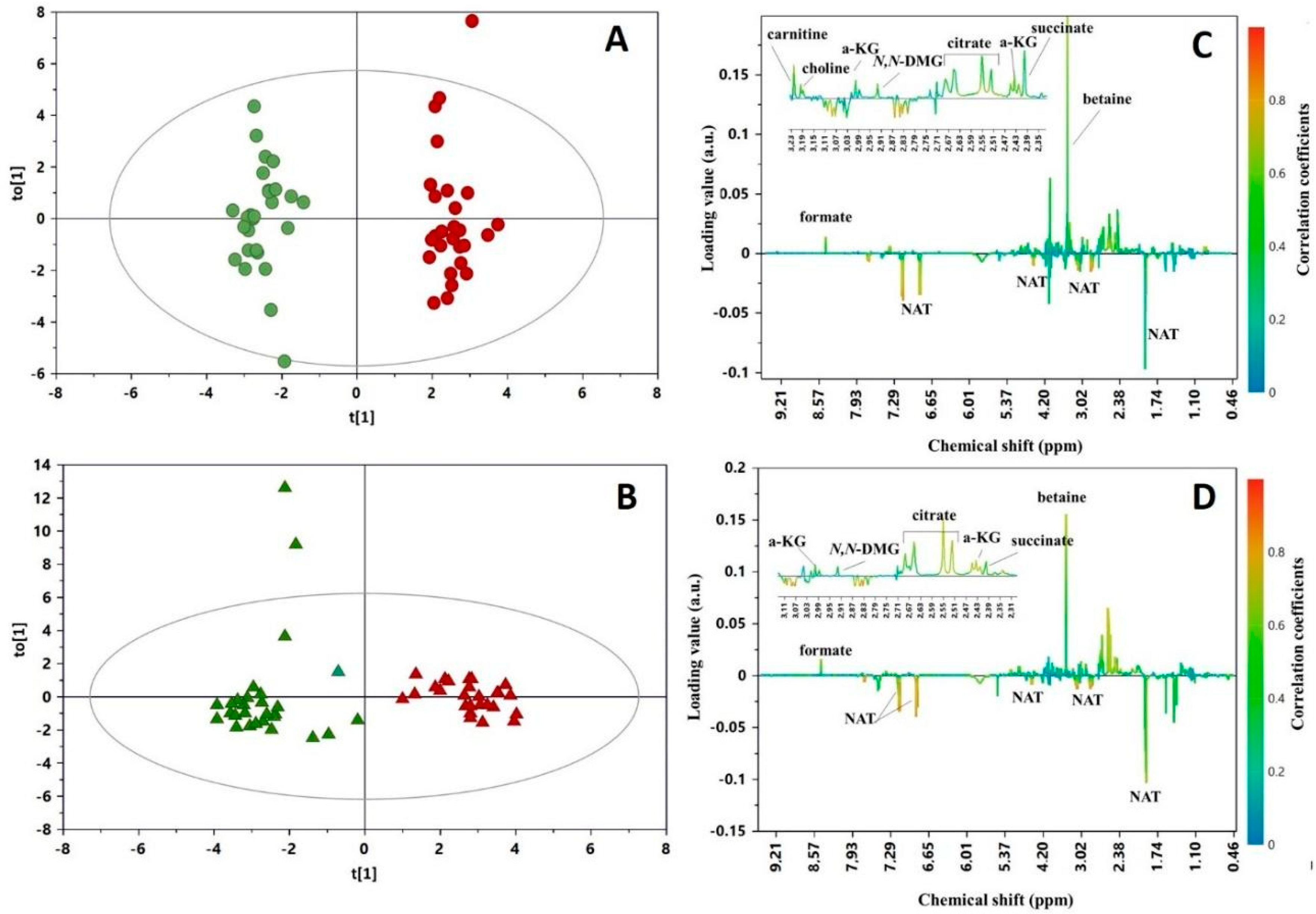
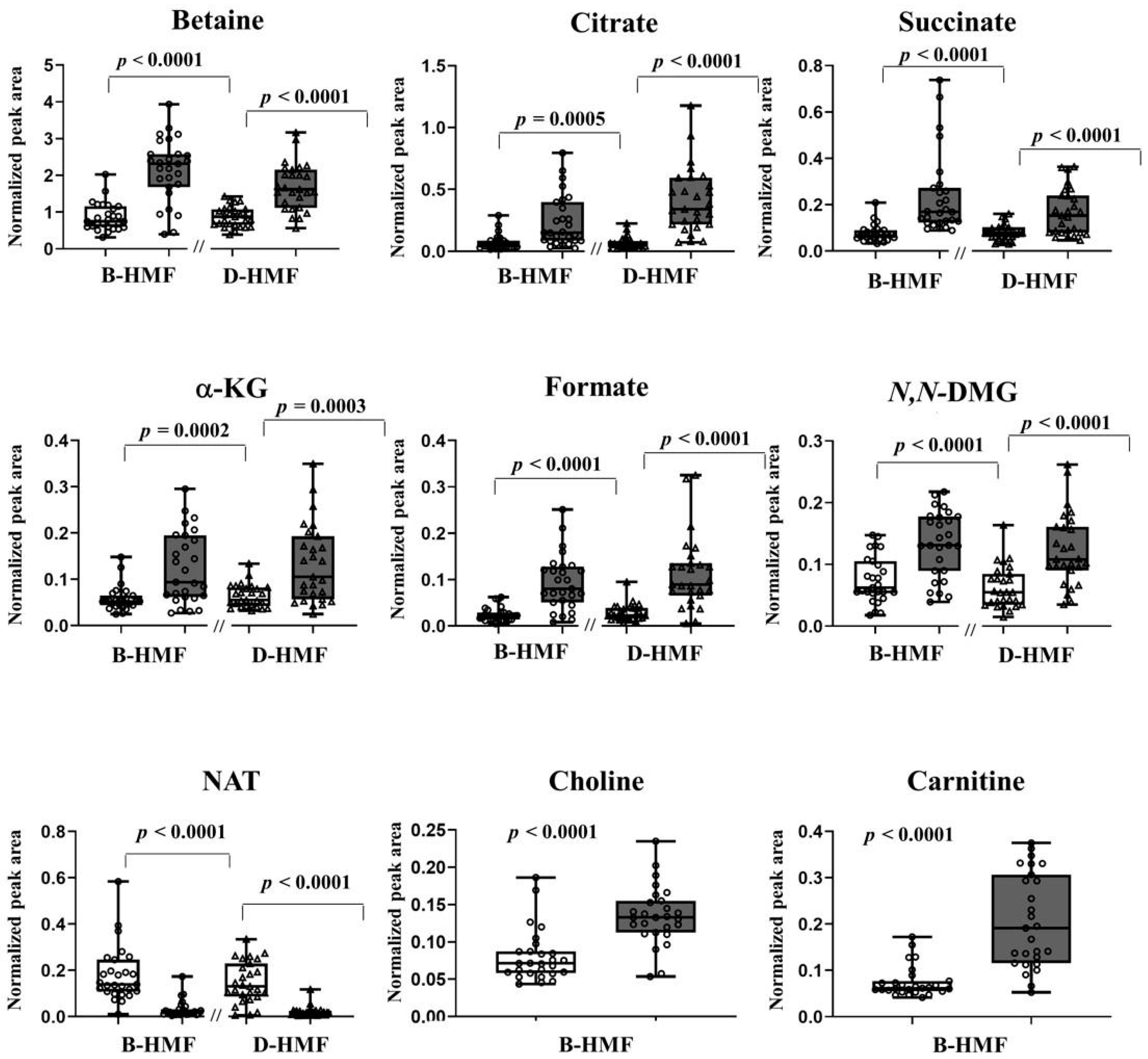
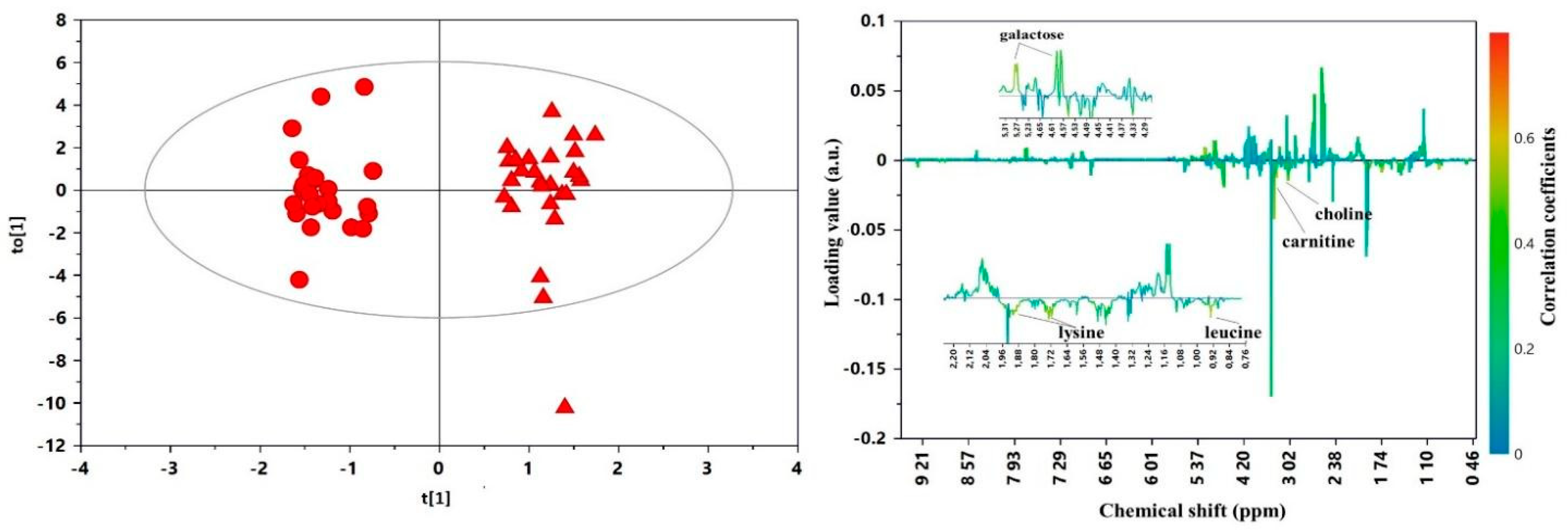
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Arslanoglu, S.; Boquien, C.Y.; King, C.; Lamireau, D.; Tonetto, P.; Barnett, D.; Bertino, E.; Gaya, A.; Gebauer, C.; Grovslien, A.; et al. Fortification of human milk for preterm infants: Update and recommendations of the European Milk Bank Association (EMBA) Working Group on Human Milk Fortification. Front. Pediatr. 2019, 7. [Google Scholar] [CrossRef]
- Chan, S.H.T.; Johnson, M.J.; Leaf, A.A.; Vollmer, B. Nutrition and neurodevelopmental outcomes in preterm infants: A systematic review. Acta Paediatr. 2016, 105, 587–599. [Google Scholar] [CrossRef]
- Ong, K.K.; Kennedy, K.; Castañeda-Gutiérrez, E.; Forsyth, S.; Godfrey, K.M.; Koletzko, B.; Latulippe, M.E.; Ozanne, S.E.; Rueda, R.; Schoemaker, M.H.; et al. Postnatal growth in preterm infants and later health outcomes: A systematic review. Acta Paediatr. 2015, 104, 974–986. [Google Scholar] [CrossRef]
- Kumar, R.K.; Singhal, A.; Vaidya, U.; Banerjee, S.; Anwar, F.; Rao, S. Optimizing nutrition in preterm low birth weight infants—Consensus summary. Front. Nutr. 2017, 4. [Google Scholar] [CrossRef]
- Kemp, J.E.; Wenhold, F.A.M. Human milk fortification strategies for improved in-hospital growth of preterm infants. S. Afr. J. Clin. Nutr. 2016, 29, 157–164. [Google Scholar] [CrossRef][Green Version]
- Wagner, J.; Hanson, C.; Anderson-Berry, A. Considerations in meeting protein needs of the human milk-fed preterm infant. Adv. Neonatal Care 2014, 14, 281–289. [Google Scholar] [CrossRef]
- Lenz, E.M.; Bright, J.; Knight, R.; Wilson, I.D.; Major, H. A metabonomic investigation of the biochemical effects of mercuric chloride in the rat using 1H NMR and HPLC-TOF/MS: Time dependant changes in the urinary profile of endogenous metabolites as a result of nephrotoxicity. Analyst 2004, 129, 535–541. [Google Scholar] [CrossRef]
- Solanky, K.S.; Bailey, N.J.C.; Holmes, E.; Lindon, J.C.; Davis, A.L.; Mulder, T.P.J.; Van Duynhoven, J.P.M.; Nicholson, J.K. NMR-based metabonomic studies on the biochemical effects of epicatechin in the rat. J. Agric. Food Chem. 2003, 51, 4139–4145. [Google Scholar] [CrossRef]
- Stella, C.; Beckwith-Hall, B.; Cloarec, O.; Holmes, E.; Lindon, J.C.; Powell, J.; Van Der Ouderaa, F.; Bingham, S.; Cross, A.J.; Nicholson, J.K. Susceptibility of human metabolic phenotypes to dietary modulation. J. Proteome Res. 2006, 5, 2780–2788. [Google Scholar] [CrossRef]
- Walsh, M.C.; Brennan, L.; Pujos-Guillot, E.; Sébédio, J.L.; Scalbert, A.; Fagan, A.; Higgins, D.G.; Gibney, M.J. Influence of acute phytochemical intake on human urinary metabolomic profiles. Am. J. Clin. Nutr. 2007, 86, 1687–1693. [Google Scholar] [CrossRef]
- Johnson, M.J.; Wootton, S.A.; Leaf, A.A.; Jackson, A.A. Preterm birth and body composition at term equivalent age: A systematic review and meta-analysis. Pediatrics 2012, 130, e640–e649. [Google Scholar] [CrossRef] [PubMed]
- Ulaszewska, M.M.; Weinert, C.H.; Trimigno, A.; Portmann, R.; Andres Lacueva, C.; Badertscher, R.; Brennan, L.; Brunius, C.; Bub, A.; Capozzi, F.; et al. Nutrimetabolomics: An integrative action for metabolomic analyses in human nutritional studies. Mol. Nutr. Food Res. 2019, 63, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Moltu, S.J.; Sachse, D.; Blakstad, E.W.; Strømmen, K.; Nakstad, B.; Almaas, A.N.; Westerberg, A.C.; Rønnestad, A.; Brække, K.; Veierød, M.B.; et al. Urinary metabolite profiles in premature infants show early postnatal metabolic adaptation and maturation. Nutrients 2014, 6, 1913–1930. [Google Scholar] [CrossRef] [PubMed]
- Cesare Marincola, F.; Corbu, S.; Lussu, M.; Noto, A.; Dessì, A.; Longo, S.; Civardi, E.; Garofoli, F.; Grenci, B.; Mongini, E.; et al. Impact of early postnatal nutrition on the NMR urinary metabolic profile of infant. J. Proteome Res. 2016, 15, 3712–3723. [Google Scholar] [CrossRef] [PubMed]
- Alexandre-Gouabau, M.C.; Moyon, T.; David-Sochard, A.; Fenaille, F.; Cholet, S.; Royer, A.L.; Guitton, Y.; Billard, H.; Darmaun, D.; Rozé, J.C.; et al. Comprehensive preterm breast milk metabotype associated with optimal infant early growth pattern. Nutrients 2019, 11, 528. [Google Scholar] [CrossRef] [PubMed]
- Morniroli, D.; Dessì, A.; Giannì, M.L.; Roggero, P.; Noto, A.; Atzori, L.; Lussu, M.; Fanos, V.; Mosca, F. Is the body composition development in premature infants associated with a distinctive nuclear magnetic resonance metabolomic profiling of urine? J. Matern. Neonatal Med. 2019, 32, 2310–2318. [Google Scholar] [CrossRef] [PubMed]
- Shoji, H.; Shimizu, T. Effect of human breast milk on biological metabolism in infants. Pediatr. Int. 2019, 61, 6–15. [Google Scholar] [CrossRef]
- Bertino, E.; Cavallarin, L.; Cresi, F.; Tonetto, P.; Peila, C.; Ansaldi, G.; Raia, M.; Varalda, A.; Giribaldi, M.; Conti, A.; et al. A novel donkey milk-derived human milk fortifier in feeding preterm infants: A randomized controlled trial. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 116–123. [Google Scholar] [CrossRef]
- Bertino, E.; Gastaldi, D.; Monti, G.; Baro, C.; Fortunato, D.; Garoffo, L.P.; Coscia, A.; Fabris, C.; Mussap, M.; Conti, A. Detailed proteomic analysis on DM: Insight into its hypoallergenicity. Front. Biosci. 2010, 2, 526–536. [Google Scholar] [CrossRef]
- Coscia, A.; Bertino, E.; Tonetto, P.; Peila, C.; Cresi, F.; Arslanoglu, S.; Moro, G.E.; Spada, E.; Milani, S.; Giribaldi, M.; et al. Nutritional adequacy of a novel human milk fortifier from donkey milk in feeding preterm infants: Study protocol of a randomized controlled clinical trial. Nutr. J. 2018, 17, 1–7. [Google Scholar] [CrossRef]
- Scalabre, A.; Jobard, E.; Demède, D.; Gaillard, S.; Pontoizeau, C.; Mouriquand, P.; Elena-Herrmann, B.; Mure, P.Y. Evolution of newborns’ urinary metabolomic profiles according to age and growth. J. Proteome Res. 2017, 16, 3732–3740. [Google Scholar] [CrossRef] [PubMed]
- Diaz, S.O.; Pinto, J.; Barros, A.S.; Morais, E.; Duarte, D.; Negrao, F.; Pita, C.; Almeida, M.D.C.; Carreira, I.M.; Spraul, M.; et al. Newborn urinary metabolic signatures of prematurity and other disorders: A case control study. J. Proteome Res. 2016, 15, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.; Trygg, J.; Wold, S. CV-ANOVA for significance testing of PLS and OPLS® models. A J. Chemom. Soc. 2008, 22, 594–600. [Google Scholar] [CrossRef]
- Bertino, E.; Giribaldi, M.; Cester, E.A.; Coscia, A.; Trapani, B.M.; Peila, C.; Arslanoglu, S.; Moro, G.E.; Cavallarin, L. New human milk fortifiers for the preterm infant. J. Pediatr. Neonatal Individ. Med. 2017, 6, e060124. [Google Scholar] [CrossRef]
- Marincola, F.C.; Dessì, A.; Pattumelli, M.G.; Corbu, S.; Ossicini, C.; Ciccarelli, S.; Agostino, R.; Mussap, M.; Fanos, V. 1H NMR-based urine metabolic profile of IUGR, LGA, and AGA newborns in the first week of life. Clin. Chim. Acta 2015, 451, 28–34. [Google Scholar] [CrossRef]
- Gubhaju, L.; Sutherland, M.R.; Black, M.J. Preterm birth and the kidney: Implications for long-term renal health. Reprod. Sci. 2011, 18, 322–333. [Google Scholar] [CrossRef]
- Lionetti, L.; Cavaliere, G.; Bergamo, P.; Trinchese, G.; De Filippo, C.; Gifuni, G.; Gaita, M.; Pignalosa, A.; Donizzetti, I.; Putti, R.; et al. Diet supplementation with donkey milk upregulates liver mitochondrial uncoupling, reduces energy efficiency and improves antioxidant and antiinflammatory defences in rats. Mol. Nutr. Food Res. 2012, 56, 1596–1600. [Google Scholar] [CrossRef]
- Borum, P.R. Carnitine in neonatal nutrition. J. Child Neurol. 1995, 10 (Suppl. 2), 2S25–2S31. [Google Scholar] [CrossRef]
- Penn, D.; Dolderer, M.; Schmidt-Sommerfeld, E. Carnitine concentrations in the milk of different species and infant formulas. Neonatology 1987, 52, 70–79. [Google Scholar] [CrossRef]
- Melegh, B. Carnitine supplementation in the premature. Neonatology 1990, 58 (Suppl. 1), 93–106. [Google Scholar] [CrossRef]
- Van Aerde, T. In preterm infants, does the supplementation of carnitine to parenteral nutrition improve the following clinical outcomes: Growth, lipid metabolism and apneic spells? Part A: Evidence-based answer and summary. Paediatr. Child Health 2004, 9, 571–572. [Google Scholar] [CrossRef][Green Version]
- Van Aerde, J.E. In preterm infants, does the supplementation of carnitine to parenteral nutrition improve the following clinical outcomes: Growth, lipid metabolism and apneic spells? Part B: Clinical commentary. Paediatr. Child Health 2004, 9, 573. [Google Scholar] [CrossRef]
- Sundekilde, U.K.; Downey, E.; O’Mahony, J.A.; O’Shea, C.A.; Ryan, C.A.; Kelly, A.L.; Bertram, H.C. The effect of gestational and lactational age on the human milk metabolome. Nutrients 2016, 8, 304. [Google Scholar] [CrossRef]
- Boehm, G.; Borte, M.; Bellstedt, K.; Moro, G.; Minoli, I. Protein quality of human milk fortifier in low birth weight infants: Effects on growth and plasma amino acid profiles. Eur. J. Pediatr. 1993, 152, 1036–1039. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, S.C.; de Figueiredo, C.M.; de Andrade, S.M.O.; Palhares, D.B. Plasma amino acids in preterm infants fed different human milk diets from a human milk bank. E-SPEN Eur. E-J. Clin. Nutr. Metab. 2007, 2, 51–56. [Google Scholar] [CrossRef][Green Version]
- Szajewska, H. Extensive and partial protein hydrolysate preterm formulas. J. Pediatr. Gastroenterol. Nutr. 2007, 45, S183–S187. [Google Scholar] [CrossRef]
- Rigo, J.; Senterre, J. Metabolic balance studies and plasma amino acid concentrations in preterm infants fed experimental protein hydrolysate preterm formulas. Acta Paediatr. 1994, 83, 98–104. [Google Scholar] [CrossRef]
- Polberger, S.K.; Axelsson, I.E.; Räihä, N.C. Urinary and serum urea as indicators of protein metabolism in very low birthweight infants fed varying human milk protein intakes. Acta Paediatr. Scand. 1990, 79, 737–742. [Google Scholar] [CrossRef]
- Pozzo, L.; Cirrincione, S.; Russo, R.; Karamać, M.; Amarowicz, R.; Coscia, A.; Antoniazzi, S.; Cavallarin, L.; Giribaldi, M. Comparison of oxidative status of human milk, human milk fortifiers and preterm infant formulas. Foods 2019, 8, 458. [Google Scholar] [CrossRef]
- He, X.; Parenti, M.; Grip, T.; Domellöf, M.; Lönnerdal, B.; Hernell, O.; Timby, N.; Slupsky, C.M. Metabolic phenotype of breast-fed infants, and infants fed standard formula or bovine MFGM supplemented formula: A randomized controlled trial. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Cresi, F.; Maggiora, E.; Pirra, A.; Tonetto, P.; Rubino, C.; Cavallarin, L.; Giribaldi, M.; Moro, G.E.; Peila, C.; Coscia, A. Effects on gastroesophageal reflux of donkey milk-derived human milk fortifier versus standard fortifier in preterm newborns: Additional data from the fortilat study. Nutrients 2020, 12, 2142. [Google Scholar] [CrossRef]

| B-HMF 1 n = 27 | D-HMF 2 n = 27 | p-Value 4 | |
|---|---|---|---|
| Maternal characteristics 3 | |||
| Pregravidic BMI in kg/m2, mean (SD) | 24.1 (5.3) | 22.4 (3.7) | 0.187 ST |
| Weight gain in kg, mean (SD) | 9.3 (4.1) | 8.8 (4.9) | 0.708 ST |
| Age in years, median (IQR) | 34 (30–40) | 32 (29–38) | 0.248 ST |
| Chronic diabetes, n (%) | 0 | 1 (3.7) | n.a. |
| Chronic hypertension, n (%) | 2 (7.4) | 2 (7.4) | 1 RR |
| Gestational diabetes, n (%) | 6 (22.2) | 6 (22.2) | 1 RR |
| Gestational hypertension, n (%) | 7 (25.9) | 5 (18.5) | 0.511 RR |
| Cesarean delivery, n (%) | 22 (81.5) | 21 (77.8) | 0.735 RR |
| Assisted reproductive technology, n (%) | 8 (29.6) | 3 (11.1) | 0.083 RR |
| Neonatal characteristics 3 | |||
| Male, n (%) | 13 (48.1) | 15 (55.6) | 0.585 RR |
| Twins, n (%) | 11 (40.7) | 9 (33.3) | 0.572 RR |
| Gestational age < 32 wk., n (%) | 20 (74.1) | 16 (59.3) | 0.242 RR |
| VLBW (<1500 g), n (%) | 25 (92.6) | 21 (77.8) | 0.117 RR |
| SGA, n (%) | 8 (29.6) | 10 (37) | 0.563 RR |
| Birth weight in g, mean (SD) | 1174 (326) | 1227 (302) | 0.541 ST |
| Birth weight in SDS, mean (SD) | −0.477 (1.092) | −0.720 (1.2) | 0.442 ST |
| Respiratory distress syndrome, n (%) | 23 (85.2) | 26 (96.3) | 0.151 RR |
| Recovered PDA, n (%) | 8 (29.6) | 4 (14.8) | 0.533 RR |
| Age at randomization in days, median (IQR) | 10 (7–16) | 9 (7–14) | 0.799 ST |
| Age at start intervention in days, median (IQR) | 10 (7–15) | 10 (7–18) | 0.613 ST |
| Clinical Outcome and Morbidities 3 | B-HMF 1 n = 27 | D-HMF 2 n = 27 | p-Value 4 |
|---|---|---|---|
| Length of hospital stay in days, median (IQR) | 48 (38–73) | 44 (33–66) | 0.532 ST |
| Early sepsis, n (%) | 6 (22.2) | 2 (7.4) | 0.117 RR |
| Late sepsis, n (%) | 3 (11.1) | 2 (7.4) | 0.638 RR |
| Necrotizing enterocolitis, n (%) | 0 | 0 | n.a. |
| Weight at end intervention in g, mean (SD) | 1505 (426) | 1596 (324) | 0.386 ST |
| Weight gain during intervention in g, mean (SD) | 424 (153) | 450 (127) | 0.518 ST |
| Length at end intervention in cm, mean (SD) | 39.8 (3.6) | 41.2 (3.1) | 0.163 ST |
| Length gain during intervention in cm, mean (SD) | 3.1 (1.3) | 3.1 (1.0) | 0.873 ST |
| Feeding intolerance, n (%) | 5 (18.5) | 2 (7.4) | 0.217 RR |
| Feeding interruptions, total hours | 300 | 183 | n.a. |
| Vomiting, n (%) | 13 (48.1) | 11 (40.7) | 0.583 RR |
| Gastric residuals, n (%) | 4 (14.8) | 3 (11.1) | 0.685 RR |
| Bile stagnation episodes, total n | 7 | 1 | n.a. |
| Breast milk at discharge, n (%) | 12 (44.4) | 19 (70.4) | 0.046 ST |
| Parenteral nutrition at end intervention, n (%) | 4 (14.8) | 1 (3.7) | 0.151 RR |
| Days to full enteral feeding, median (IQR) | 23 (17–31) | 21 (13–24) | 0.021 ST |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giribaldi, M.; Peila, C.; Coscia, A.; Cavallarin, L.; Antoniazzi, S.; Corbu, S.; Maiocco, G.; Sottemano, S.; Cresi, F.; Moro, G.E.; et al. Urinary Metabolomic Profile of Preterm Infants Receiving Human Milk with Either Bovine or Donkey Milk-Based Fortifiers. Nutrients 2020, 12, 2247. https://doi.org/10.3390/nu12082247
Giribaldi M, Peila C, Coscia A, Cavallarin L, Antoniazzi S, Corbu S, Maiocco G, Sottemano S, Cresi F, Moro GE, et al. Urinary Metabolomic Profile of Preterm Infants Receiving Human Milk with Either Bovine or Donkey Milk-Based Fortifiers. Nutrients. 2020; 12(8):2247. https://doi.org/10.3390/nu12082247
Chicago/Turabian StyleGiribaldi, Marzia, Chiara Peila, Alessandra Coscia, Laura Cavallarin, Sara Antoniazzi, Sara Corbu, Giulia Maiocco, Stefano Sottemano, Francesco Cresi, Guido E. Moro, and et al. 2020. "Urinary Metabolomic Profile of Preterm Infants Receiving Human Milk with Either Bovine or Donkey Milk-Based Fortifiers" Nutrients 12, no. 8: 2247. https://doi.org/10.3390/nu12082247
APA StyleGiribaldi, M., Peila, C., Coscia, A., Cavallarin, L., Antoniazzi, S., Corbu, S., Maiocco, G., Sottemano, S., Cresi, F., Moro, G. E., Bertino, E., Fanos, V., & Cesare Marincola, F. (2020). Urinary Metabolomic Profile of Preterm Infants Receiving Human Milk with Either Bovine or Donkey Milk-Based Fortifiers. Nutrients, 12(8), 2247. https://doi.org/10.3390/nu12082247







