Impact of Varying Dosages of Fish Oil on Recovery and Soreness Following Eccentric Exercise
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Approach
2.2. Participants
2.3. Visit 1: Initial Screening and Supplement Distribution
Supplementation and Diet
2.4. Visit 2: One-Repetition Maximum (1RM), Familiarization, and Diet Counseling
2.5. Visit 3: Investigational Protocol (Muscle-Damaging Exercise Visit)
Resistance Exercise Protocol
2.6. Blood Collection, Perceived Soreness, and Muscle Performance Indices
2.6.1. Blood Collection and Assessments
2.6.2. Perceived Soreness
2.6.3. Vertical Jump
2.6.4. T-Test Agility Test
2.6.5. Forty-Yard Sprint
2.6.6. Maximal Voluntary Isometric Contraction
2.7. Statistical Analysis
3. Results
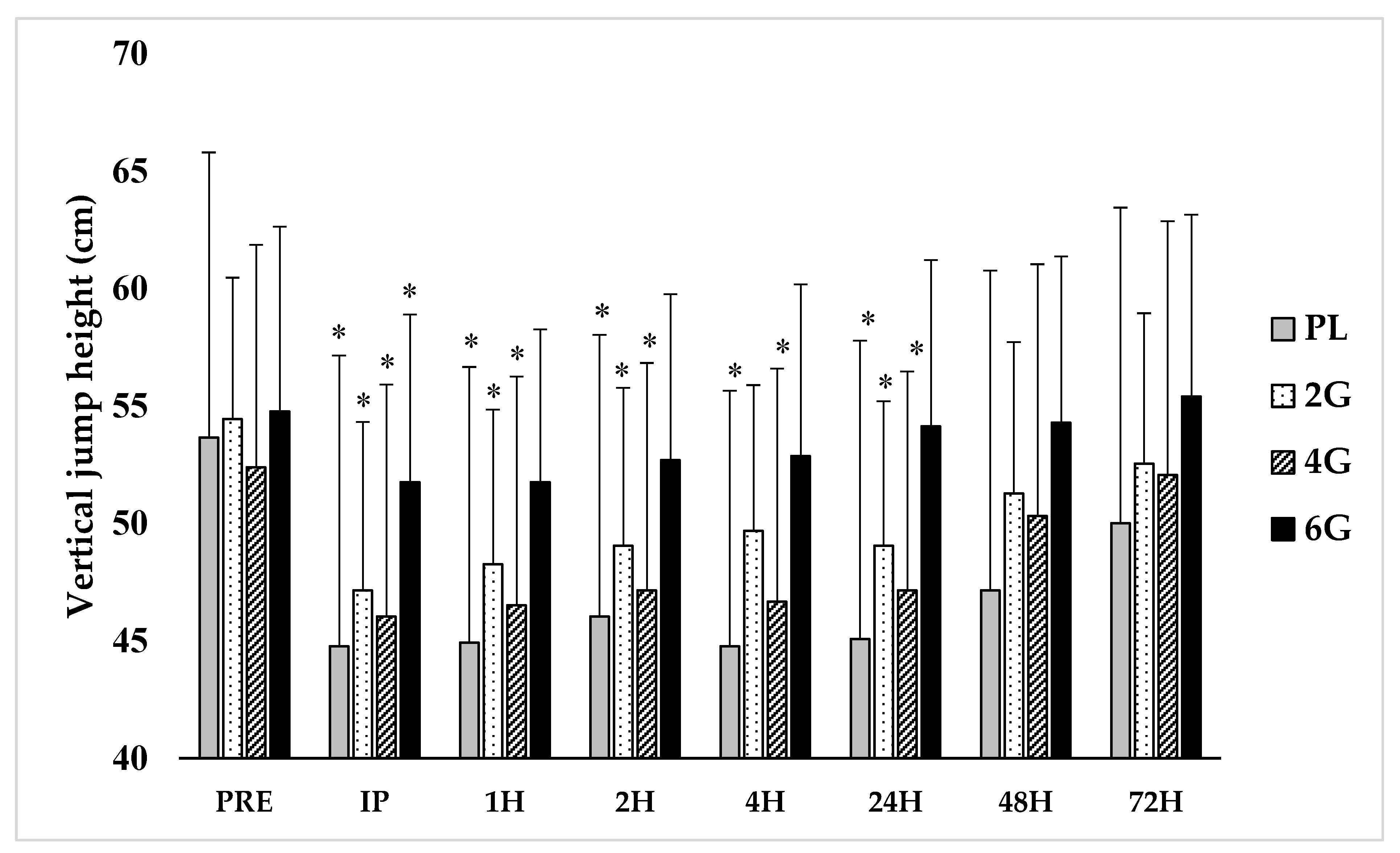
3.1. Performance Measures
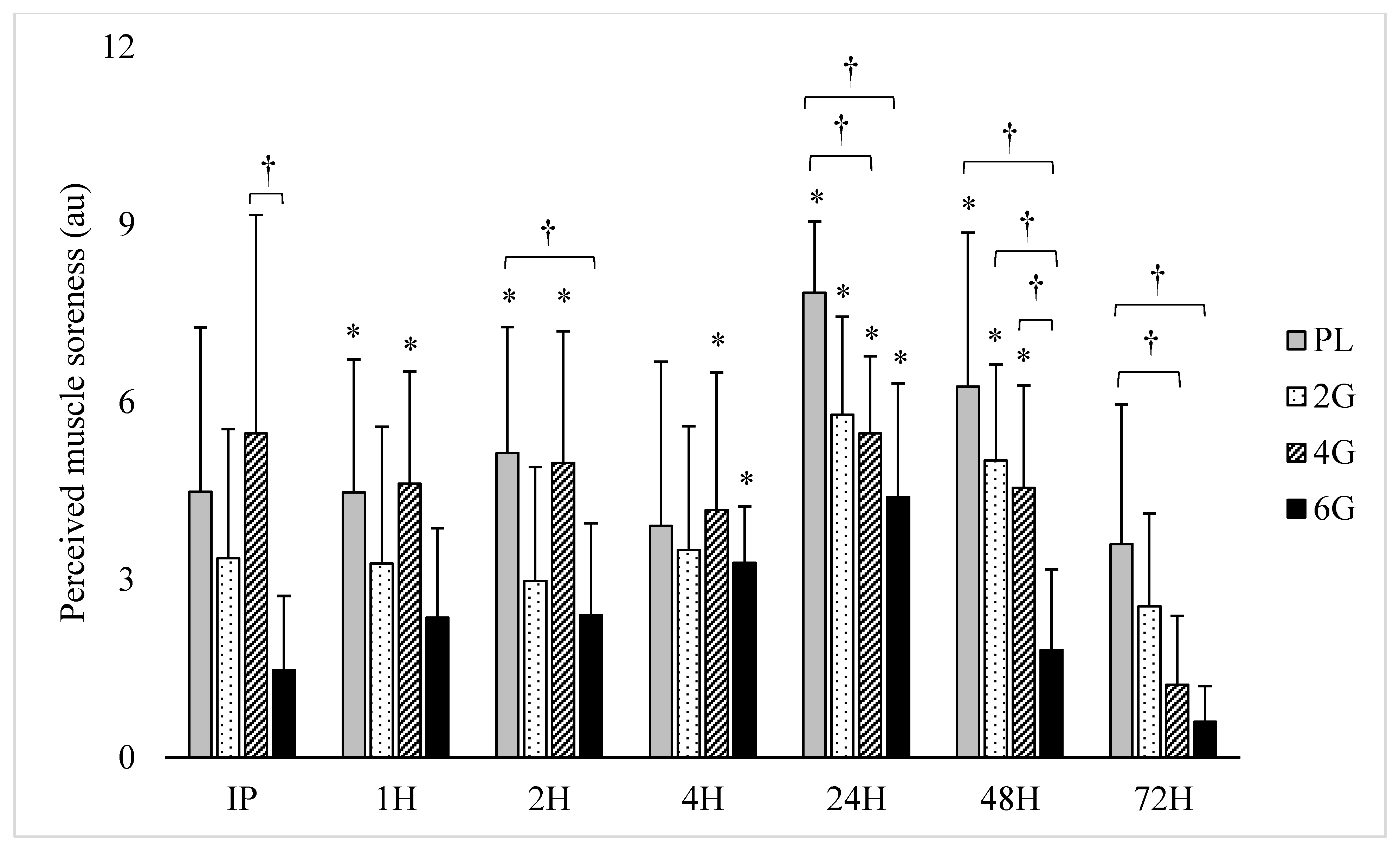
3.2. Perceived Soreness
3.3. Indirect Markers of Muscle Damage
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Freitas, M.C.; Gerosa-Neto, J.; Zanchi, N.E.; Lira, F.S.; Rossi, F.E. Role of metabolic stress for enhancing muscle adaptations: Practical applications. World J. Methodol. 2017, 7, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Friden, J.; Lieber, R.L. Eccentric exercise-induced injuries to contractile and cytoskeletal muscle fibre components. Acta Physiol. Scand. 2001, 171, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Manfredi, T.J.; Ding, W.; Fiatarone, M.A.; Evans, W.J.; Cannon, J.G. Acute phase response in exercise. III. Neutrophil and IL-1 beta accumulation in skeletal muscle. Am. J. Physiol. 1993, 265, R166–R172. [Google Scholar] [PubMed]
- Cannon, J.G.; Meydani, S.N.; Fielding, R.A.; Fiatarone, M.A.; Meydani, M.; Farhangmehr, M.; Orencole, S.F.; Blumberg, J.B.; Evans, W.J. Acute phase response in exercise. II. Associations between vitamin E, cytokines, and muscle proteolysis. Am. J. Physiol. 1991, 260, R1235–R1240. [Google Scholar] [CrossRef] [PubMed]
- Hyldahl, R.D.; Nelson, B.; Xin, L.; Welling, T.; Groscost, L.; Hubal, M.J.; Chipkin, S.; Clarkson, P.M.; Parcell, A.C. Extracellular matrix remodeling and its contribution to protective adaptation following lengthening contractions in human muscle. FASEB J. 2015, 29, 2894–2904. [Google Scholar] [CrossRef] [PubMed]
- Sayers, S.P.; Clarkson, P.M. Force recovery after eccentric exercise in males and females. Eur. J. Appl. Physiol. 2001, 84, 122–126. [Google Scholar] [CrossRef]
- Byrne, C.; Eston, R. The effect of exercise-induced muscle damage on isometric and dynamic knee extensor strength and vertical jump performance. J. Sports Sci. 2002, 20, 417–425. [Google Scholar] [CrossRef]
- Miles, M.P.; Clarkson, P.M. Exercise-induced muscle pain, soreness, and cramps. J. Sports Med. Phys. Fit. 1994, 34, 203–216. [Google Scholar]
- Clarkson, P.M.; Newham, D.J. Associations between muscle soreness, damage, and fatigue. Adv. Exp. Med. Biol. 1995, 384, 457–469. [Google Scholar]
- Morgan, D.L.; Allen, D.G. Early events in stretch-induced muscle damage. J. Appl. Physiol. 1999, 87, 2007–2015. [Google Scholar] [CrossRef]
- Peake, J.; Nosaka, K.; Suzuki, K. Characterization of inflammatory responses to eccentric exercise in humans. Exerc. Immunol. Rev. 2005, 11, 64–85. [Google Scholar] [PubMed]
- Gomez-Cabrera, M.C.; Vina, J.; Ji, L.L. Interplay of oxidants and antioxidants during exercise: Implications for muscle health. Phys. Sportsmed. 2009, 37, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Jamurtas, A.Z. Exercise-Induced Muscle Damage and Oxidative Stress. Antioxidants 2018, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Howatson, G.; van Someren, K.A. The prevention and treatment of exercise-induced muscle damage. Sports Med. 2008, 38, 483–503. [Google Scholar] [CrossRef]
- Poppendieck, W.; Wegmann, M.; Ferrauti, A.; Kellmann, M.; Pfeiffer, M.; Meyer, T. Massage and Performance Recovery: A Meta-Analytical Review. Sports Med. 2016, 46, 183–204. [Google Scholar] [CrossRef]
- Cheatham, S.W.; Kolber, M.J.; Cain, M.; Lee, M. The Effects of Self-Myofascial Release Using a Foam Roll or Roller Massager on Joint Range of Motion, Muscle Recovery, and Performance: A Systematic Review. Int. J. Sports Phys. Ther. 2015, 10, 827–838. [Google Scholar]
- Apostolopoulos, N.C.; Lahart, I.M.; Plyley, M.J.; Tauton, J.; Nevill, A.M.; Koutedakis, Y.; Wyon, M.; Metsios, G.S. The effects of different passive static stretching intensities on recovery from unaccustomed eccentric exercise—A randomized controlled trial. Appl. Physiol. Nutr. Metab. 2018, 43, 806–815. [Google Scholar] [CrossRef]
- Morelli, K.M.; Brown, L.B.; Warren, G.L. Effect of NSAIDs on Recovery from Acute Skeletal Muscle Injury: A Systematic Review and Meta-analysis. Am. J. Sports Med. 2018, 46, 224–233. [Google Scholar] [CrossRef]
- Marqués-Jiménez, D.; Calleja-González, J.; Arratibel, I.; Delextrat, A.; Terrados, N. Are compression garments effective for the recovery of exercise-induced muscle damage? A systematic review with meta-analysis. Physiol. Behav. 2016, 153, 133–148. [Google Scholar]
- Fonda, B.; Sarabon, N. Effects of whole-body cryotherapy on recovery after hamstring damaging exercise: A crossover study. Scand. J. Med. Sci. Sports 2013, 23, e270–e278. [Google Scholar] [CrossRef]
- Harty, P.S.; Cottet, M.L.; Malloy, J.K.; Kerksick, C.M. Nutritional and Supplementation Strategies to Prevent and Attenuate Exercise-Induced Muscle Damage: A Brief. Review. Sports Med. Open 2019, 5, 1. [Google Scholar] [CrossRef]
- Heileson, J.L.; Funderburk, K. The effect of fish oil supplementation on the promotion and preservation of lean body mass, strength, and recovery from physiological stress in young, healthy adults: A systematic review. Nutr. Rev. 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Adkins, Y.; Kelley, D.S. Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J. Nutr. Biochem. 2010, 21, 781–792. [Google Scholar] [CrossRef]
- Sekikawa, A.; Cui, C.; Sugiyama, D.; Fabio, A.; Harris, W.S.; Zhang, X. Effect of High.-Dose Marine Omega-3 Fatty Acids on Atherosclerosis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Nutrients 2019, 11, 2599. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.H. Mechanisms for the hypotriglyceridemic effect of marine omega-3 fatty acids. Am. J. Cardiol. 2006, 98, 27i–33i. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.Y.; Jacobson, T.A. Effects of eicosapentaenoic acid versus docosahexaenoic acid on serum lipids: A systematic review and meta-analysis. Curr. Atheroscler. Rep. 2011, 13, 474–483. [Google Scholar] [CrossRef]
- Lopez-Huertas, E. The effect of EPA and DHA on metabolic syndrome patients: A systematic review of randomised controlled trials. Br. J. Nutr. 2012, 107 (Suppl. S2), S185–S194. [Google Scholar] [CrossRef]
- Allaire, J.; Couture, P.; Leclerc, M.; Charest, A.; Marin, J.; Lépine, M.C.; Talbot, D.; Tchernof, A.; Lamarche, B. A randomized, crossover, head-to-head comparison of eicosapentaenoic acid and docosahexaenoic acid supplementation to reduce inflammation markers in men and women: The Comparing EPA to DHA (ComparED) Study. Am. J.Clin. Nutr. 2016, 104, 280–287. [Google Scholar] [CrossRef]
- Calder, P.C. N-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83 (Suppl. S6), 1505S–1519S. [Google Scholar] [CrossRef]
- Mori, T.A.; Beilin, L.J. Omega-3 fatty acids and inflammation. Curr. Atheroscler. Rep. 2004, 6, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Jouris, K.B.; McDaniel, J.L.; Weiss, E.P. The Effect of Omega-3 Fatty Acid Supplementation on the Inflammatory Response to eccentric strength exercise. J. Sports Sci. Med. 2011, 10, 432–438. [Google Scholar] [PubMed]
- Tinsley, G.M.; Gann, J.J.; Huber, S.R.; Andre, T.L.; La Bounty, P.M.; Bowden, R.G.; Gordon, P.M.; Granjean, P.W. Effects of Fish. Oil Supplementation on Postresistance Exercise Muscle Soreness. J. Diet Suppl. 2017, 14, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Gray, P.; Chappell, A.; Jenkinson, A.M.; Thies, F.; Gray, S.R. Fish oil supplementation reduces markers of oxidative stress but not muscle soreness after eccentric exercise. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 206–214. [Google Scholar] [CrossRef]
- Lembke, P.; Capodice, J.; Hebert, K.; Swenson, T. Influence of omega-3 (n3) index on performance and wellbeing in young adults after heavy eccentric exercise. J. Sports Sci. Med. 2014, 13, 151–156. [Google Scholar] [PubMed]
- Da Boit, M.; Mastalurova, I.; Brazaite, G.; McGovern, N.; Thompson, K.; Gray, S.R. The Effect of Krill Oil Supplementation on Exercise Performance and Markers of Immune Function. PLoS ONE 2015, 10, e0139174. [Google Scholar] [CrossRef]
- McGlory, C.; Gorissen, S.H.M.; Kamal, M.; Bahniwal, R.; Hector, A.J.; Baker, S.K.; Chabowski, A.; Phillips, S.M. Omega-3 fatty acid supplementation attenuates skeletal muscle disuse atrophy during two weeks of unilateral leg immobilization in healthy young women. FASEB J. 2019, 33, 4586–4597. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Yanagimoto, K.; Ueda, H.; Ochi, E. Supplementation of eicosapentaenoic acid-rich fish oil attenuates muscle stiffness after eccentric contractions of human elbow flexors. J. Int. Soc. Sports Nutr. 2019, 16, 19. [Google Scholar] [CrossRef]
- Black, K.E.; Witard, O.C.; Baker, D.; Healey, P.; Lewis, V.; Tavares, F.; Christensen, S.; Pease, T.; Smith, B. Adding omega-3 fatty acids to a protein-based supplement during pre-season training results in reduced muscle soreness and the better maintenance of explosive power in professional Rugby Union players. Eur. J. Sport Sci. 2018, 18, 1357–1367. [Google Scholar] [CrossRef]
- McKinley-Barnard, S.K.; Andrew, T.L.; Gann, J.J.; Hwang, P.S.; Willoughby, D.S. Effectiveness of Fish Oil Supplementation in Attenuating Exercise-Induced Muscle Damage in Women During Midfollicular and Midluteal Menstrual Phases. J. Strength Cond. Res. 2018, 32, 1601–1612. [Google Scholar] [CrossRef]
- Lenn, J.; Uhl, T.; Mattacola, C.; Boissonneault, G.; Yates, J.; Ibrahim, W.; Bruckner, G. The effects of fish oil and isoflavones on delayed onset muscle soreness. Med. Sci. Sports Exerc. 2002, 34, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- Gravina, L.; Brown, F.F.; Alexander, L.; Dick, J.; Bell, G.; Witard, O.C.; Galloway, S.D.R. n-3 Fatty Acid Supplementation During 4 Weeks of Training Leads to Improved Anaerobic Endurance Capacity, but not Maximal Strength, Speed, or Power in Soccer Players. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 305–313. [Google Scholar] [CrossRef][Green Version]
- Calder, P.C. n-3 fatty acids, inflammation and immunity: New mechanisms to explain old actions. Proc. Nutr. Soc. 2013, 72, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.S.; Pollock, M.L.; Ward, A. Generalized equations for predicting body density of women. Med. Sci. Sports Exerc. 1980, 12, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.S.; Pollock, M.L. Generalized equations for predicting body density of men. Br. J. Nutr. 1978, 40, 497–504. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 1988; pp. 284–288. [Google Scholar]
- VanDusseldorp, T.A.; Escobar, K.A.; Johnson, K.E.; Stratton, M.T.; Moriarty, T.; Cole, N.; McCormick, J.J.; Kerksick, C.M.; Vaughan, R.A.; Dokladny, K.; et al. Effect of Branched-Chain Amino Acid Supplementation on Recovery Following Acute Eccentric Exercise. Nutrients 2018, 10, 1389. [Google Scholar] [CrossRef]
- Jakeman, J.R.; Lambrick, D.M.; Wooley, B.; Babraj, J.A.; Faulkner, J.A. Effect of an acute dose of omega-3 fish oil following exercise-induced muscle damage. Eur. J. Appl. Physiol. 2017, 117, 575–582. [Google Scholar] [CrossRef]
- Armstrong, R.B. Initial events in exercise-induced muscular injury. Med. Sci. Sports Exerc. 1990, 22, 429–435. [Google Scholar]
- Smith, L.L. Acute inflammation: The underlying mechanism in delayed onset muscle soreness? Med. Sci. Sports Exerc. 1991, 23, 542–551. [Google Scholar] [CrossRef]
- Brocklehurst, W.E. Role of kinins and prostaglandins in inflammation. Proc. R. Soc. Med. 1971, 64, 4–6. [Google Scholar]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Philpott, J.D.; Donnelly, C.; Walshe, I.H.; MacKinley, E.E.; Dick, J.; Galloway, S.D.R.; Tipton, K.D.; Witard, O.C. Adding Fish. Oil to Whey Protein, Leucine, and Carbohydrate over a Six-Week Supplementation Period. Attenuates Muscle Soreness Following Eccentric Exercise in Competitive Soccer Players. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Yanagimoto, K.; Nakazato, K.; Hayamizu, K.; Ochi, E. Eicosapentaenoic and docosahexaenoic acids-rich fish oil supplementation attenuates strength loss and limited joint range of motion after eccentric contractions: A randomized, double-blind, placebo-controlled, parallel-group trial. Eur. J. Appl. Physiol. 2016, 116, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Ochi, E.; Yanagimoto, K.; Morishima, T.; Tsuchiya, Y. Eicosapentaenoic Acid-Rich Fish. Oil Supplementation Inhibits the Decrease in Concentric Work Output and Muscle Swelling of the Elbow Flexors. J. Am. Coll. Nutr. 2019, 38, 125–131. [Google Scholar]
- Ochi, E.; Tsuchiya, Y.; Yanagimoto, K. Effect of eicosapentaenoic acids-rich fish oil supplementation on motor nerve function after eccentric contractions. J. Int. Soc. Sports Nutr. 2017, 14, 23. [Google Scholar] [CrossRef]
- Tartibian, B.; Maleki, B.H.; Abbasi, A. The effects of ingestion of omega-3 fatty acids on perceived pain and external symptoms of delayed onset muscle soreness in untrained men. Clin. J. Sport Med. 2009, 19, 115–119. [Google Scholar] [CrossRef]
- McGlory, C.; Galloway, S.D.R.; Hamilton, D.L.; McClintock, C.; Breen, L.; Dick, J.R.; Bell, J.G.; Tipton, K.D. Temporal changes in human skeletal muscle and blood lipid composition with fish oil supplementation. Prostaglandins Leukot. Essent. Fat. Acids 2014, 90, 199–206. [Google Scholar] [CrossRef]
- Browning, L.M.; Walker, C.G.; Mander, A.P.; West, A.L.; Madden, J.; Gambell, J.M.; Young, S.; Wang, L.; Jebb, S.A.; Calder, P.C. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am. J. Clin. Nutr. 2012, 96, 748–758. [Google Scholar] [CrossRef]
- Wright, G.A.; Pustina, A.A.; Mikat, R.P.; Kernozek, T.W. Predicting lower body power from vertical jump prediction equations for loaded jump squats at different intensities in men and women. J. Strength Cond. Res. 2012, 26, 648–655. [Google Scholar] [CrossRef]
- Gammone, M.A.; Riccioni, G.; Parrinello, G.; D’Orazio, N.D. Omega-3 Polyunsaturated Fatty Acids: Benefits and Endpoints in Sport. Nutrients 2018, 11, 46. [Google Scholar] [CrossRef]
 = damaging bout of eccentric exercise;
= damaging bout of eccentric exercise;  = perceived muscle soreness;
= perceived muscle soreness;  = blood collection for assessment of creatine kinase and lactate dehydrogenase;
= blood collection for assessment of creatine kinase and lactate dehydrogenase;  = muscular performance (vertical jump, 40-yard dash, T-test agility, maximal voluntary isometric contraction).
= muscular performance (vertical jump, 40-yard dash, T-test agility, maximal voluntary isometric contraction).
 = damaging bout of eccentric exercise;
= damaging bout of eccentric exercise;  = perceived muscle soreness;
= perceived muscle soreness;  = blood collection for assessment of creatine kinase and lactate dehydrogenase;
= blood collection for assessment of creatine kinase and lactate dehydrogenase;  = muscular performance (vertical jump, 40-yard dash, T-test agility, maximal voluntary isometric contraction).
= muscular performance (vertical jump, 40-yard dash, T-test agility, maximal voluntary isometric contraction).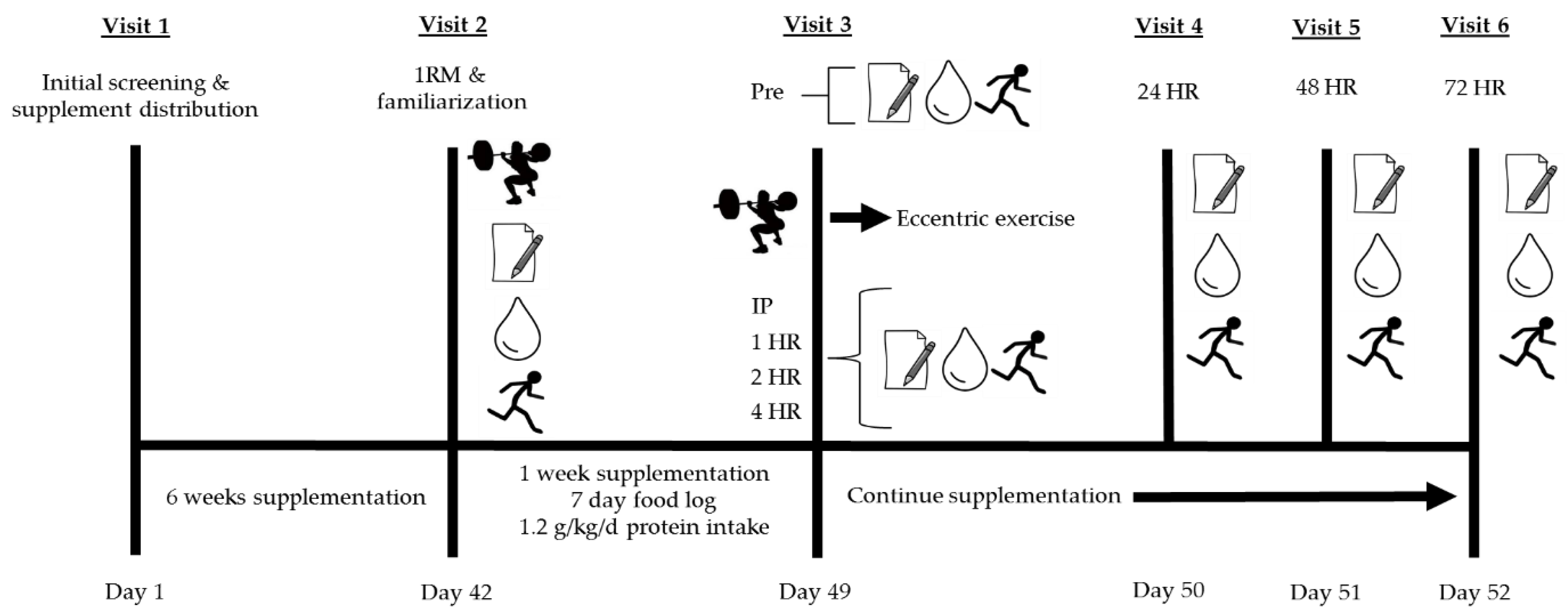
| Participant Characteristics | 2G | 4G | 6G | Placebo |
|---|---|---|---|---|
| Age (year) | 23.5 ± 3.3 | 23.3 ± 3.0 | 23.8 ± 2.8 | 23.0 ± 3.0 |
| Height (cm) | 170.9 ± 6.9 | 172.9 ± 4.7 | 173.8 ± 7.6 | 173.6 ± 6.2 |
| Body Mass (kg) | 76.1 ± 14.2 | 69.7 ± 15.9 | 72.8 ± 13.5 | 67.9 ± 10.7 |
| Body Fat (%) | 20.8 ± 4.1 | 19.0 ± 6.2 | 19.4 ± 6.1 | 20.6 ± 7.2 |
| Calorie Intake (kcal/day)-Familiarization-Experimental Trial (Day 42–Day 52) | 2363.25 ± 489.13 | 2283.88 ± 375.98 | 2050.88 ± 552.04 | 2160.13 ± 415.21 |
| Protein Intake (g/kg)-Familiarization-Experimental Trial (Day 42–Day 52) | 1.28 ± 0.10 | 1.21 ± 0.24 | 1.23 ± 0.11 | 1.19 ± 0.17 |
| Supplementation | EPA (mg) | DHA (mg) | Total EPA+DHA (mg) |
|---|---|---|---|
| 2G FO | 800 | 600 | 1400 |
| 4G FO | 1600 | 1200 | 2800 |
| 6G FO | 2400 | 1800 | 4200 |
| Time Point | 2G | 4G | 6G | Placebo |
|---|---|---|---|---|
| PRE | 233.26 ± 63.45 | 221.76 ± 50.21 | 250.74 ± 85.51 | 230.95 ± 70.36 |
| IP | 182.00 ± 63.79 | 172.55 ± 61.28 | 217.16 ± 70.46 | 169.64 ± 65.47 |
| 1H | 182.41 ± 51.02 | 174.56 ± 54.36 | 196.23 ± 64.08 | 169.25 ± 65.89 |
| 2H | 183.06 ± 53.45 | 174.85 ± 53.53 | 210.64 ± 68.17 | 172.95 ± 60.08 |
| 4H | 189.03 ± 42.78 | 171.20 ± 56.02 | 222.51 ± 79.28 | 170.38 ± 62.35 |
| 24H | 191.46 ± 57.54 | 178.75 ± 57.00 | 229.79 ± 74.16 | 175.64 ± 66.94 |
| 48H | 204.10 ± 59.57 | 196.05 ± 65.85 | 231.13 ± 73.67 | 191.59 ± 68.22 |
| 72H | 224.93 ± 62.66 | 205.76 ± 54.48 | 251.28 ± 89.69 | 211.43 ± 67.93 |
| Time Point | 2G | 4G | 6G | Placebo |
|---|---|---|---|---|
| PRE | 5.69 ± 0.38 | 5.63 ± 0.55 | 5.51 ± 0.41 | 5.68 ± 0.61 |
| IP | 6.36 ± 0.53 | 6.17 ± 0.83 | 5.81 ± 0.31 | 6.46 ± 0.87 |
| 1H | 6.25 ± 0.57 | 6.03 ± 0.64 | 5.74 ± 0.39 | 6.38 ± 0.84 |
| 2H | 6.19 ± 0.49 | 6.04 ± 0.64 | 5.75 ± 0.45 | 6.31 ± 0.74 |
| 4H | 6.19 ± 0.50 | 6.04 ± 0.61 | 5.73 ± 0.43 | 6.56 ± 1.02 |
| 24H | 6.19 ± 0.57 | 6.13 ± 0.92 | 5.72 ± 0.31 | 6.48 ± 1.03 |
| 48H | 5.93 ± 0.37 | 5.90 ± 0.70 | 5.68 ± 0.32 | 6.27 ± 0.92 |
| 72H | 5.79 ± 0.44 | 5.79 ± 0.67 | 5.52 ± 0.41 | 5.96 ± 0.79 |
| Baseline | PRE | 2-HR | 4-HR | 24-HR | 48-HR | 72-HR | |
|---|---|---|---|---|---|---|---|
| Creatine Kinase (IU/L) | |||||||
| PL | 115.8 ± 70.5 | 116.1 ± 70.4 | 218.1 ± 67.9 | 355.8 ± 141.7 | 1751.9 ± 1397.3 | 1784.5 ± 1713.5 | 1804.9 ± 2034.8 |
| 2G | 89.9 ± 44.1 | 89.6 ± 42.2 | 193.3 ± 93.9 | 427.6 ± 198.4 | 3020.6 ± 1753.4 | 2060.6 ± 1353.3 | 956.8 ± 692.7 |
| 4G | 108.8 ± 59.2 | 101.9 ± 56.6 | 181.3 ± 72.4 | 435.8 ± 290.0 | 2058.0 ± 2217.7 | 2188.3 ± 2110.3 | 1371.1 ± 1309.9 |
| 6G | 106.0 ± 17.0 | 102.9 ± 19.3 | 128.0 ± 58.8 | 285.1 ± 121.3 | 544.5 ± 150.8 | 261.1 ± 103.5 | 114.3 ± 21.1 |
| Lactate Dehydrogenase (IU/L) | |||||||
| PL | 108.3 ± 52.6 | 109.5 ± 46.7 | 167.3 ± 74.7 | 193.9 ± 106.8 | 401.2 ± 165.3 | 423.3 ± 186.2 | 410.0 ± 200.3 |
| 2G | 121.6 ± 16.5 | 121.4 ± 17.8 | 151.0 ± 39.9 | 188.6 ± 108.1 | 364.0 ± 139.7 | 397.5 ± 139.9 | 412.1 ± 135.0 |
| 4G | 167.6 ± 87.5 | 149.6 ± 70.7 | 197.1 ± 73.2 | 210.3 ± 79.5 | 376.1 ± 212.5 | 375.9 ± 233.9 | 237.7 ± 180.1 |
| 6G | 114.3 ± 15.6 | 114.5 ± 17.0 | 152.9 ± 45.5 | 181.4 ± 53.8 | 194.2 ± 49.2 | 198.0 ± 55.0 | 130.9 ± 28.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
VanDusseldorp, T.A.; Escobar, K.A.; Johnson, K.E.; Stratton, M.T.; Moriarty, T.; Kerksick, C.M.; Mangine, G.T.; Holmes, A.J.; Lee, M.; Endito, M.R.; et al. Impact of Varying Dosages of Fish Oil on Recovery and Soreness Following Eccentric Exercise. Nutrients 2020, 12, 2246. https://doi.org/10.3390/nu12082246
VanDusseldorp TA, Escobar KA, Johnson KE, Stratton MT, Moriarty T, Kerksick CM, Mangine GT, Holmes AJ, Lee M, Endito MR, et al. Impact of Varying Dosages of Fish Oil on Recovery and Soreness Following Eccentric Exercise. Nutrients. 2020; 12(8):2246. https://doi.org/10.3390/nu12082246
Chicago/Turabian StyleVanDusseldorp, Trisha A., Kurt A. Escobar, Kelly E. Johnson, Matthew T. Stratton, Terence Moriarty, Chad M. Kerksick, Gerald T. Mangine, Alyssa J. Holmes, Matthew Lee, Marvin R. Endito, and et al. 2020. "Impact of Varying Dosages of Fish Oil on Recovery and Soreness Following Eccentric Exercise" Nutrients 12, no. 8: 2246. https://doi.org/10.3390/nu12082246
APA StyleVanDusseldorp, T. A., Escobar, K. A., Johnson, K. E., Stratton, M. T., Moriarty, T., Kerksick, C. M., Mangine, G. T., Holmes, A. J., Lee, M., Endito, M. R., & Mermier, C. M. (2020). Impact of Varying Dosages of Fish Oil on Recovery and Soreness Following Eccentric Exercise. Nutrients, 12(8), 2246. https://doi.org/10.3390/nu12082246








