Dietary Patterns Are Associated with Risk of Prostate Cancer in a Population-Based Case-Control Study in Montreal, Canada
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
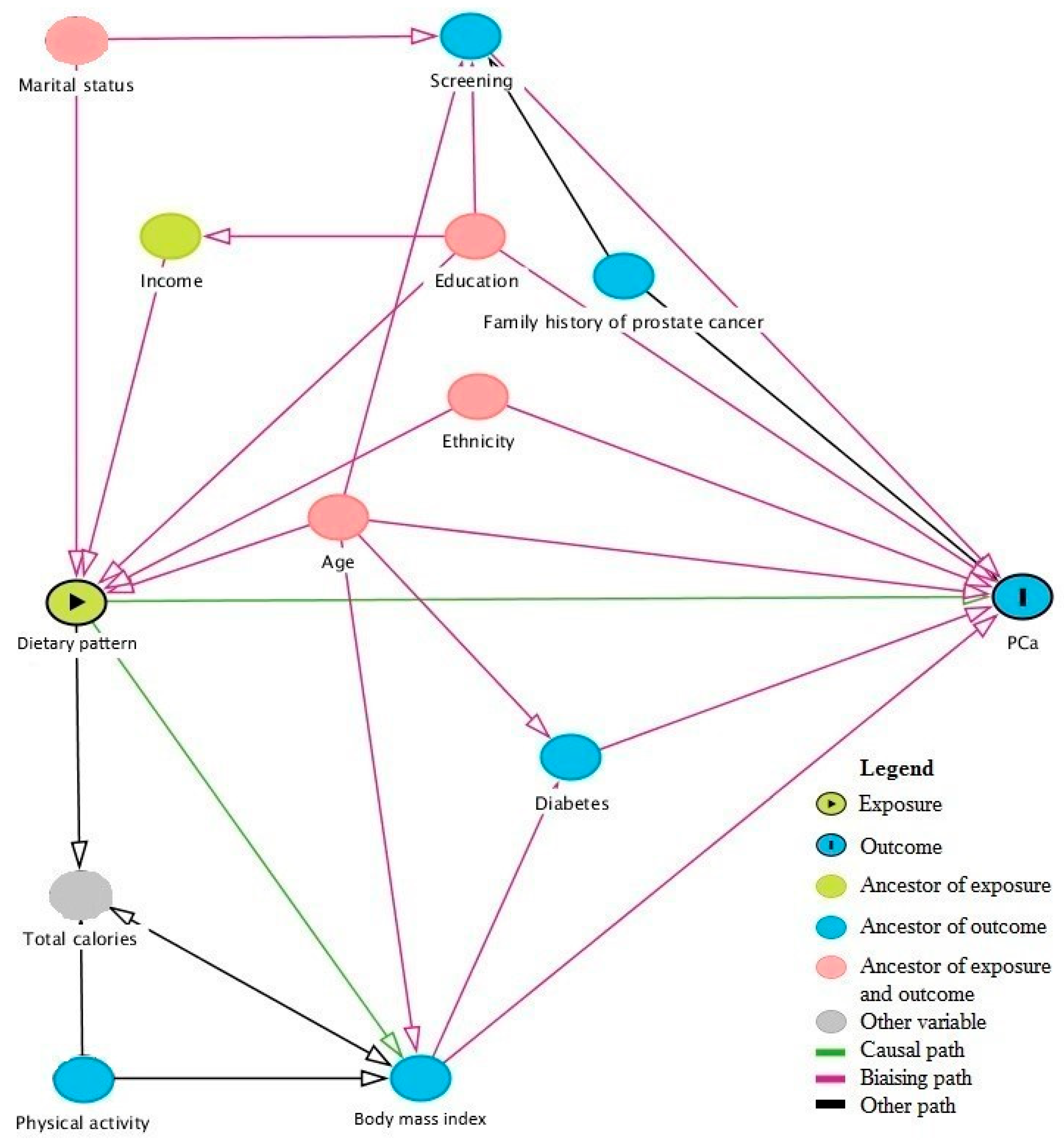
2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. Identification of Dietary Patterns
3.3. Association between Dietary Patterns and PCa Risk
4. Discussion
4.1. Previous Studies
4.1.1. Cohort Studies
4.1.2. Case-Control Studies
4.2. Methodological Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hsing, A.W.; Chokkalingam, A.P. Prostate cancer epidemiology. Front. Biosci. 2006, 11, 1388–1413. [Google Scholar] [CrossRef] [PubMed]
- Rebbeck, T.R. Prostate Cancer Genetics: Variation by Race, Ethnicity, and Geography. Semin. Radiat. Oncol. 2017, 27, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Schottenfeld, D.; Fraumeni, J.F. Cancer Epidemiology and Prevention, 3rd ed.; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Wilson, K.M.; Giovannucci, E.L.; Mucci, L.A. Lifestyle and dietary factors in the prevention of lethal prostate cancer. Asian J. Androl. 2012, 14, 365–374. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund International/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Continuous Update Project Expert Report 2018; World Cancer Research Fund International: London, UK, 2018. [Google Scholar]
- Giovannucci, E. Does prostate-specific antigen screening influence the results of studies of tomatoes, lycopene, and prostate cancer risk? J. Natl. Cancer Inst. 2007, 99, 1060–1062. [Google Scholar] [CrossRef]
- Masko, E.M.; Allott, E.H.; Freedland, S.J. The Relationship Between Nutrition and Prostate Cancer: Is More Always Better? Eur. Urol. 2013, 63, 810–820. [Google Scholar] [CrossRef]
- Platz, E.A. Energy imbalance and prostate cancer. J. Nutr. 2002, 132 (Suppl. 11), 3471S–3481S. [Google Scholar] [CrossRef]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef]
- Jacobs, D.R., Jr.; Tapsell, L.C. Food, not nutrients, is the fundamental unit in nutrition. Nutr. Rev. 2007, 65, 439–450. [Google Scholar] [CrossRef]
- Hoffmann, K.; Schulze, M.B.; Schienkiewitz, A.; Nothlings, U.; Boeing, H. Application of a new statistical method to derive dietary patterns in nutritional epidemiology. Am. J. Epidemiol. 2004, 159, 935–944. [Google Scholar] [CrossRef]
- Ambrosini, G.L.; Fritschi, L.; de Klerk, N.H.; Mackerras, D.; Leavy, J. Dietary patterns identified using factor analysis and prostate cancer risk: a case control study in Western Australia. Ann. Epidemiol. 2008, 18, 364–370. [Google Scholar] [CrossRef]
- Bosire, C.; Stampfer, M.J.; Subar, A.F.; Park, Y.; Kirkpatrick, S.I.; Chiuve, S.E.; Hollenbeck, A.R.; Reedy, J. Index-based dietary patterns and the risk of prostate cancer in the NIH-AARP diet and health study. Am. J. Epidemiol. 2013, 177, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Capurso, C.; Vendemiale, G. The Mediterranean Diet Reduces the Risk and Mortality of the Prostate Cancer: A Narrative Review. Front. Nutr. 2017, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Castello, A.; Boldo, E.; Amiano, P.; Castano-Vinyals, G.; Aragones, N.; Gomez-Acebo, I.; Peiro, R.; Jimenez-Moleon, J.J.; Alguacil, J.; Tardon, A.; et al. Mediterranean Dietary Pattern is Associated with Low Risk of Aggressive Prostate Cancer: MCC-Spain Study. J. Urol. 2018, 199, 430–437. [Google Scholar] [CrossRef] [PubMed]
- De Stefani, E.; Ronco, A.L.; Deneo-Pellegrini, H.; Boffetta, P.; Aune, D.; Acosta, G.; Brennan, P.; Ferro, G.; Mendilaharsu, M. Dietary patterns and risk of advanced prostate cancer: A principal component analysis in Uruguay. Cancer Causes Control. 2010, 21, 1009–1016. [Google Scholar] [CrossRef]
- Jackson, M.; Tulloch-Reid, M.; Walker, S.; McFarlane-Anderson, N.; Bennett, F.; Francis, D.; Coard, K. Dietary patterns as predictors of prostate cancer in Jamaican men. Nutr. Cancer 2013, 65, 367–374. [Google Scholar] [CrossRef]
- Jalilpiran, Y.; Dianatinasab, M.; Zeighami, S.; Bahmanpour, S.; Ghiasvand, R.; Mohajeri, S.A.R.; Faghih, S. Western Dietary Pattern, But not Mediterranean Dietary Pattern, Increases the Risk of Prostate Cancer. Nutr. Cancer 2018, 70, 851–859. [Google Scholar] [CrossRef]
- Muller, D.C.; Severi, G.; Baglietto, L.; Krishnan, K.; English, D.R.; Hopper, J.L.; Giles, G.G. Dietary Patterns and Prostate Cancer Risk. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 3126–3129. [Google Scholar] [CrossRef]
- Niclis, C.; Roman, M.D.; Osella, A.R.; Eynard, A.R.; Diaz Mdel, P. Traditional Dietary Pattern Increases Risk of Prostate Cancer in Argentina: Results of a Multilevel Modeling and Bias Analysis from a Case-Control Study. J. Cancer Epidemiol. 2015, 2015, 179562. [Google Scholar] [CrossRef]
- Rosato, V.; Edefonti, V.; Bravi, F.; Bosetti, C.; Bertuccio, P.; Talamini, R.; Dal Maso, L.; Montella, M.; Ferraroni, M.; La Vecchia, C. Nutrient-based dietary patterns and prostate cancer risk: A case-control study from Italy. Cancer Causes Control CCC 2014, 25, 525–532. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Adherence to Mediterranean diet and risk of cancer: An updated systematic review and meta-analysis of observational studies. Cancer Med. 2015, 4, 1933–1947. [Google Scholar] [CrossRef]
- Shin, S.; Saito, E.; Sawada, N.; Ishihara, J.; Takachi, R.; Nanri, A.; Shimazu, T.; Yamaji, T.; Iwasaki, M.; Sasazuki, S.; et al. Dietary patterns and prostate cancer risk in Japanese: The Japan Public Health Center-based Prospective Study (JPHC Study). Cancer Causes Control 2018, 29, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Tseng, M.; Breslow, R.A.; DeVellis, R.F.; Ziegler, R.G. Dietary patterns and prostate cancer risk in the National Health and Nutrition Examination Survey Epidemiological Follow-up Study cohort. Cancer Epidemiol. Biomarkers Prev. 2004, 13, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.; Aronson, K.J.; King, W.; Wilson, J.W.; Fan, W.; Heaton, J.P.; MacNeily, A.; Nickel, J.C.; Morales, A. Dietary patterns and risk of prostate cancer in Ontario, Canada. Int. J. Cancer 2005, 116, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Hu, F.B.; Willett, W.C.; Giovannucci, E. Dietary patterns and risk of prostate cancer in U.S. men. Cancer Epidemiol. Biomarkers Prev. 2006, 15, 167–171. [Google Scholar] [CrossRef]
- Société Canadienne du Cancer: Comité consultatif de la Société canadienne du cancer. Statistiques canadiennes sur le cancer 2019; Société canadienne du cancer: Toronto, ON, Canada, 2019. [Google Scholar]
- Blanc-Lapierre, A.; Spence, A.; Karakiewicz, P.I.; Aprikian, A.; Saad, F.; Parent, M.E. Metabolic syndrome and prostate cancer risk in a population-based case-control study in Montreal, Canada. BMC Public Health 2015, 15, 913. [Google Scholar] [CrossRef]
- Pan, S.Y.; Ugnat, A.M.; Mao, Y.; Wen, S.W.; Johnson, K.C.; Canadian Cancer Registries Epidemiology Research, G. A case-control study of diet and the risk of ovarian cancer. Cancer Epidemiol. Biomarkers Prev. 2004, 13, 1521–1527. [Google Scholar]
- Wright, J.L.; Salinas, C.A.; Lin, D.W.; Kolb, S.; Koopmeiners, J.; Feng, Z.; Stanford, J.L. Prostate cancer specific mortality and Gleason 7 disease differences in prostate cancer outcomes between cases with Gleason 4 + 3 and Gleason 3 + 4 tumors in a population based cohort. J. Urol. 2009, 182, 2702–2707. [Google Scholar] [CrossRef]
- Trudeau, K.; Rousseau, M.C.; Csizmadi, I.; Parent, M.E. Dietary patterns among French-speaking men residing in Montreal, Canada. Prev Med. Rep. 2019, 13, 205–213. [Google Scholar] [CrossRef]
- Norman, G.R.; Streiner, D.L. Biostatistics: The Bare Essentials; B.C. Decker: New York, NC, USA, 2008. [Google Scholar]
- Blanchet, C.; Plante, C.; Rochette, L. La Consommation Alimentaire et les Apports Nutritionnels des Adultes Québécois; Institut national de santé publique du Québec: Quebec City, QC, Canada, 2009; p. 140. [Google Scholar]
- Textor, J.; Hardt, J.; Knuppel, S. DAGitty: A graphical tool for analyzing causal diagrams. Epidemiology 2011, 22, 745. [Google Scholar] [CrossRef]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic; Report of a WHO consultation. World Health Organ. Tech. Report Series 894; World Health Organization: Geneva, Switzerland, 2000; p. 9. [Google Scholar]
- Kaiser, H.F.; Rice, J. Little Jiffy, Mark Iv. Educ. Psychol. Meas. 1974, 34, 111–117. [Google Scholar] [CrossRef]
- Castello, A.; Pollan, M.; Buijsse, B.; Ruiz, A.; Casas, A.M.; Baena-Canada, J.M.; Lope, V.; Antolin, S.; Ramos, M.; Munoz, M.; et al. Spanish Mediterranean diet and other dietary patterns and breast cancer risk: case-control EpiGEICAM study. Br. J. Cancer 2014, 111, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Willet, W. Nutritional Epidemiology, 3nd ed.; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- Newby, P.K.; Tucker, K.L. Empirically derived eating patterns using factor or cluster analysis: A review. Nutr. Rev. 2004, 62, 177–203. [Google Scholar] [CrossRef] [PubMed]
- Canada’s Food Guide. Eat Well. Live Well. Available online: http://www.hc-sc.gc.ca/fn-an/food-guide-aliment/index-eng.php (accessed on 7 March 2019).
- Xu, M.; Richardson, L.; Campbell, S.; Pintos, J.; Siemiatycki, J. Response rates in case-control studies of cancer by era of fieldwork and by characteristics of study design. Ann. Epidemiol. 2018, 28, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Shephard, R.J. Physical activity and prostate cancer: An updated review. Sports Med. 2017, 47, 1055–1073. [Google Scholar] [CrossRef] [PubMed]
- Joseph, M.S.; Tincopa, M.A.; Walden, P.; Jackson, E.; Conte, M.L.; Rubenfire, M. The impact of structured exercise programs on metabolic syndrome and its components: A systematic review. Diabetes Metab. Syndr. Obes. 2019, 12, 2395–2404. [Google Scholar] [CrossRef] [PubMed]
- Streiner, D.L.; Norman, G.R. Correction for multiple testing: Is there a resolution? Chest 2011, 140, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Rothman, K.J. No adjustments are needed for multiple comparisons. Epidemiology 1990, 1, 43–46. [Google Scholar] [CrossRef]
- World Health Organization. WHO Fruit and Vegetable Promotion Initiative—Report of the Meeting; WHO: Geneva, Switzerland, 2003; p. 29. [Google Scholar]
- Demoury, C.; Karakiewicz, P.; Parent, M.E. Association between lifetime alcohol consumption and prostate cancer risk: A case-control study in Montreal, Canada. Cancer Epidemiol. 2016, 45, 11–17. [Google Scholar] [CrossRef]
- VanderWeele, D.J.; Brown, C.D.; Taxy, J.B.; Gillard, M.; Hatcher, D.M.; Tom, W.R.; Stadler, W.M.; White, K.P. Low-grade prostate cancer diverges early from high grade and metastatic disease. Cancer Sci. 2014, 105, 1079–1085. [Google Scholar] [CrossRef]
| Characteristics | Cases | Controls | p-Value | ||
|---|---|---|---|---|---|
| (n = 1919) | (n = 1991) | ||||
| Age in years, mean (SD) | 64 | (6.8) | 65 | (6.9) | <0.001 |
| Ancestry, n (%) | <0.001 | ||||
| Black | 128 | (6.7) | 89 | (4.5) | |
| Asian | 24 | (1.3) | 72 | (3.6) | |
| European | 1693 | (88.2) | 1699 | (85.3) | |
| Greater Middle Eastern | 45 | (2.3) | 100 | (5.0) | |
| Latino | 29 | (1.5) | 31 | (1.6) | |
| Family income in $ CAD, n (%) | 0.54 | ||||
| <20,000 | 223 | (11.6) | 245 | (12.3) | |
| 20,000–29,999 | 262 | (13.7) | 252 | (12.7) | |
| 30,000–49,999 | 445 | (23.2) | 462 | (23.2) | |
| 50,000–79,999 | 422 | (22.0) | 410 | (20.6) | |
| >80,000 | 425 | (22.1) | 428 | (21.5) | |
| Unknown | 142 | (7.4) | 194 | (9.7) | |
| Education, n (%) | 0.34 | ||||
| Primary school of less | 443 | (23.1) | 426 | (21.4) | |
| High school | 572 | (29.8) | 578 | (29.0) | |
| College | 313 | (16.3) | 375 | (18.8) | |
| University | 588 | (30.6) | 610 | (30.6) | |
| Other | 3 | (0.2) | 2 | (0.1) | |
| Body mass index (kg/m2), mean (SD) | 26.8 | (4.0) | 27.2 | (4.4) | 0.003 |
| Ever smoked, n (%) | 0.23 | ||||
| No | 514 | (26.8) | 514 | (25.8) | |
| Yes | 1404 | (73.2) | 1477 | (74.2) | |
| Overall physical activity, n (%) | 0.55 | ||||
| Not very active | 432 | (22.5) | 488 | (24.5) | |
| Moderately active | 522 | (27.2) | 558 | (28.0) | |
| Very active | 965 | (50.3) | 945 | (47.5) | |
| Last prostate screening test, n (%) | < 0.001 | ||||
| ≤2 years before index date | 1903 | (99.2) | 1510 | (75.8) | |
| >2 years before index date | 1 | (0.02) | 235 | (11.8) | |
| Never screened | 2 | (0.1) | 190 | (9.5) | |
| Unknown | 13 | (0.7) | 56 | (2.8) | |
| First-degree relative with prostate cancer, n (%) | < 0.001 | ||||
| No | 1409 | (73.4) | 1736 | (87.2) | |
| Yes | 447 | (23.3) | 199 | (10.0) | |
| Unknown | 63 | (3.3) | 56 | (2.8) | |
| Use of vitamins or mineral supplements, n (%) | 0.08 | ||||
| No | 1184 | (61.7) | 1222 | (61.4) | |
| Yes | 735 | (38.3) | 768 | (38.6) | |
| Total calories 2 years ago (kcal/day), mean (SD) | 1989.0 | (663.4) | 1916.9 | (645.6) | 0.08 |
| Proxy respondent, n (%) | 49.0 | (2.6) | 76.0 | (3.8) | 0.16 |
| Servings per | Rotated Factor Loadings | |||||
|---|---|---|---|---|---|---|
| Food and Beverage Items | Week 1 | Pattern 1 | Pattern 2 | Pattern 3 | ||
| Banana | 2.69 | ± | 2.71 | 0.29 | 0.22 | |
| Apple, pear | 2.84 | ± | 3.38 | 0.55 | ||
| Orange, grapefruit, other citrus fruits | 2.32 | ± | 2.94 | 0.47 | ||
| Peaches, nectarine | 0.61 | ± | 1.31 | 0.54 | ||
| Canned fruit, fruit sauce, fruit salad | 0.62 | ± | 1.50 | 0.45 | ||
| Apricots | 0.26 | ± | 0.95 | 0.44 | ||
| Cantaloupe | 0.48 | ± | 0.89 | 0.52 | ||
| Watermelon, honeydew melon | 0.38 | ± | 0.65 | 0.48 | ||
| Strawberries, raspberries, blueberries | 1.10 | ± | 1.52 | 0.45 | 0.28 | |
| Other fresh fruit | 1.58 | ± | 1.84 | 0.50 | ||
| Potatoes, fried or pan fried | 0.72 | ± | 0.98 | 0.43 | 0.28 | |
| Potatoes, not fried | 2.51 | ± | 2.24 | 0.33 | ||
| Sweet potatoes | 0.17 | ± | 0.59 | 0.29 | ||
| Baked beans, other legumes or lentils | 0.86 | ± | 1.16 | 0.37 | ||
| Broccoli | 1.27 | ± | 1.34 | 0.50 | ||
| Carrots | 2.01 | ± | 1.87 | 0.33 | 0.28 | |
| Spinach | 0.51 | ± | 0.81 | 0.56 | ||
| Coleslaw, cabbage, cauliflower, Brussel’s sprouts | 0.93 | ± | 1.12 | 0.35 | ||
| Dark lettuce | 2.42 | ± | 2.26 | 0.48 | ||
| Tomatoes | 3.08 | ± | 2.45 | 0.37 | ||
| Sweet red peppers | 0.97 | ± | 1.43 | 0.49 | ||
| Other vegetables | 2.81 | ± | 2.16 | 0.34 | ||
| Tomato soup or cream of tomato | 0.34 | ± | 0.68 | 0.40 | ||
| Vegetable soup | 1.25 | ± | 1.50 | 0.22 | 0.30 | |
| Tofu, soybeans | 0.17 | ± | 0.66 | 0.26 | −0.23 | |
| Ketchup, salsa | 0.83 | ± | 1.35 | 0.22 | 0.43 | |
| Salad dressing, mayonnaise (excl. low fat) | 2.24 | ± | 2.36 | 0.27 | 0.27 | |
| Beef | 1.89 | ± | 1.42 | 0.49 | ||
| Pork | 1.03 | ± | 0.90 | 0.46 | ||
| Chicken, turkey, or other poultry | 1.89 | ± | 1.10 | 0.21 | ||
| Veal, lamb | 0.40 | ± | 0.61 | 0.39 | 0.26 | |
| Liver | 0.17 | ± | 0.29 | |||
| Hot-dogs or sausage | 0.42 | ± | 0.66 | 0.50 | 0.27 | |
| BBQ | 1.14 | ± | 1.40 | 0.23 | 0.48 | |
| Cold cuts | 1.23 | ± | 1.64 | 0.48 | 0.28 | |
| Bacon, breakfast sausage | 0.49 | ± | 0.90 | 0.46 | 0.31 | |
| Fish, seafood | 1.29 | ± | 1.02 | 0.43 | ||
| Eggs, omelets, or quiche | 1.79 | ± | 1.87 | 0.28 | ||
| Cheese | 3.80 | ± | 2.76 | 0.22 | 0.26 | |
| Pasta with tomato sauce | 1.07 | ± | 0.93 | 0.25 | 0.29 | |
| Pasta with cheese without tomato sauce | 0.23 | ± | 0.41 | 0.21 | ||
| Pizza | 0.40 | ± | 0.56 | 0.35 | 0.23 | |
| Cookies, muffins | 2.44 | ± | 3.31 | 0.50 | ||
| White bread | 5.52 | ± | 8.16 | −0.30 | 0.35 | |
| Brown bread | 5.86 | ± | 7.33 | 0.45 | −0.24 | |
| Rice | 1.68 | ± | 1.91 | 0.37 | ||
| Donuts, cakes, pastries, and pies | 1.39 | ± | 2.24 | 0.50 | ||
| Oatmeal or cream of wheat | 0.63 | ± | 1.48 | −0.23 | 0.32 | |
| Breakfast cereal | 1.92 | ± | 2.49 | 0.21 | −0.23 | 0.43 |
| Real fruit juice | 3.76 | ± | 4.05 | 0.28 | ||
| Tomato or vegetable juice | 1.07 | ± | 1.84 | 0.39 | ||
| Glass of milk or milk in cereal | 4.64 | ± | 5.74 | −0.23 | 0.45 | |
| Cream or milk in coffee or tea | 12.07 | ± | 13.89 | 0.20 | ||
| Dark carbonated soft drinks | 2.46 | ± | 6.50 | −0.27 | 0.26 | 0.31 |
| Other carbonated soft drinks | 0.85 | ± | 2.66 | 0.21 | ||
| Fried food | 0.28 | ± | 0.58 | 0.21 | ||
| Nuts or peanuts butter | 2.74 | ± | 2.86 | 0.26 | 0.23 | |
| Chips, corn chips, popcorn, tortillas | 0.82 | ± | 1.55 | 0.32 | 0.40 | |
| Chocolate | 0.83 | ± | 1.75 | 0.35 | ||
| Yoghurt | 2.33 | ± | 2.97 | 0.42 | 0.20 | |
| Ice cream | 0.82 | ± | 1.38 | 0.40 | ||
| Fat of beef or pork | 1.44 | ± | 31.66 | 0.28 | ||
| Meat slightly blackened | 1.01 | ± | 1.77 | 0.42 | ||
| Coffee | 14.90 | ± | 14.58 | 0.33 | ||
| Green tea | 1.31 | ± | 4.23 | 0.29 | ||
| Beer | 3.52 | ± | 9.21 | 0.38 | ||
| Wine | 4.05 | ± | 6.99 | 0.35 | 0.39 | |
| Spirits | 1.08 | ± | 4.72 | 0.21 | 0.27 | |
| Proportion of variance explained (%) | 8.6 | 7.2 | 3.7 | |||
| Cumulative variance explained (%) | 8.6 | 15.8 | 19.5 | |||
| Quartiles of Dietary Pattern Score | 1991 Controls | All Prostate Cancers 1917 Cases | Low-Grade Prostate Cancers 2 1385 Cases | High-Grade Prostate Cancers 3 529 Cases |
|---|---|---|---|---|
| n | n Cases/OR (95% CI) | n Cases/OR (95% CI) | n Cases/OR (95% CI) | |
| Healthy Eating | ||||
| 1 | 497 | 499/1.00 (reference) | 335/1.00 (reference) | 163/1.00 (reference) |
| 2 | 499 | 505/0.95 (0.78–1.15) | 378/1.07 (0.86–1.32) | 126/0.75 (0.57–0.99) |
| 3 | 497 | 477/0.84 (0.68–1.02) | 343/0.91 (0.73–1.14) | 133/0.77 (0.58–1.02) |
| 4 | 498 | 436/0.76 (0.61–0.93) | 329/0.90 (0.71–1.13) | 107/0.66 (0.48–0.89) |
| ptrend = 0.004 | ptrend = 0.16 | ptrend = 0.008 | ||
| Western Salty and Alcohol | ||||
| 1 | 498 | 457/1.00 (reference) | 325/1.00 (reference) | 132/1.00 (reference) |
| 2 | 498 | 482/0.92 (0.76–1.13) | 331/0.88 (0.70–1.10) | 149/0.99 (0.75–1.32) |
| 3 | 497 | 482/0.87 (0.71–1.06) | 355/0.88 (0.71–1.10) | 126/0.82 (0.61–1.10) |
| 4 | 498 | 496/0.89 (0.72–1.09) | 374/0.91 (0.73–1.14) | 122/0.78 (0.58–1.06) |
| ptrend = 0.21 | ptrend = 0.59 | ptrend = 0.05 | ||
| Western Sweet and Beverages | ||||
| 1 | 498 | 388/1.00 (reference) | 285/1.00 (reference) | 103/1.00 (reference) |
| 2 | 498 | 442/1.01 (0.82–1.24) | 325/0.96 (0.76–1.20) | 117/0.96 (0.70–1.30) |
| 3 | 498 | 494/1.12 (0.91–1.38) | 360/1.01 (0.80–1.27) | 134/1.06 (0.78–1.45) |
| 4 | 497 | 593/1.35 (1.10–1.66) | 415/1.14 (0.91–1.44) | 175/1.32 (0.98–1.80) |
| ptrend = 0.002 | ptrend = 0.23 | ptrend = 0.02 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trudeau, K.; Rousseau, M.-C.; Barul, C.; Csizmadi, I.; Parent, M.-É. Dietary Patterns Are Associated with Risk of Prostate Cancer in a Population-Based Case-Control Study in Montreal, Canada. Nutrients 2020, 12, 1907. https://doi.org/10.3390/nu12071907
Trudeau K, Rousseau M-C, Barul C, Csizmadi I, Parent M-É. Dietary Patterns Are Associated with Risk of Prostate Cancer in a Population-Based Case-Control Study in Montreal, Canada. Nutrients. 2020; 12(7):1907. https://doi.org/10.3390/nu12071907
Chicago/Turabian StyleTrudeau, Karine, Marie-Claude Rousseau, Christine Barul, Ilona Csizmadi, and Marie-Élise Parent. 2020. "Dietary Patterns Are Associated with Risk of Prostate Cancer in a Population-Based Case-Control Study in Montreal, Canada" Nutrients 12, no. 7: 1907. https://doi.org/10.3390/nu12071907
APA StyleTrudeau, K., Rousseau, M.-C., Barul, C., Csizmadi, I., & Parent, M.-É. (2020). Dietary Patterns Are Associated with Risk of Prostate Cancer in a Population-Based Case-Control Study in Montreal, Canada. Nutrients, 12(7), 1907. https://doi.org/10.3390/nu12071907





