Rice Bran Phenolic Extracts Modulate Insulin Secretion and Gene Expression Associated with β-Cell Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Rice Bran Phenolic Extract Preparation
2.3. Cell Culture Conditions
2.4. Cytotoxicity Assay
2.5. Expression of Genes Associated with β-Cell Function
2.5.1. Experimental Design
2.5.2. Gene Expression Analysis
2.6. Glucose-Stimulated Insulin Secretion
2.7. Statistical Analysis
3. Results
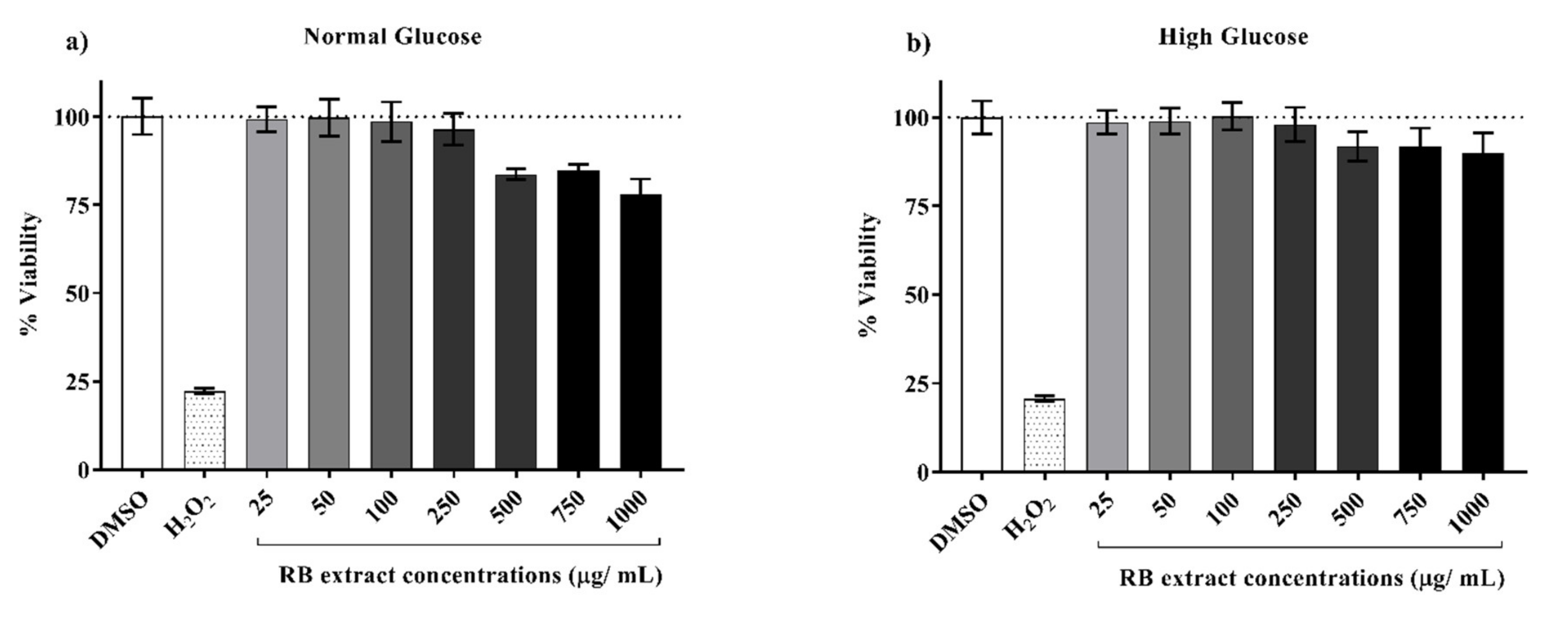
3.1. Cytotoxicity of RB Phenolic Extracts on INS-1E Cells
3.2. Effect of RB Phenolic Extracts on Expression of Genes Associated with β-Cell Function
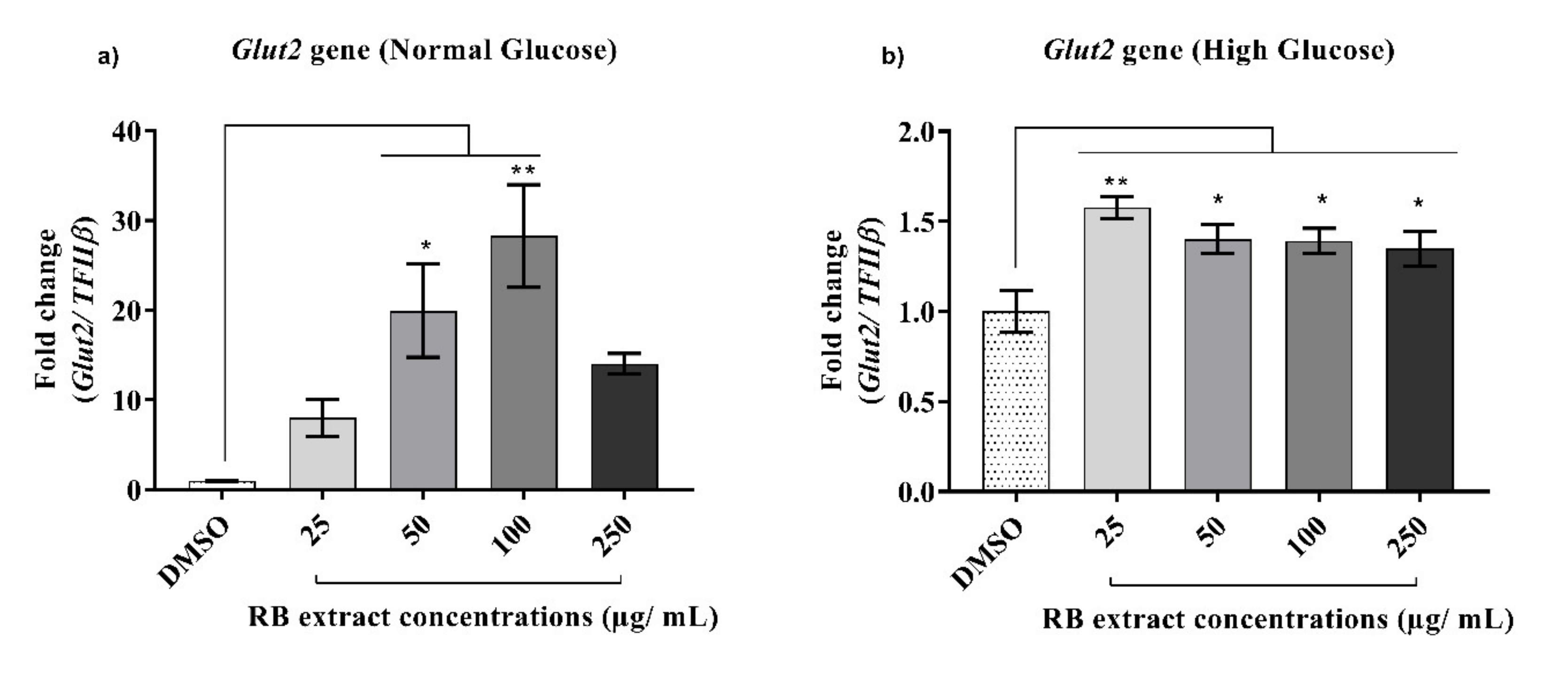
3.2.1. Expression of the Glut2 Gene
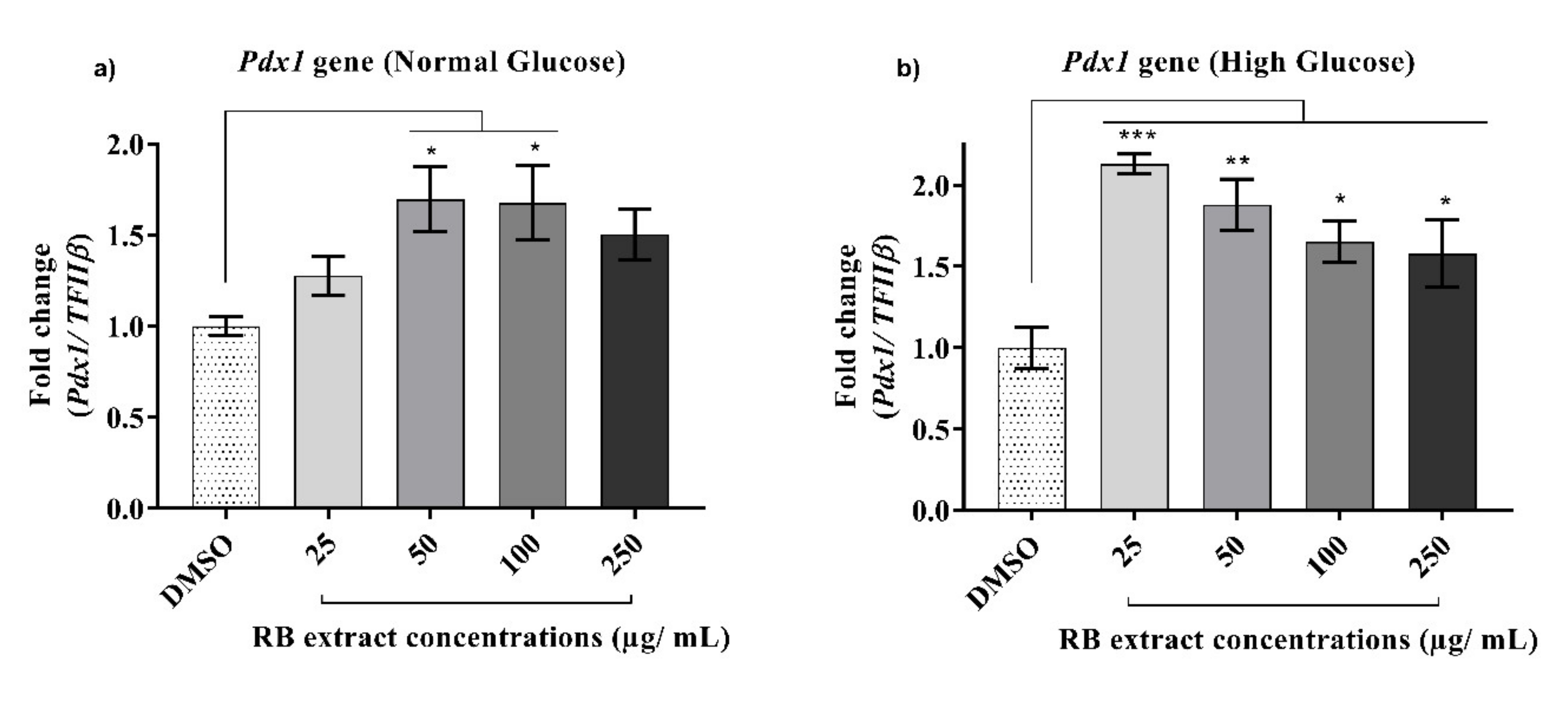
3.2.2. Expression of the Pdx1 Gene
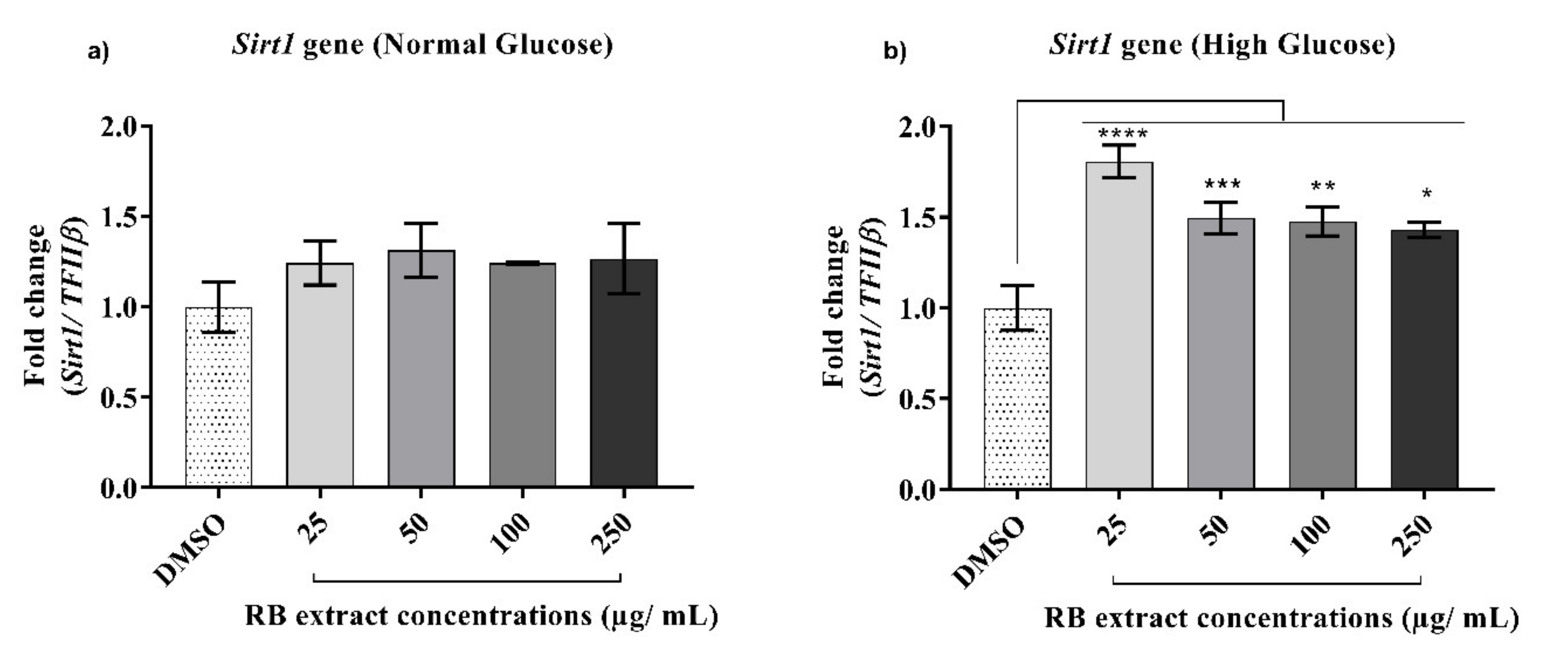
3.2.3. Expression of the Sirt1 Gene
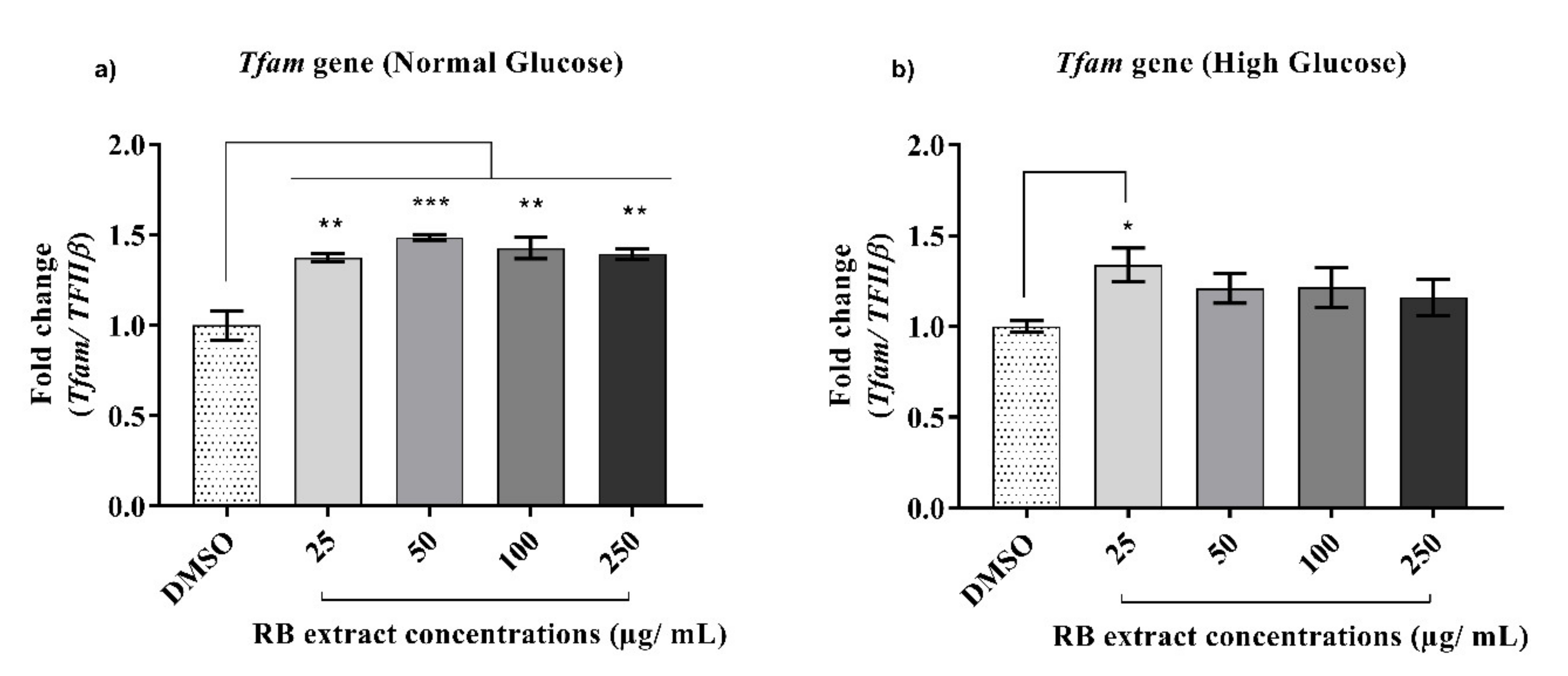
3.2.4. Expression of the Tfam Gene
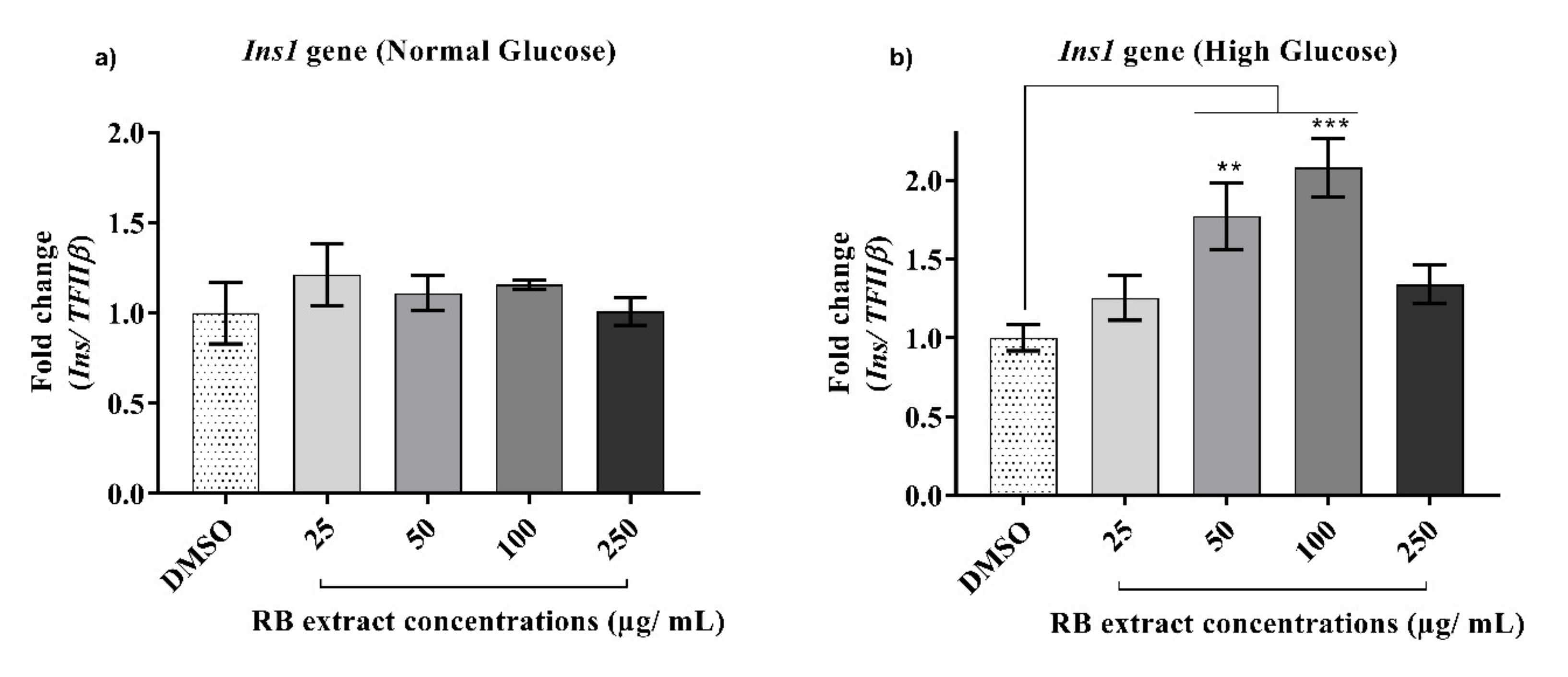
3.2.5. Expression of the Ins1 Gene
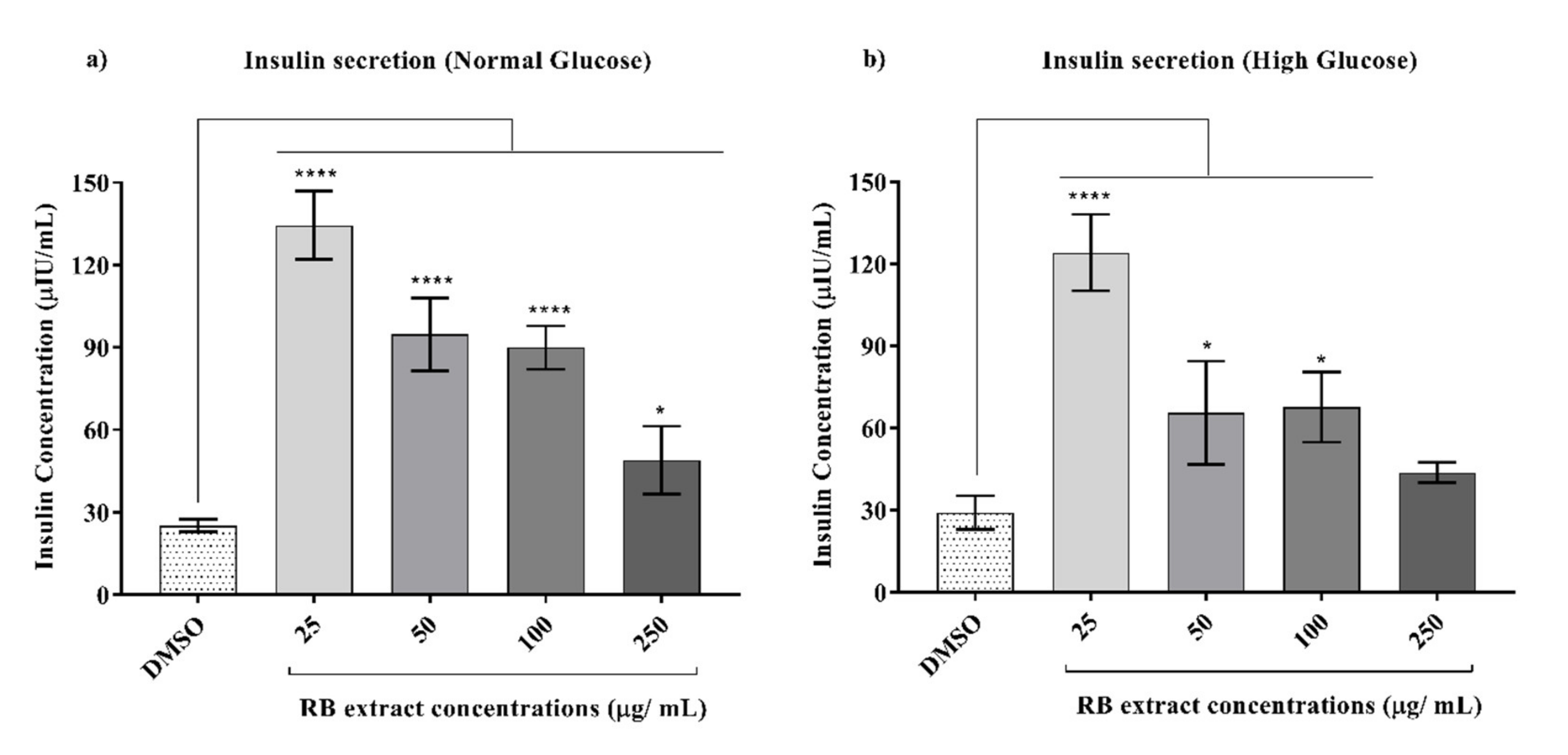
3.3. Glucose-Stimulated Insulin Secretion
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Leko, V.; Varnum-Finney, B.; Li, H.; Gu, Y.; Flowers, D.; Nourigat, C.; Bernstein, I.D.; Bedalov, A. SIRT1 is dispensable for function of hematopoietic stem cells in adult mice. Blood J. Am. Soc. Hematol. 2012, 119, 1856–1860. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.C.; Williams, D.; Vicogne, J.; Pessin, J.E. The glucose transporter 2 undergoes plasma membrane endocytosis and lysosomal degradation in a secretagogue-dependent manner. Endocrinology 2009, 150, 4056–4064. [Google Scholar] [CrossRef] [PubMed]
- Keane, K.N.; Cruzat, V.F.; Carlessi, R.; de Bittencourt, P.I.H.; Newsholme, P. Molecular events linking oxidative stress and inflammation to insulin resistance and β-cell dysfunction. Oxidative Med. Cell. Longev. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.G.; Francis, N.; Hill, R.; LE Waters, D.; Blanchard, C.L.; Santhakumar, A.B. Coloured rice phenolic extracts increase expression of genes associated with insulin secretion in rat pancreatic insulinoma β-cells. Int. J. Mol. Sci. 2020, 21, 3314. [Google Scholar] [CrossRef] [PubMed]
- Thorens, B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia 2015, 58, 221–232. [Google Scholar] [CrossRef]
- Gao, N.; LeLay, J.; Vatamaniuk, M.Z.; Rieck, S.; Friedman, J.R.; Kaestner, K.H. Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development. Genes Dev. 2008, 22, 3435–3448. [Google Scholar] [CrossRef]
- Imai, S.-i.; Kiess, W. Therapeutic potential of SIRT1 and NAMPT-mediated NAD biosynthesis in type 2 diabetes. Front. Biosci. J. Virtual Libr. 2009, 14, 2983. [Google Scholar] [CrossRef]
- Lezza, A.M. Mitochondrial transcription factor A (TFAM): One actor for different roles. Front. Biol. 2012, 7, 30–39. [Google Scholar] [CrossRef]
- Kataoka, K.; Han, S.-i.; Shioda, S.; Hirai, M.; Nishizawa, M.; Handa, H. MafA is a glucose-regulated and pancreatic β-cell-specific transcriptional activator for the insulin gene. J. Biol. Chem. 2002, 277, 49903–49910. [Google Scholar] [CrossRef]
- Campbell, S.C.; Macfarlane, W.M. Regulation of the pdx1 gene promoter in pancreatic β-cells. Biochem. Biophys. Res. Commun. 2002, 299, 277–284. [Google Scholar] [CrossRef]
- Gemma, C.; Sookoian, S.; Dieuzeide, G.; García, S.I.; Gianotti, T.F.; González, C.D.; Pirola, C.J. Methylation of TFAM gene promoter in peripheral white blood cells is associated with insulin resistance in adolescents. Mol. Genet. Metab. 2010, 100, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Gianotti, T.F.; Sookoian, S.; Dieuzeide, G.; García, S.I.; Gemma, C.; González, C.D.; Pirola, C.J. A decreased mitochondrial DNA content is related to insulin resistance in adolescents. Obesity 2008, 16, 1591–1595. [Google Scholar] [CrossRef] [PubMed]
- Mehran, A.E.; Templeman, N.M.; Brigidi, G.S.; Lim, G.E.; Chu, K.-Y.; Hu, X.; Botezelli, J.D.; Asadi, A.; Hoffman, B.G.; Kieffer, T.J. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab. 2012, 16, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Kaup, R.M.; Khayyal, M.T.; Verspohl, E.J. Antidiabetic effects of a standardized egyptian rice bran extract. Phytother. Res. 2013. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Oksbjerg, N.; Young, J.; Jeppesen, P. Caffeic acid, naringenin and quercetin enhance glucose-stimulated insulin secretion and glucose sensitivity in INS-1E cells. Diabetes Obes. Metab. 2014, 16, 602–612. [Google Scholar] [CrossRef]
- Cheng, A.-S.; Cheng, Y.-H.; Chang, T.-L. Resveratrol protects RINm5F pancreatic cells from methylglyoxal-induced apoptosis. J. Funct. Foods 2013, 5, 1774–1783. [Google Scholar] [CrossRef]
- Dall’Asta, M.; Bayle, M.; Neasta, J.; Scazzina, F.; Bruni, R.; Cros, G.; Del Rio, D.; Oiry, C. Protection of pancreatic β-cell function by dietary polyphenols. Phytochem. Rev. 2015, 14, 933–959. [Google Scholar] [CrossRef]
- Saji, N.; Schwarz, L.J.; Santhakumar, A.B.; Blanchard, C.L. Stabilization treatment of rice bran alters phenolic content and antioxidant activity. Cereal Chem. 2020, 97, 281–292. [Google Scholar] [CrossRef]
- Saji, N.; Francis, N.; Schwarz, L.J.; Blanchard, C.L.; Santhakumar, A.B. Rice bran derived bioactive compounds modulate risk factors of cardiovascular disease and type 2 diabetes mellitus: An updated review. Nutrients 2019, 11, 2736. [Google Scholar] [CrossRef]
- Saji, N.; Francis, N.; Blanchard, C.L.; Schwarz, L.J.; Santhakumar, A.B. Rice Bran phenolic compounds regulate genes associated with antioxidant and anti-inflammatory activity in human umbilical vein endothelial cells with induced oxidative stress. Int. J. Mol. Sci. 2019, 20, 4715. [Google Scholar] [CrossRef]
- Vetterli, L.; Brun, T.; Giovannoni, L.; Bosco, D.; Maechler, P. Resveratrol potentiates glucose-stimulated insulin secretion in INS-1E β-cells and human islets through a SIRT1-dependent mechanism. J. Biol. Chem. 2011, 286, 6049–6060. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.; Janovjak, H.; Miserez, A.; Dobbie, Z. Processing of gene expression data generated by quantitative real-time RT PCR (vol 32, pg 1378, 2002). Biotechniques 2002, 33, 514. [Google Scholar]
- Dharmani, M.; Kamarulzaman, K.; Giribabu, N.; Choy, K.; Zuhaida, M.; Aladdin, N.; Jamal, J.; Mustafa, M. Effect of Marantodes pumilum Blume (Kuntze) var. alata on β-cell function and insulin signaling in ovariectomised diabetic rats. Phytomedicine 2019, 65, 153101. [Google Scholar] [CrossRef] [PubMed]
- Henquin, J.-C. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 2000, 49, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Grondin, M.; Robinson, I.; Do Carmo, S.; Ali-Benali, M.A.; Ouellet, F.; Mounier, C.; Sarhan, F.; Averill-Bates, D.A. Cryopreservation of insulin-secreting INS832/13 cells using a wheat protein formulation. Cryobiology 2013, 66, 136–143. [Google Scholar] [CrossRef]
- Tabatabaie, P.S.; Yazdanparast, R. Teucrium polium extract reverses symptoms of streptozotocin-induced diabetes in rats via rebalancing the Pdx1 and FoxO1 expressions. Biomed. Pharmacother. 2017, 93, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Sameermahmood, Z.; Raji, L.; Saravanan, T.; Vaidya, A.; Mohan, V.; Balasubramanyam, M. Gallic acid protects RINm5F β-cells from glucolipotoxicity by its antiapoptotic and insulin-secretagogue actions. Phytother. Res. 2010, 24, S83–S94. [Google Scholar] [CrossRef] [PubMed]
- Kawamori, D.; Kaneto, H.; Nakatani, Y.; Matsuoka, T.-a.; Matsuhisa, M.; Hori, M.; Yamasaki, Y. The forkhead transcription factor Foxo1 bridges the JNK pathway and the transcription factor PDX-1 through its intracellular translocation. J. Biol. Chem. 2006, 281, 1091–1098. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, F.; Ge, X.; Yan, T.; Chen, X.; Shi, X.; Zhai, Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007, 6, 307–319. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; Fernández-Quintela, A.; Aguirre, L.; Macarulla, M.; Rimando, A.; Portillo, M. Pterostilbene improves glycaemic control in rats fed an obesogenic diet: Involvement of skeletal muscle and liver. Food Funct. 2015, 6, 1968–1976. [Google Scholar] [CrossRef]
- Lu, C.; Zhu, W.; Shen, C.-L.; Gao, W. Green tea polyphenols reduce body weight in rats by modulating obesity-related genes. PLoS ONE 2012, 7, e38332. [Google Scholar] [CrossRef] [PubMed]
- Puddu, A.; Storace, D.; Odetti, P.; Viviani, G. Advanced glycation end-products affect transcription factors regulating insulin gene expression. Biochem. Biophys. Res. Commun. 2010, 395, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Park, H.-M.; Ji, H.-S.; Han, J.; Kim, S.-K.; Park, H.-Y.; Jeong, T.-S. Phenolic-enriched blueberry-leaf extract attenuates glucose homeostasis, pancreatic β-cell function, and insulin sensitivity in high-fat diet–induced diabetic mice. Nutr. Res. 2020, 73, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of dietary polyphenols on carbohydrate metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef]
- Pramote, K.; Nucha, S.; Suched, S.; Parinda, P.; Prasong, S.; Shuji, A. Subcritical water extraction of flavoring and phenolic compounds from cinnamon bark (Cinnamomum zeylanicum). J. Oleo Sci. 2012, 61, 349–355. [Google Scholar]
- Woods, S.C.; Lutz, T.A.; Geary, N.; Langhans, W. Pancreatic signals controlling food intake; insulin, glucagon and amylin. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1219–1235. [Google Scholar] [CrossRef]
- Nomura, E.; Kashiwada, A.; Hosoda, A.; Nakamura, K.; Morishita, H.; Tsuno, T.; Taniguchi, H. Synthesis of amide compounds of ferulic acid, and their stimulatory effects on insulin secretion In vitro. Bioorganic Med. Chem. 2003, 11, 3807–3813. [Google Scholar] [CrossRef]
- Zabad, O.M.; Samra, Y.A.; Eissa, L.A. P-Coumaric acid alleviates experimental diabetic nephropathy through modulation of Toll like receptor-4 in rats. Life Sci. 2019, 238, 116965. [Google Scholar] [CrossRef]
- Mattson, M.P. Hormesis defined. Ageing Res. Rev. 2008, 7, 1–7. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Trovato-Salinaro, A.; Cambria, M.T.; Locascio, M.; Rienzo, L.; Condorelli, D.; Mancuso, C.; De Lorenzo, A.; Calabrese, E. The hormetic role of dietary antioxidants in free radical-related diseases. Curr. Pharm. Des. 2010, 16, 877–883. [Google Scholar] [CrossRef]
- Karakaya, S. Bioavailability of phenolic compounds. Crit. Rev. Food Sci. Nutr. 2004, 44, 453–464. [Google Scholar] [CrossRef] [PubMed]
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Glut2 | TCAGCCAGCCTGTGTATGCA | TCCACAAGCAGCACAGAGACA |
| Pdx1 | CCGCGTTCATCTCCCTTT C | CTCCTGCCCACTGGCTTT T |
| Sirt1 | CAGTGTCATGGTTCCTTTGC | CACCGAGGAACTACCTGA T |
| Tfam | GGGAAGAGCAAATGGCTGAA | TCACACTGCGACGGATGA GA |
| Ins1 | TGCTCACCCGCGACCTT | GTTCATATGCACCACTGGACTGAA |
| TfIIβ | GTTCTGCTCCAACCTTTGCCT | TGTGTAGCTGCCATCTGCACT T |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saji, N.; Francis, N.; Schwarz, L.J.; Blanchard, C.L.; Santhakumar, A.B. Rice Bran Phenolic Extracts Modulate Insulin Secretion and Gene Expression Associated with β-Cell Function. Nutrients 2020, 12, 1889. https://doi.org/10.3390/nu12061889
Saji N, Francis N, Schwarz LJ, Blanchard CL, Santhakumar AB. Rice Bran Phenolic Extracts Modulate Insulin Secretion and Gene Expression Associated with β-Cell Function. Nutrients. 2020; 12(6):1889. https://doi.org/10.3390/nu12061889
Chicago/Turabian StyleSaji, Nancy, Nidhish Francis, Lachlan J. Schwarz, Christopher L. Blanchard, and Abishek B. Santhakumar. 2020. "Rice Bran Phenolic Extracts Modulate Insulin Secretion and Gene Expression Associated with β-Cell Function" Nutrients 12, no. 6: 1889. https://doi.org/10.3390/nu12061889
APA StyleSaji, N., Francis, N., Schwarz, L. J., Blanchard, C. L., & Santhakumar, A. B. (2020). Rice Bran Phenolic Extracts Modulate Insulin Secretion and Gene Expression Associated with β-Cell Function. Nutrients, 12(6), 1889. https://doi.org/10.3390/nu12061889







