Associations of Urinary Phytoestrogen Concentrations with Sleep Disorders and Sleep Duration among Adults
Abstract
1. Introduction
2. Materials and Methods
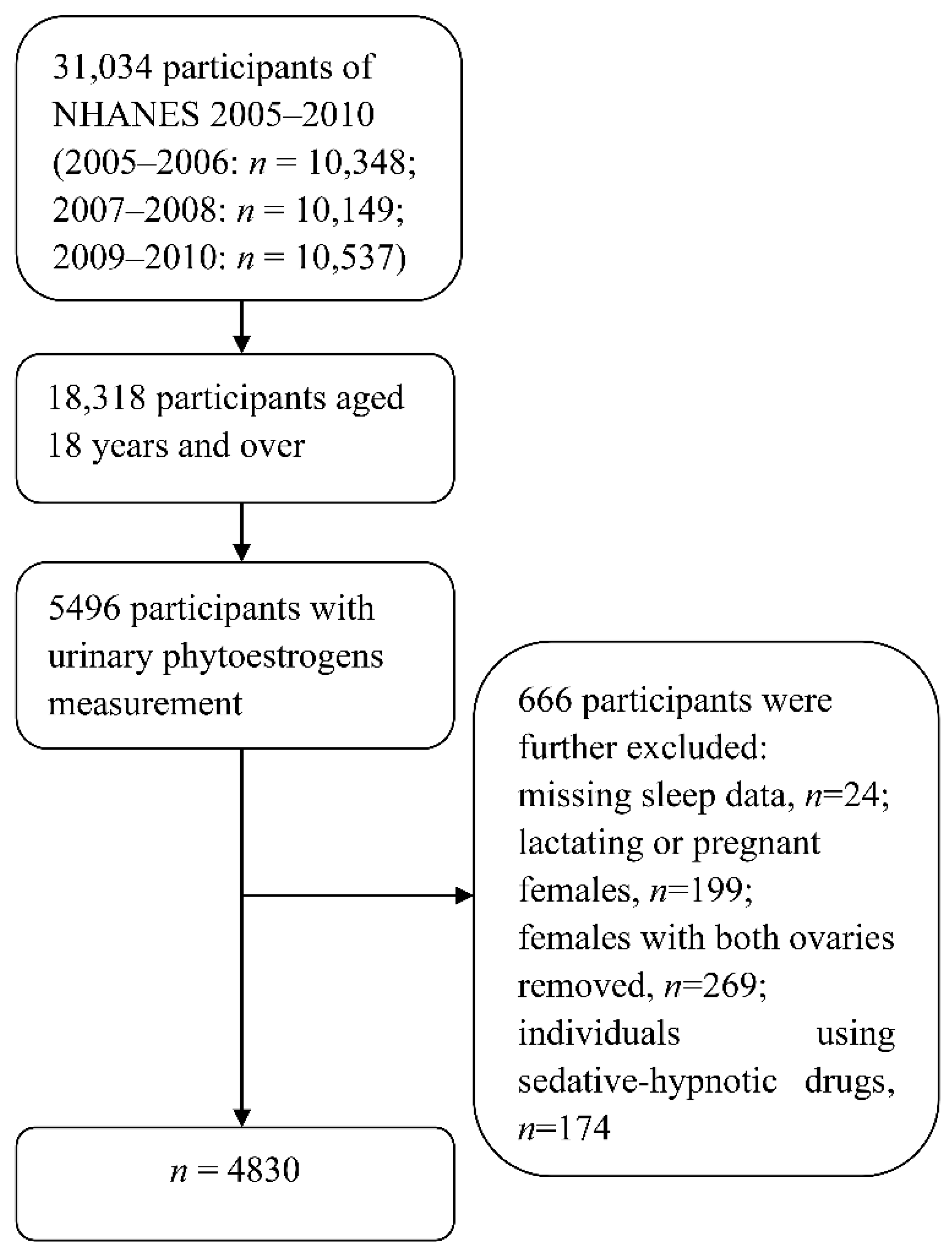
2.1. Study Population
2.2. Urinary Phytoestrogens Measurement
2.3. Sleep Disorders and Sleep Duration Assessments
2.4. Covariates
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sateia, M.J. International classification of sleep disorders-third edition: Highlights and modifications. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, M.K.; Latreille, V. Sleep Disorders. Am. J. Med. 2019, 132, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S.; Lin, L.; Austin, D.; Young, T.; Mignot, E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004, 1, e62. [Google Scholar] [CrossRef] [PubMed]
- Hoevenaar-Blom, M.P.; Spijkerman, A.M.; Kromhout, D.; Verschuren, W.M. Sufficient sleep duration contributes to lower cardiovascular disease risk in addition to four traditional lifestyle factors: The MORGEN study. Eur. J. Prev. Cardiol. 2014, 21, 1367–1375. [Google Scholar] [CrossRef]
- Ayas, N.T.; White, D.P.; Al-Delaimy, W.K.; Manson, J.E.; Stampfer, M.J.; Speizer, F.E.; Patel, S.; Hu, F.B. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care 2003, 26, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Zheng, Y.; Hui, R. The relationship of sleep duration and insomnia to risk of hypertension incidence: A meta-analysis of prospective cohort studies. Hypertens. Res. 2013, 36, 985–995. [Google Scholar] [CrossRef]
- Gozal, D.; Farre, R.; Nieto, F.J. Obstructive sleep apnea and cancer: Epidemiologic links and theoretical biological constructs. Sleep Med. Rev. 2016, 27, 43–55. [Google Scholar] [CrossRef]
- Yin, J.; Jin, X.; Shan, Z.; Li, S.; Huang, H.; Li, P.; Peng, X.; Peng, Z.; Yu, K.; Bao, W.; et al. Relationship of sleep duration with all-cause mortality and cardiovascular events: A systematic review and dose-response meta-analysis of prospective cohort studies. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- McEwen, B.S.; Alves, S.E. Estrogen actions in the central nervous system. Endocr. Rev. 1999, 20, 279–307. [Google Scholar] [CrossRef]
- Wiklund, I.; Karlberg, J.; Mattsson, L.A. Quality of life of postmenopausal women on a regimen of transdermal estradiol therapy: A double-blind placebo-controlled study. Am. J. Obstet. Gynecol. 1993, 168, 824–830. [Google Scholar] [CrossRef]
- Ensrud, K.E.; Guthrie, K.A.; Hohensee, C.; Caan, B.; Carpenter, J.S.; Freeman, E.W.; LaCroix, A.Z.; Landis, C.A.; Manson, J.; Newton, K.M.; et al. Effects of estradiol and venlafaxine on insomnia symptoms and sleep quality in women with hot flashes. Sleep 2015, 38, 97–108. [Google Scholar] [CrossRef]
- Polo-Kantola, P.; Erkkola, R.; Helenius, H.; Irjala, K.; Polo, O. When does estrogen replacement therapy improve sleep quality? Am. J. Obstet. Gynecol. 1998, 178, 1002–1009. [Google Scholar] [CrossRef]
- Canonico, M.; Plu-Bureau, G.; Lowe, G.D.; Scarabin, P.Y. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: Systematic review and meta-analysis. Brit. Med. J. 2008, 336, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- Furness, S.; Roberts, H.; Marjoribanks, J.; Lethaby, A. Hormone therapy in postmenopausal women and risk of endometrial hyperplasia. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef] [PubMed]
- Benson, V.S.; Kirichek, O.; Beral, V.; Green, J. Menopausal hormone therapy and central nervous system tumor risk: Large UK prospective study and meta-analysis. Int. J. Cancer 2015, 136, 2369–2377. [Google Scholar] [CrossRef]
- Greiser, C.M.; Greiser, E.M.; Doren, M. Menopausal hormone therapy and risk of breast cancer: A meta-analysis of epidemiological studies and randomized controlled trials. Hum. Reprod. Update 2005, 11, 561–573. [Google Scholar] [CrossRef][Green Version]
- Greiser, C.M.; Greiser, E.M.; Doren, M. Menopausal hormone therapy and risk of ovarian cancer: Systematic review and meta-analysis. Hum. Reprod. Update 2007, 13, 453–463. [Google Scholar] [CrossRef]
- Nie, Q.; Xing, M.; Hu, J.; Hu, X.; Nie, S.; Xie, M. Metabolism and health effects of phyto-estrogens. Crit. Rev. Food Sci. Nutr. 2017, 57, 2432–2454. [Google Scholar] [CrossRef]
- Mueller, S.O.; Simon, S.; Chae, K.; Metzler, M.; Korach, K.S. Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (ERalpha) and ERbeta in human cells. Toxicol. Sci. 2004, 80, 14–25. [Google Scholar] [CrossRef]
- Patisaul, H.B.; Jefferson, W. The pros and cons of phytoestrogens. Front. Neuroendocrinol. 2010, 31, 400–419. [Google Scholar] [CrossRef]
- Thompson, L.U.; Robb, P.; Serraino, M.; Cheung, F. Mammalian lignan production from various foods. Nutr. Cancer 1991, 16, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Knight, D.C.; Eden, J.A. Phytoestrogens—A short review. Maturitas 1995, 22, 167–175. [Google Scholar] [CrossRef]
- Lampe, J.W. Isoflavonoid and lignan phytoestrogens as dietary biomarkers. J. Nutr. 2003, 133 (Suppl. 3), 956s–964s. [Google Scholar] [CrossRef] [PubMed]
- Lampe, J.W.; Atkinson, C.; Hullar, M.A. Assessing exposure to lignans and their metabolites in humans. J. AOAC Int. 2006, 89, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.; Frankenfeld, C.L.; Lampe, J.W. Gut bacterial metabolism of the soy isoflavone daidzein: Exploring the relevance to human health. Exp. Biol. Med. 2005, 230, 155–170. [Google Scholar] [CrossRef]
- Kuhnle, G.G.; Dell‘Aquila, C.; Aspinall, S.M.; Runswick, S.A.; Mulligan, A.A.; Bingham, S.A. Phytoestrogen content of foods of animal origin: Dairy products, eggs, meat, fish, and seafood. J. Agric. Food Chem. 2008, 56, 10099–10104. [Google Scholar] [CrossRef] [PubMed]
- Frankenfeld, C.L. Dairy consumption is a significant correlate of urinary equol concentration in a representative sample of US adults. Am. J. Clin. Nutr. 2011, 93, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Albert, A.; Altabre, C.; Baró, F.; Buendía, E.; Cabero, A.; Cancelo, M.J.; Castelo-Branco, C.; Chantre, P.; Duran, M.; Haya, J.; et al. Efficacy and safety of a phytoestrogen preparation derived from Glycine max (L.) Merr in climacteric symptomatology: A multicentric, open, prospective and non-randomized trial. Phytomedicine 2002, 9, 85–92. [Google Scholar] [CrossRef]
- Hachul, H.; Brandao, L.C.; D‘Almeida, V.; Bittencourt, L.R.; Baracat, E.C.; Tufik, S. Isoflavones decrease insomnia in postmenopause. Menopause 2011, 18, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Scapagnini, G.; Marzatico, F.; Nobile, V.; Ferrara, N.; Corbi, G. Influence of equol and resveratrol supplementation on health-related quality of life in menopausal women: A randomized, placebo-controlled study. Maturitas 2017, 96, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Hirose, A.; Terauchi, M.; Akiyoshi, M.; Owa, Y.; Kato, K.; Kubota, T. Low-dose isoflavone aglycone alleviates psychological symptoms of menopause in Japanese women: A randomized, double-blind, placebo-controlled study. Arch. Gynecol. Obstet. 2016, 293, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Uesugi, S.; Watanabe, S.; Ishiwata, N.; Uehara, M.; Ouchi, K. Effects of isoflavone supplements on bone metabolic markers and climacteric symptoms in Japanese women. Biofactors 2004, 22, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Wisniewski, A.; Braga-Basaria, M.; Xu, X.; Yep, M.; Denmeade, S.; Dobs, A.S.; DeWeese, T.; Carducci, M.; Basaria, S. Lack of an effect of high dose isoflavones in men with prostate cancer undergoing androgen deprivation therapy. J. Urol. 2009, 182, 2265–2272. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.G.; Giolo, J.S.; Mariano, I.M.; Batista, J.P.; Ribeiro, A.; Souza, T.; de Oliveira, E.P.; Resende, A.; Puga, G.M. Combined exercise training reduces climacteric symptoms without the additive effects of isoflavone supplementation: A clinical, controlled, randomised, double-blind study. Nutr. Health 2017, 23, 271–279. [Google Scholar] [CrossRef]
- Balk, J.L.; Whiteside, D.A.; Naus, G.; DeFerrari, E.; Roberts, J.M. A pilot study of the effects of phytoestrogen supplementation on postmenopausal endometrium. J. Soc. Gynecol. Investig. 2002, 9, 238–242. [Google Scholar] [CrossRef]
- Cui, Y.; Niu, K.; Huang, C.; Momma, H.; Guan, L.; Kobayashi, Y.; Guo, H.; Chujo, M.; Otomo, A.; Nagatomi, R. Relationship between daily isoflavone intake and sleep in Japanese adults: A cross-sectional study. Nutr. J. 2015, 14, 127. [Google Scholar] [CrossRef]
- Cao, Y.; Taylor, A.W.; Zhen, S.; Adams, R.; Appleton, S.; Shi, Z. Soy isoflavone intake and sleep parameters over 5 years among Chinese adults: Longitudinal analysis from the Jiangsu Nutrition Study. J. Acad. Nutr. Diet. 2017, 117, 536–544 e532. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, L.; Wang, G.; He, Z.; Zhao, Y.; Xu, Y.; Gao, Y.; Zhang, L. Sedative and hypnotic effects of supercritical carbon dioxide fluid extraction from Schisandra chinensis in mice. J. Food Drug Anal. 2016, 24, 831–838. [Google Scholar] [CrossRef]
- Li, N.; Liu, J.; Wang, M.; Yu, Z.; Zhu, K.; Gao, J.; Wang, C.; Sun, J.; Chen, J.; Li, H. Sedative and hypnotic effects of Schisandrin B through increasing GABA/Glu ratio and upregulating the expression of GABAA in mice and rats. Biomed. Pharmacother. 2018, 103, 509–516. [Google Scholar] [CrossRef]
- Hu, C.; Yuan, Y.V.; Kitts, D.D. Antioxidant activities of the flaxseed lignan secoisolariciresinol diglucoside, its aglycone secoisolariciresinol and the mammalian lignans enterodiol and enterolactone in vitro. Food Chem. Toxicol. 2007, 45, 2219–2227. [Google Scholar] [CrossRef]
- Muthyala, R.S.; Ju, Y.H.; Sheng, S.; Williams, L.D.; Doerge, D.R.; Katzenellenbogen, B.S.; Helferich, W.G.; Katzenellenbogen, J.A. Equol, a natural estrogenic metabolite from soy isoflavones: Convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg. Med. Chem. 2004, 12, 1559–1567. [Google Scholar] [CrossRef]
- Montalesi, E.; Cipolletti, M.; Cracco, P.; Fiocchetti, M.; Marino, M. Divergent effects of daidzein and its metabolites on estrogen-induced survival of breast cancer cells. Cancers 2020, 12, 167. [Google Scholar] [CrossRef] [PubMed]
- Carreau, C.; Flouriot, G.; Bennetau-Pelissero, C.; Potier, M. Enterodiol and enterolactone, two major diet-derived polyphenol metabolites have different impact on ERalpha transcriptional activation in human breast cancer cells. J. Steroid Biochem. Mol. Biol. 2008, 110, 176–185. [Google Scholar] [CrossRef]
- Reger, M.K.; Zollinger, T.W.; Liu, Z.; Jones, J.; Zhang, J. Urinary phytoestrogens and cancer, cardiovascular, and all-cause mortality in the continuous National Health and Nutrition Examination Survey. Eur. J. Nutr. 2016, 55, 1029–1040. [Google Scholar] [CrossRef]
- Richard, A.; Rohrmann, S.; Mohler-Kuo, M.; Rodgers, S.; Moffat, R.; Güth, U.; Eichholzer, M. Urinary phytoestrogens and depression in perimenopausal US women: NHANES 2005–2008. J. Affect. Disord. 2014, 156, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, Q.; Zhang, Q.; Gu, A.; Jiang, Z.Y. Urinary enterolactone is associated with obesity and metabolic alteration in men in the US National Health and Nutrition Examination Survey 2001-10. Br. J. Nutr. 2015, 113, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. Available online: https://www.cdc.gov/nchs/nhanes/index.htm (accessed on 28 April 2020).
- Zipf, G.; Chiappa, M.; Porter, K.S.; Ostchega, Y.; Lewis, B.G.; Dostal, J. National health and nutrition examination survey: Plan and operations, 1999–2010. Vital Health Stat 1 2013, 56, 1–37. [Google Scholar]
- Centers for Disease Control and Prevention, National Health and Nutrition Examination Survey. Laboratory Procedure Manual, Phytoestrogens. Available online: http://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/Phyto_F_met_phytoestrogens.pdf (accessed on 28 April 2020).
- Grace, P.B.; Taylor, J.I.; Low, Y.L.; Luben, R.N.; Mulligan, A.A.; Botting, N.P.; Dowsett, M.; Welch, A.A.; Khaw, K.T.; Wareham, N.J.; et al. Phytoestrogen concentrations in serum and spot urine as biomarkers for dietary phytoestrogen intake and their relation to breast cancer risk in European prospective investigation of cancer and nutrition-norfolk. Cancer Epidemiol. Biomarkers Prev. 2004, 13, 698–708. [Google Scholar]
- Seow, A.; Shi, C.Y.; Franke, A.A.; Hankin, J.H.; Lee, H.P.; Yu, M.C. Isoflavonoid levels in spot urine are associated with frequency of dietary soy intake in a population-based sample of middle-aged and older Chinese in Singapore. Cancer Epidemiol. Biomarkers Prev. 1998, 7, 135–140. [Google Scholar]
- Struja, T.; Richard, A.; Linseisen, J.; Eichholzer, M.; Rohrmann, S. The association between urinary phytoestrogen excretion and components of the metabolic syndrome in NHANES. Eur. J. Nutr. 2014, 53, 1371–1381. [Google Scholar] [CrossRef]
- National Health and Nutrition Examination Survey 2007–2008 Data Documentation. Analytic Notes. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2007-2008/ALB_CR_E.htm (accessed on 28 April 2020).
- Beydoun, H.A.; Beydoun, M.A.; Jeng, H.A.; Zonderman, A.B.; Eid, S.M. Bisphenol—A and sleep adequacy among adults in the national health and nutrition examination surveys. Sleep 2016, 39, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Shiff, B.; Kohn, T.P.; Ramasamy, R. Impaired sleep is associated with low testosterone in US adult males: Results from the National Health and Nutrition Examination Survey. World J. Urol. 2019, 37, 1449–1453. [Google Scholar] [CrossRef] [PubMed]
- Grandner, M.A.; Jackson, N.; Gerstner, J.R.; Knutson, K.L. Dietary nutrients associated with short and long sleep duration. Data from a nationally representative sample. Appetite 2013, 64, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Scinicariello, F.; Buser, M.C.; Feroe, A.G.; Attanasio, R. Antimony and sleep-related disorders: NHANES 2005-2008. Environ. Res 2017, 156, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Continuous NHANES Web Tutorial. Available online: https://www.cdc.gov/nchs/tutorials/NHANES/index_continuous.htm (accessed on 28 April 2020).
- Vaz Fragoso, C.A.; Van Ness, P.H.; Araujo, K.L.; Iannone, L.P.; Klar Yaggi, H. Age-related differences in sleep-wake symptoms of adults undergoing polysomnography. J. Am. Geriatr. Soc. 2015, 63, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Roehrs, T.; Kapke, A.; Roth, T.; Breslau, N. Sex differences in the polysomnographic sleep of young adults: A community-based study. Sleep Med. 2006, 7, 49–53. [Google Scholar] [CrossRef]
- Atkinson, C.; Newton, K.M.; Bowles, E.J.; Yong, M.; Lampe, J.W. Demographic, anthropometric, and lifestyle factors and dietary intakes in relation to daidzein-metabolizing phenotypes among premenopausal women in the United States. Am. J. Clin. Nutr. 2008, 87, 679–687. [Google Scholar] [CrossRef]
- Sherwin, B.B. Hormones, mood, and cognitive functioning in postmenopausal women. Obstet. Gynecol. 1996, 87, 20s–26s. [Google Scholar] [CrossRef]
- Zeitzer, J.M.; Maidment, N.T.; Behnke, E.J.; Ackerson, L.C.; Fried, I.; Engel, J.; Jr Wilson, C.L. Ultradian sleep-cycle variation of serotonin in the human lateral ventricle. Neurology 2002, 59, 1272–1274. [Google Scholar] [CrossRef] [PubMed]
- Brzezinski, A.; Debi, A. Phytoestrogens: The “natural” selective estrogen receptor modulators? Eur. J. Obstet. Gynecol. Reprod. Biol. 1999, 85, 47–51. [Google Scholar] [CrossRef]
- Deschasaux, M.; Bouter, K.E.; Prodan, A.; Levin, E.; Groen, A.K.; Herrema, H.; Tremaroli, V.; Bakker, G.J.; Attaye, I.; Pinto-Sietsma, S.J.; et al. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat. Med. 2018, 24, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Clerici, C. Equol: History, chemistry, and formation. J. Nutr. 2010, 140, 1355S–1362S. [Google Scholar] [CrossRef]
- Gaya, P.; Medina, M.; Sánchez-Jiménez, A.; Landete, J.M. Phytoestrogen metabolism by adult human gut microbiota. Molecules 2016, 21, 1034. [Google Scholar] [CrossRef]
- Lee, S.A.; Wen, W.; Xiang, Y.B.; Barnes, S.; Liu, D.; Cai, Q.; Zheng, W.; Shu, X.O. Assessment of dietary isoflavone intake among middle-aged Chinese men. J. Nutr. 2007, 137, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Ryan, H.H.; Jones, E.; Simas, T.A.; Lichtenstein, A.H.; Sun, Q.; Hayman, L.L. Urinary isoflavone concentrations are inversely associated with cardiometabolic risk markers in pregnant U.S. women. J. Nutr. 2014, 144, 344–351. [Google Scholar] [CrossRef] [PubMed]
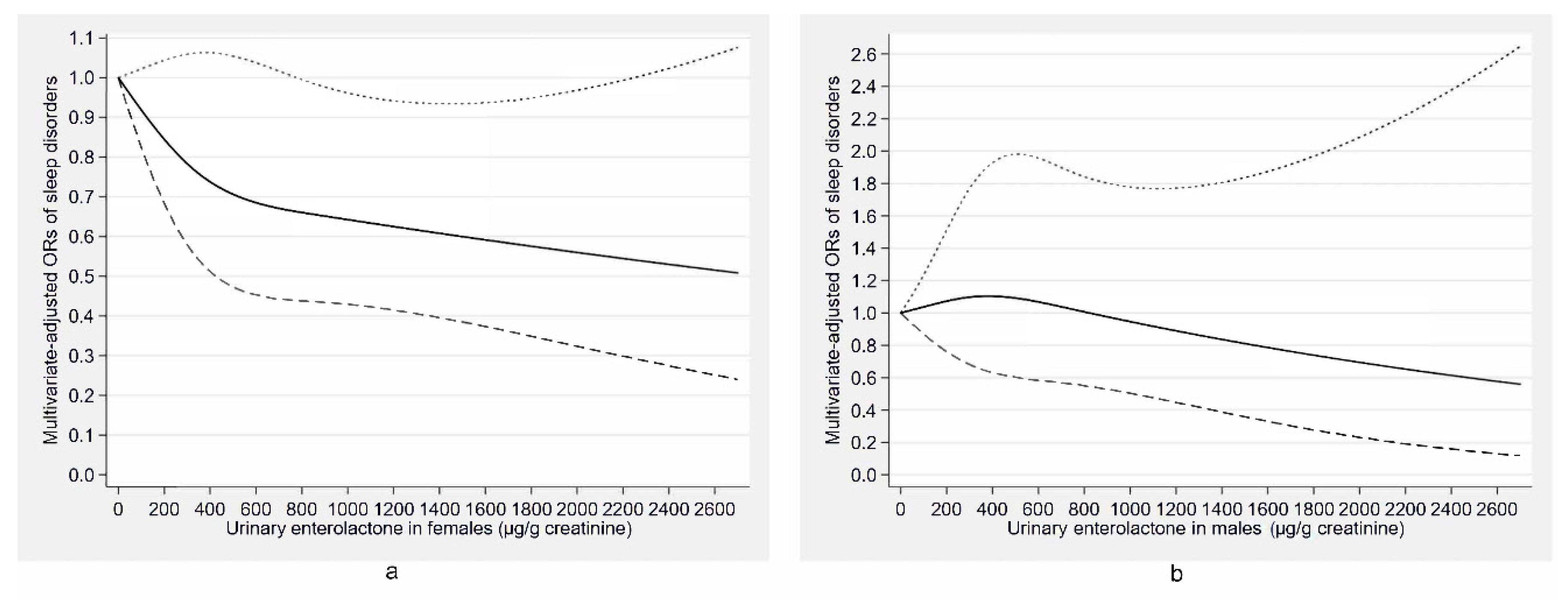
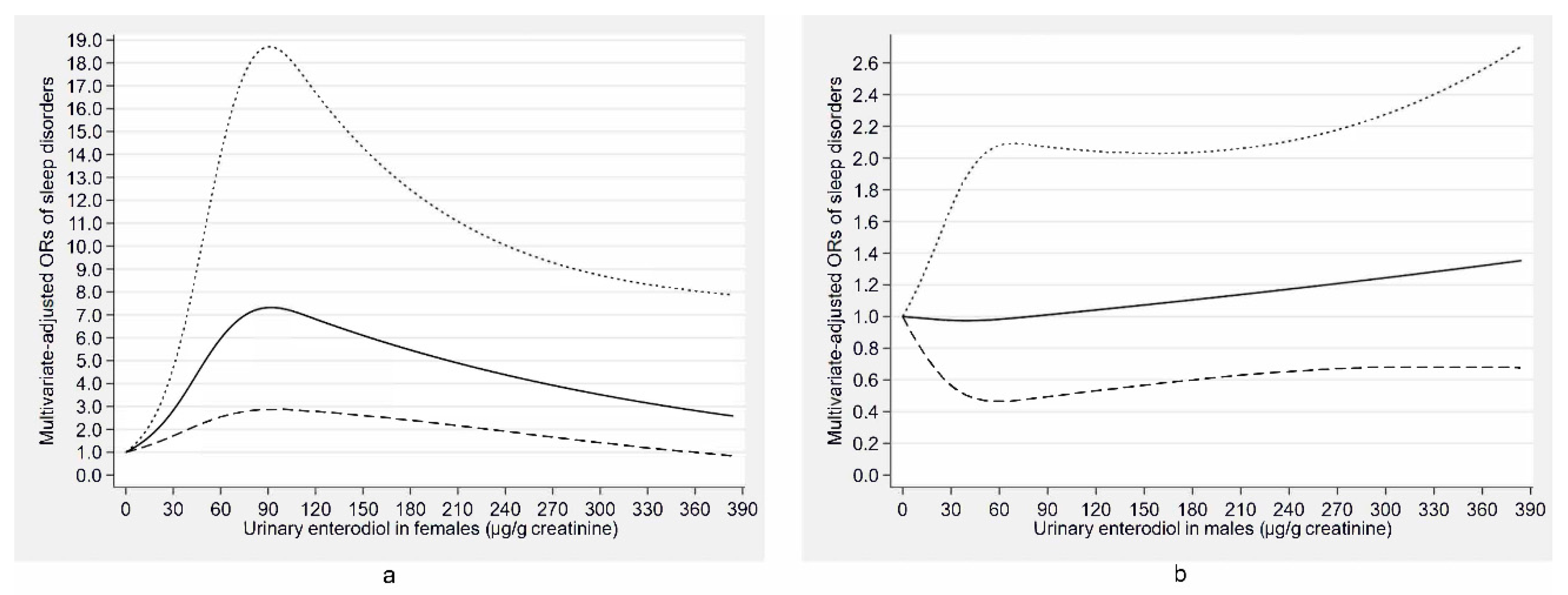
| Characteristics | Total | Males | Females | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No Sleep Disorders | Sleep Disorders | p-Value | No Sleep Disorders | Sleep Disorders | p-Value | No Sleep Disorders | Sleep Disorders | p-Value | |
| Number of individuals (%) | 4528 (93.75) | 302 (6.25) | 2396 (92.80) | 186 (7.20) | 2132 (94.84) | 116 (5.16) | |||
| Gender (%) | 0.003 | - | - | ||||||
| Female | 2132 (47.08) | 116 (38.41) | - | - | - | - | |||
| Male | 2396 (52.92) | 186 (61.59) | - | - | - | - | |||
| Age (%) | <0.001 | <0.001 | 0.005 | ||||||
| 18–39 years | 1846 (40.77) | 71 (23.51) | 957 (39.94) | 40 (21.51) | 889 (41.70) | 31 (26.72) | |||
| 40–59 years | 1378 (30.43) | 109 (36.09) | 707 (29.51) | 66 (35.48) | 671 (31.47) | 43 (37.07) | |||
| ≥60 years | 1304 (28.80) | 122 (40.40) | 732 (30.55) | 80 (43.01) | 572 (26.83) | 42 (36.21) | |||
| Race (%) | <0.001 | <0.001 | 0.287 | ||||||
| Mexican American | 914 (20.19) | 39 (12.91) | 466 (19.45) | 16 (8.60) | 448 (21.01) | 23 (19.83) | |||
| Non-Hispanic White | 2040 (45.05) | 173 (57.28) | 1118 (46.66) | 116 (62.37) | 922 (43.25) | 57 (49.14) | |||
| Non-Hispanic Black | 975 (21.53) | 54 (17.88) | 529 (22.08) | 34 (18.28) | 446 (20.92) | 20 (17.24) | |||
| Other Hispanic | 385 (8.50) | 28 (9.27) | 180 (7.51) | 14 (7.53) | 205 (9.62) | 14 (12.07) | |||
| Other race | 214 (4.73) | 8 (2.65) | 103 (4.30) | 6 (3.23) | 111 (5.21) | 2 (1.72) | |||
| Education (%) | 0.006 | 0.004 | 0.444 | ||||||
| High school | 1117 (24.69) | 63 (20.86) | 619 (25.86) | 41 (22.04) | 498 (23.37) | 22 (18.97) | |||
| Below high school | 1317 (29.10) | 71 (23.51) | 708 (29.57) | 39 (20.97) | 609 (28.58) | 32 (27.59) | |||
| Above high school | 2091 (46.21) | 168 (55.63) | 1067 (44.57) | 106 (56.99) | 1024 (48.05) | 62 (53.45) | |||
| Occupation (%) | 0.002 | <0.001 | 0.626 | ||||||
| No work | 1878 (41.48) | 156 (51.66) | 898 (37.49) | 100 (53.76) | 980 (45.97) | 56 (48.28) | |||
| Regular night or evening shift/rotating shift/other | 735 (16.24) | 41 (13.58) | 428 (17.87) | 22 (11.83) | 307 (14.40) | 19 (16.38) | |||
| Regular daytime schedule | 1914 (42.28) | 105 (34.77) | 1069 (44.63) | 64 (34.41) | 845 (39.63) | 41 (35.34) | |||
| Family income/year (%) | 0.721 | 0.932 | 0.536 | ||||||
| $20,000 and over | 3019 (74.07) | 201 (73.09) | 1630 (75.74) | 126 (75.45) | 1389 (72.19) | 75 (69.44) | |||
| Below $20,000 | 1057 (25.93) | 74 (26.91) | 522 (24.26) | 41 (24.55) | 535 (27.81) | 33 (30.56) | |||
| Marital status (%) | 0.032 | 0.006 | 0.712 | ||||||
| Living with partner/married | 2578 (59.40) | 197 (65.67) | 1454 (63.52) | 136 (73.51) | 1124 (54.80) | 61 (53.04) | |||
| Never married/widowed/separated/divorced | 1762 (40.60) | 103 (34.33) | 835 (36.48) | 49 (26.49) | 927 (45.20) | 54 (46.96) | |||
| Body mass index (%) | <0.001 | <0.001 | 0.009 | ||||||
| 18.5 to <25 kg/m2 | 1371 (30.56) | 43 (14.63) | 688 (28.97) | 21 (11.67) | 683 (32.35) | 22 (19.30) | |||
| <18.5 kg/m2 | 83 (1.85) | 2 (0.68) | 37 (1.56) | 0 (0.00) | 46 (2.18) | 2 (1.75) | |||
| 25 to <30 kg/m2 | 1499 (33.42) | 82 (27.89) | 896 (37.73) | 50 (27.78) | 603 (28.56) | 32 (28.07) | |||
| ≥30 kg/m2 | 1533 (34.17) | 167 (56.80) | 754 (31.75) | 109 (60.56) | 779 (36.90) | 58 (50.88) | |||
| Physical activity | 0.047 | 0.017 | 0.234 | ||||||
| Moderate | 1267 (28.26) | 88 (29.53) | 573 (24.17) | 54 (29.67) | 694 (32.84) | 34 (29.31) | |||
| Vigorous | 1720 (38.36) | 94 (31.54) | 1118 (47.15) | 66 (36.26) | 602 (28.49) | 28 (24.14) | |||
| Other | 1497 (33.39) | 116 (38.93) | 680 (28.68) | 62 (34.07) | 817 (38.67) | 54 (46.55) | |||
| Smoked at least 100 cigarettes in life (%) | 1948 (46.47) | 163 (54.70) | 0.006 | 1222 (55.37) | 115 (62.84) | 0.050 | 726 (36.57) | 48 (41.74) | 0.264 |
| Had at least 12 alcohol drinks/year (%) | 2810 (73.52) | 196 (71.01) | 0.363 | 1735 (84.63) | 140 (82.35) | 0.430 | 1075 (60.67) | 56 (52.83) | 0.109 |
| Use of female hormones (%) | 247 (5.94) | 27 (9.28) | 0.022 | - | - | - | 247 (14.00) | 27 (25.71) | 0.001 |
| Hypertension (%) | 1973 (44.91) | 188 (63.09) | <0.001 | 1154 (49.34) | 128 (70.33) | <0.001 | 819 (39.87) | 60 (51.72) | 0.011 |
| Depressive symptoms (%) | 240 (5.86) | 57 (20.58) | <0.001 | 78 (3.54) | 23 (13.29) | <0.001 | 162 (8.56) | 34 (32.69) | <0.001 |
| Diabetes (%) | 432 (9.70) | 60 (20.98) | <0.001 | 209 (8.89) | 39 (22.29) | <0.001 | 223 (10.60) | 21 (18.92) | 0.006 |
| C-reactive protein (mg/dL), median (interquartile range) | 0.17 (0.36) | 0.24 (0.44) | <0.001 | 0.14 (0.27) | 0.25 (0.41) | <0.001 | 0.21 (0.44) | 0.22 (0.51) | 0.225 |
| Caffeine intake (mg/day), median (interquartile range) | 92.00 (173.00) | 128.00 (212.13) | 0.001 | 103.50 (186.50) | 132.75 (219.88) | 0.012 | 82.00 (150.75) | 103.25 (208.38) | 0.057 |
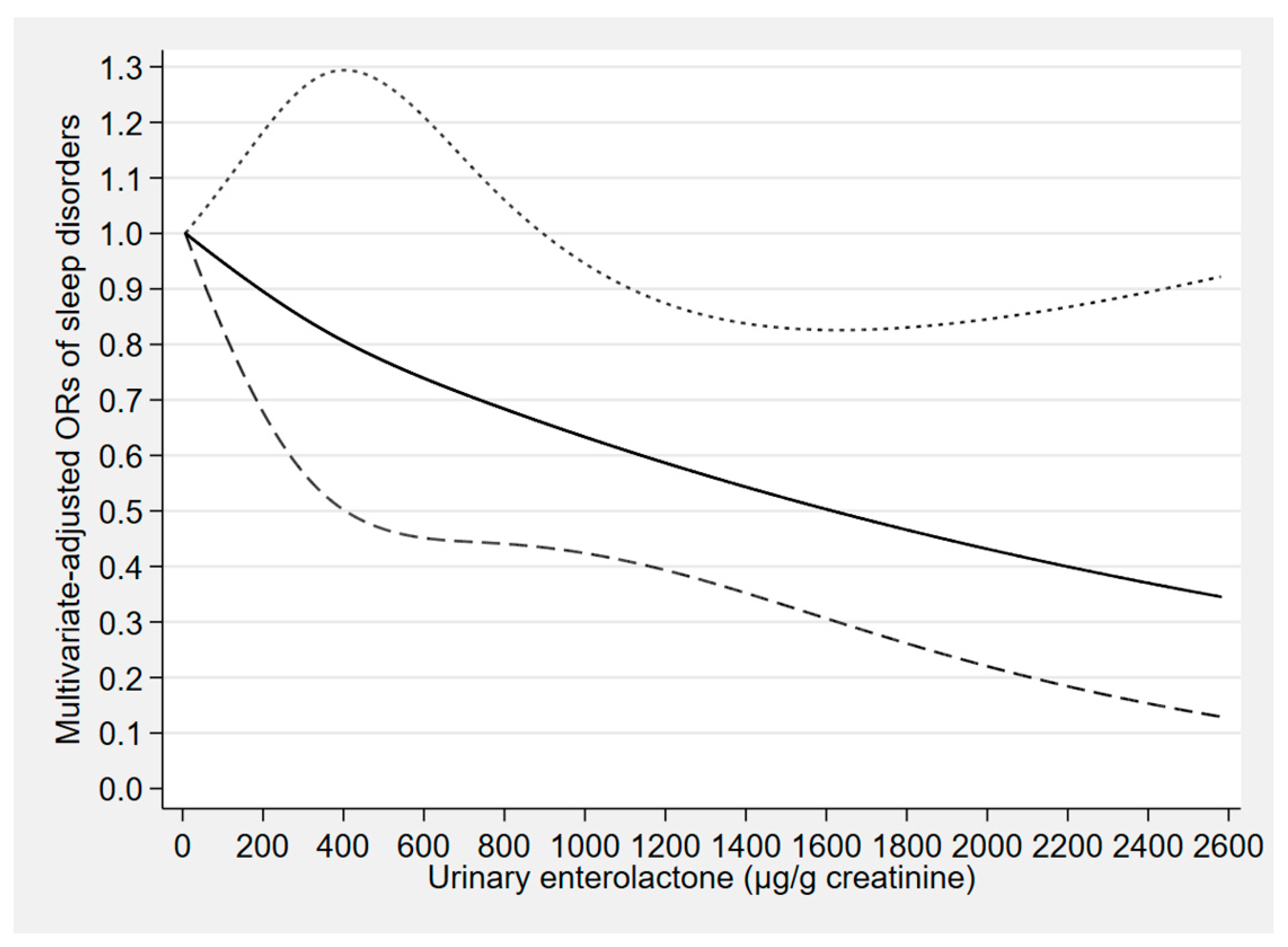
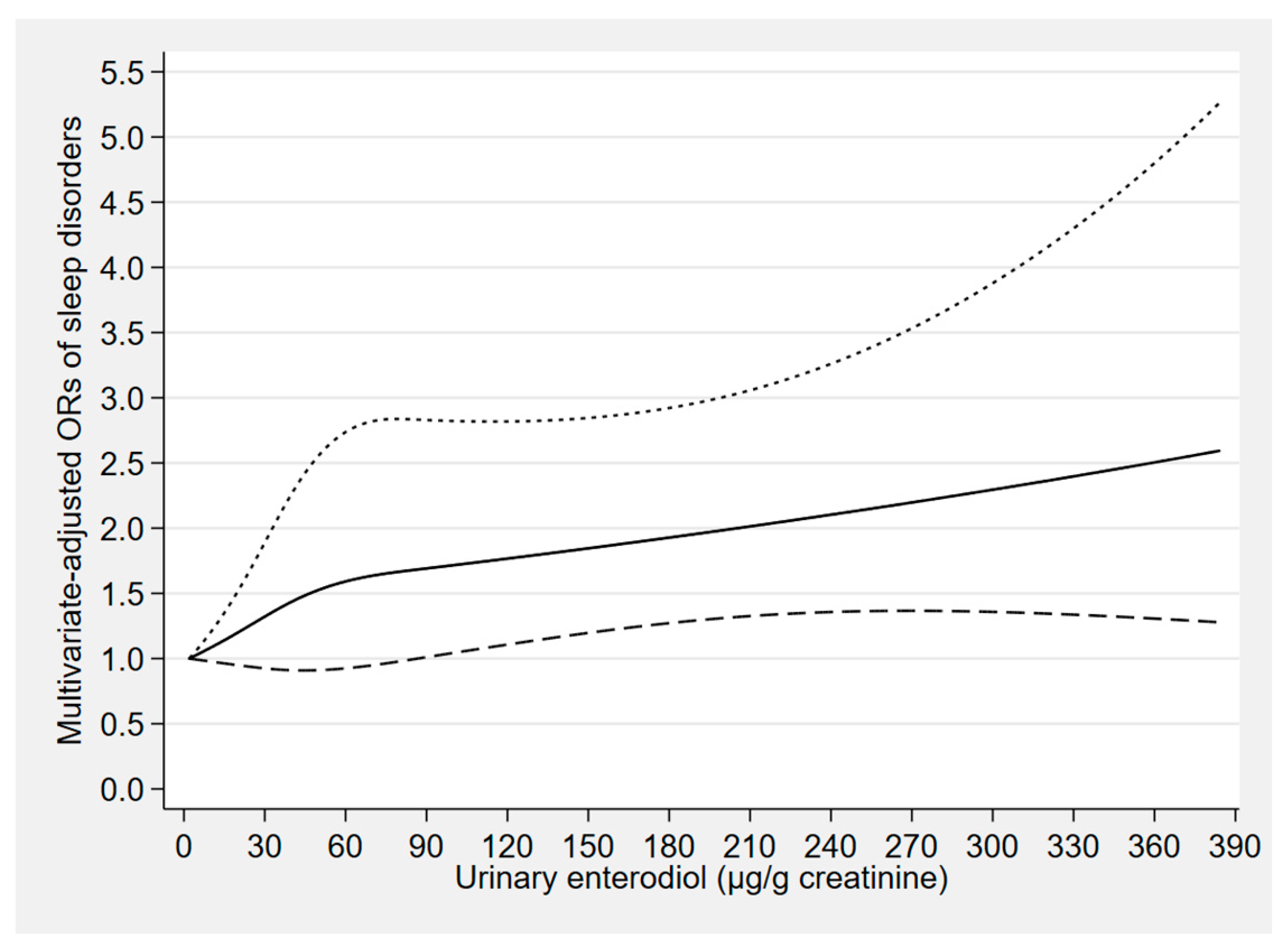
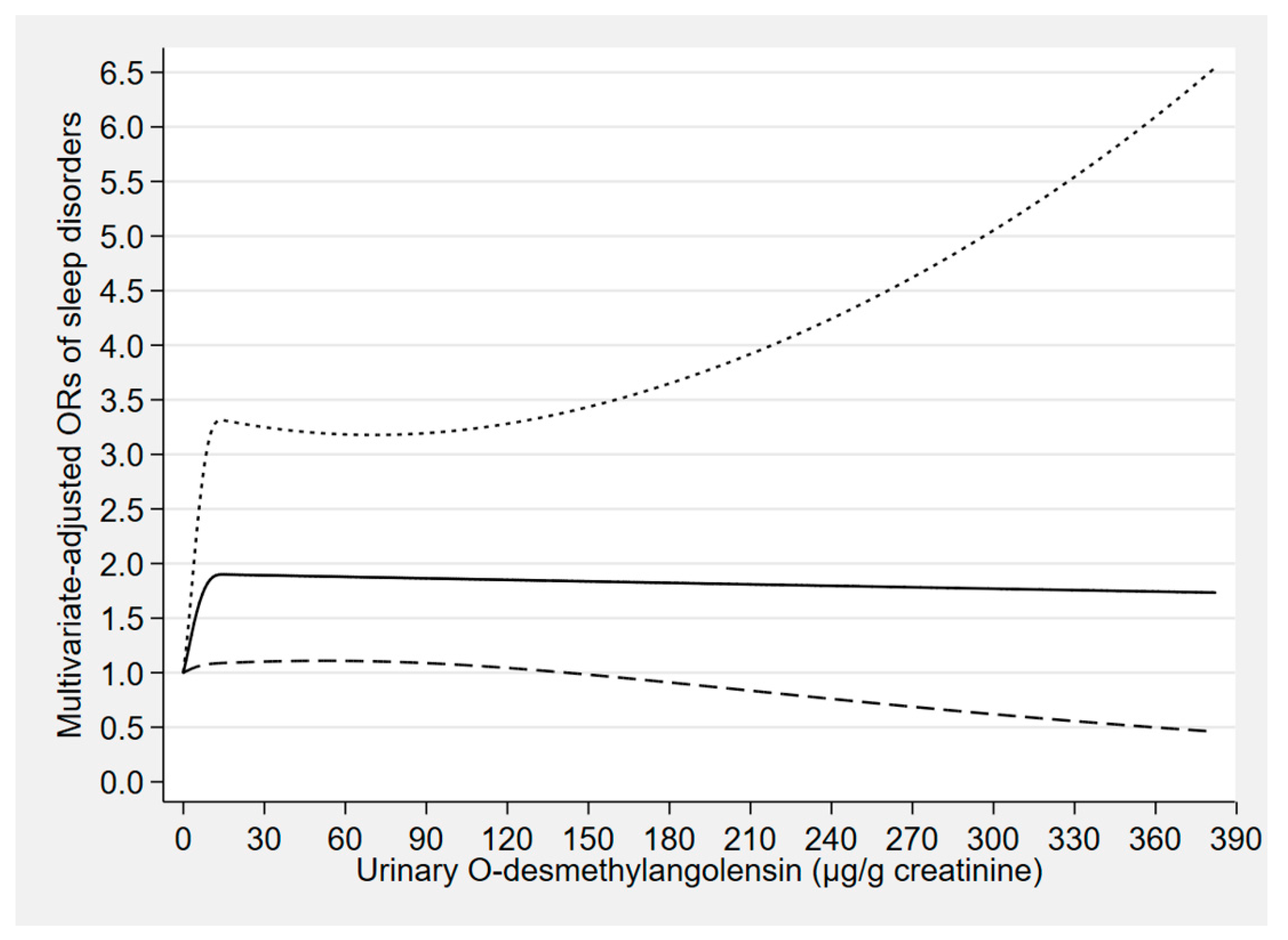
| Phytoestrogen Concentrations (μg/g Creatinine) | Cases/Participants | Model 1 a | Model 2 b | Model 3 c |
|---|---|---|---|---|
| Enterolactone | ||||
| Tertile 1 (<160.53) | 107/1612 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (160.53 to <621.68) | 96/1609 | 0.83 (0.54–1.25) | 0.78 (0.52–1.18) | 0.99 (0.63–1.56) |
| Tertile 3 (≥621.68) | 99/1609 | 0.64 (0.42–0.96) * | 0.57 (0.38–0.85) ** | 0.64 (0.41–1.00) * |
| Enterodiol | ||||
| Tertile 1 (<21.52) | 91/1611 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (21.52 to <70.27) | 103/1609 | 1.21 (0.88–1.65) | 1.18 (0.86–1.62) | 1.28 (0.86–1.91) |
| Tertile 3 (≥70.27) | 108/1610 | 1.34 (0.88–2.05) | 1.35 (0.89–2.07) | 1.54 (1.07–2.21) * |
| Daidzein | ||||
| Tertile 1 (<24.22) | 83/1611 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (24.22 to <108.62) | 106/1609 | 1.46 (0.82–2.61) | 1.48 (0.83–2.63) | 1.18 (0.65–2.15) |
| Tertile 3 (≥108.62) | 113/1610 | 1.30 (0.61–2.79) | 1.32 (0.62–2.81) | 1.12 (0.54–2.30) |
| O-Desmethylangolensin | ||||
| Tertile 1 (<1.04) | 84/1624 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (1.04 to <9.06) | 102/1613 | 1.33 (0.88–2.02) | 1.32 (0.86–2.03) | 1.38 (0.84–2.27) |
| Tertile 3 (≥9.06) | 116/1593 | 1.65 (1.05–2.61) * | 1.68 (1.06–2.65) * | 1.89 (1.26–2.85) ** |
| Equol | ||||
| Tertile 1 (<3.75) | 84/1613 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (3.75 to <9.73) | 119/1612 | 1.21 (0.83–1.74) | 1.16 (0.79–1.71) | 1.01 (0.66–1.56) |
| Tertile 3 (≥9.73) | 99/1605 | 0.86 (0.57–1.30) | 0.84 (0.55–1.28) | 0.76 (0.52–1.12) |
| Genistein | ||||
| Tertile 1 (<12.35) | 104/1626 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (12.35 to <50.00) | 85/1601 | 0.60 (0.40–0.91) * | 0.58 (0.38–0.88) * | 0.68 (0.41–1.12) |
| Tertile 3 (≥50.00) | 113/1603 | 0.80 (0.46–1.38) | 0.74 (0.42–1.29) | 0.78 (0.41–1.46) |
| Phytoestrogens Concentrations (μg/g Creatinine) | Cases/Participants | Model 1 a | Model 2 b | Model 3 c |
|---|---|---|---|---|
| Age (18–39 years) | ||||
| Enterolactone | ||||
| Tertile 1 (<160.53) | 33/767 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (160.53 to <621.68) | 23/683 | 0.60 (0.26–1.38) | 0.60 (0.26–1.38) | 0.86 (0.36–2.02) |
| Tertile 3 (≥621.68) | 15/467 | 0.40 (0.16–0.97) * | 0.40 (0.16–1.01) | 0.65 (0.27–1.56) |
| Enterodiol | ||||
| Tertile 1 (<21.52) | 28/732 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (21.52 to <70.27) | 24/620 | 1.55 (0.79–3.03) | 1.57 (0.81–3.05) | 1.62 (0.73–3.57) |
| Tertile 3 (≥70.27) | 19/565 | 1.35 (0.63–2.92) | 1.39 (0.64–3.00) | 1.21 (0.46–3.15) |
| Daidzein | ||||
| Tertile 1 (<24.22) | 16/679 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (24.22 to <108.62) | 32/661 | 1.88 (0.77–4.57) | 1.89 (0.78–4.58) | 1.17 (0.58–2.36) |
| Tertile 3 (≥108.62) | 23/577 | 0.86 (0.30–2.49) | 0.87 (0.31–2.47) | 0.88 (0.18–4.30) |
| O-Desmethylangolensin | ||||
| Tertile 1 (<1.04) | 19/707 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (1.04 to <9.06) | 21/621 | 1.23 (0.59–2.58) | 1.24 (0.59–2.60) | 1.34 (0.68–2.66) |
| Tertile 3 (≥9.06) | 31/589 | 3.27 (1.39–7.70) ** | 3.28 (1.40–7.68) ** | 4.16 (1.58–10.97) ** |
| Equol | ||||
| Tertile 1 (<3.75) | 27/694 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (3.75 to <9.73) | 22/631 | 0.89 (0.41–1.94) | 0.89 (0.42–1.93) | 0.78 (0.36–1.70) |
| Tertile 3 (≥9.73) | 22/592 | 0.72 (0.32–1.64) | 0.73 (0.32–1.65) | 0.44 (0.16–1.15) |
| Genistein | ||||
| Tertile 1 (<12.35) | 19/701 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (12.35 to <50.00) | 30/650 | 1.08 (0.52–2.24) | 1.08 (0.52–2.24) | 1.18 (0.45–3.09) |
| Tertile 3 (≥50.00) | 22/566 | 1.20 (0.49–2.93) | 1.20 (0.49–2.94) | 1.29 (0.31–5.39) |
| Age (40–59 years) | ||||
| Enterolactone | ||||
| Tertile 1 (<160.53) | 39/533 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (160.53 to <621.68) | 37/468 | 0.89 (0.48–1.66) | 0.89 (0.48–1.64) | 1.00 (0.54–1.83) |
| Tertile 3 (≥ 621.68) | 33/486 | 0.63 (0.31–1.29) | 0.67 (0.33–1.38) | 0.58 (0.28–1.22) |
| Enterodiol | ||||
| Tertile 1 (<21.52) | 30/470 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (21.52 to <70.27) | 37/515 | 0.97 (0.53–1.75) | 1.00 (0.56–1.78) | 1.33 (0.62–2.87) |
| Tertile 3 (≥70.27) | 42/502 | 1.29 (0.69–2.40) | 1.36 (0.73–2.53) | 2.56 (1.12–5.84) * |
| Daidzein | ||||
| Tertile 1 (<24.22) | 36/521 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (24.22 to <108.62) | 30/469 | 1.20 (0.49–2.94) | 1.14 (0.47–2.79) | 1.37 (0.46–4.05) |
| Tertile 3 (≥108.62) | 43/497 | 1.37 (0.43–4.34) | 1.32 (0.42–4.19) | 1.22 (0.31–4.74) |
| O-Desmethylangolensin | ||||
| Tertile 1 (<1.04) | 31/522 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (1.04 to <9.06) | 35/481 | 1.84 (0.89–3.80) | 1.86 (0.90–3.83) | 1.65 (0.64–4.27) |
| Tertile 3 (≥9.06) | 43/484 | 1.90 (0.92–3.93) | 2.00 (0.98–4.05) | 1.92 (0.78–4.76) |
| Equol | ||||
| Tertile 1 (<3.75) | 32/534 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (3.75 to <9.73) | 40/487 | 1.17 (0.62–2.19) | 1.20 (0.64–2.26) | 1.07 (0.46–2.51) |
| Tertile 3 (≥9.73) | 37/466 | 0.90 (0.48–1.70) | 0.95 (0.50–1.80) | 1.07 (0.51–2.24) |
| Genistein | ||||
| Tertile 1 (<12.35) | 47/526 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (12.35 to <50.00) | 19/474 | 0.35 (0.16–0.77) * | 0.34 (0.15–0.78) * | 0.34 (0.13–0.88) * |
| Tertile 3 (≥50.00) | 43/487 | 0.65 (0.21–1.97) | 0.65 (0.21–2.00) | 0.50 (0.17–1.48) |
| Age (≥60 years) | ||||
| Enterolactone | ||||
| Tertile 1 (<160.53) | 35/312 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (160.53 to <621.68) | 36/458 | 0.87 (0.46–1.63) | 0.80 (0.42–1.53) | 1.21 (0.53–2.77) |
| Tertile 3 (≥621.68) | 51/656 | 0.60 (0.34–1.06) | 0.58 (0.32–1.03) | 0.84 (0.38–1.90) |
| Enterodiol | ||||
| Tertile 1 (<21.52) | 33/409 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (21.52 to <70.27) | 42/474 | 1.21 (0.67–2.16) | 1.22 (0.68–2.20) | 1.01 (0.52–1.96) |
| Tertile 3 (≥70.27) | 47/543 | 1.26 (0.67–2.37) | 1.34 (0.71–2.50) | 0.88 (0.43–1.78) |
| Daidzein | ||||
| Tertile 1 (<24.22) | 31/411 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (24.22 to <108.62) | 44/479 | 1.93 (0.85–4.36) | 1.88 (0.82–4.29) | 1.27 (0.48–3.35) |
| Tertile 3 (≥108.62) | 47/536 | 2.36 (0.65–8.53) | 2.26 (0.63–8.16) | 1.59 (0.43–5.93) |
| O-Desmethylangolensin | ||||
| Tertile 1 (<1.04) | 34/395 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (1.04 to <9.06) | 46/511 | 0.73 (0.38–1.38) | 0.75 (0.39–1.44) | 0.78 (0.33–1.80) |
| Tertile 3 (≥9.06) | 42/520 | 0.66 (0.34–1.29) | 0.70 (0.35–1.39) | 0.87 (0.34–2.28) |
| Equol | ||||
| Tertile 1 (<3.75) | 25/385 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (3.75 to <9.73) | 57/494 | 1.41 (0.79–2.54) | 1.41 (0.78–2.55) | 1.01 (0.42–2.45) |
| Tertile 3 (≥9.73) | 40/547 | 0.75 (0.36–1.56) | 0.76 (0.37–1.59) | 0.56 (0.25–1.24) |
| Genistein | ||||
| Tertile 1 (<12.35) | 38/399 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (12.35 to <50.00) | 36/477 | 0.60 (0.29–1.26) | 0.60 (0.29–1.25) | 0.77 (0.29–2.06) |
| Tertile 3 (≥50.00) | 48/550 | 0.51 (0.16–1.61) | 0.53 (0.17–1.64) | 0.86 (0.19–3.85) |
| Phytoestrogen Concentrations (μg/g Creatinine) | Cases/Participants | Model 1 a | Model 2 b | Model 3 c |
|---|---|---|---|---|
| Females | ||||
| Enterolactone | ||||
| Tertile 1 (<160.53) | 40/698 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (160.53 to <621.68) | 36/706 | 0.52 (0.24–1.14) | 0.53 (0.24–1.16) | 0.56 (0.25–1.26) |
| Tertile 3 (≥621.68) | 40/844 | 0.36 (0.19–0.68) ** | 0.32 (0.17–0.61) ** | 0.45 (0.21–0.94) * |
| Enterodiol | ||||
| Tertile 1 (<21.52) | 28/649 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (21.52 to <70.27) | 33/728 | 1.90 (1.04–3.48) * | 1.81 (0.98–3.32) | 2.70 (1.60–4.55) *** |
| Tertile 3 (≥70.27) | 55/871 | 2.89 (1.54–5.42) ** | 2.79 (1.46–5.31) ** | 3.79 (1.83–7.87) ** |
| Daidzein | ||||
| Tertile 1 (<24.22) | 21/708 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (24.22 to <108.62) | 51/745 | 2.16 (0.93–5.00) | 2.33 (1.01–5.39) * | 1.82 (0.77–4.30) |
| Tertile 3 (≥108.62) | 44/795 | 1.71 (0.56–5.20) | 1.82 (0.59–5.55) | 1.44 (0.48–4.28) |
| O-Desmethylangolensin | ||||
| Tertile 1 (<1.04) | 27/697 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (1.04 to <9.06) | 41/753 | 1.70 (0.82–3.52) | 1.68 (0.79–3.57) | 2.04 (0.95–4.37) |
| Tertile 3 (≥9.06) | 48/798 | 2.00 (1.00–3.99) | 1.94 (1.00–3.76) | 2.09 (0.96–4.56) |
| Equol | ||||
| Tertile 1 (<3.75) | 31/669 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (3.75 to <9.73) | 47/752 | 1.36 (0.74–2.48) | 1.30 (0.71–2.37) | 1.25 (0.57–2.73) |
| Tertile 3 (≥9.73) | 38/827 | 0.85 (0.41–1.78) | 0.80 (0.38–1.68) | 0.62 (0.28–1.35) |
| Genistein | ||||
| Tertile 1 (<12.35) | 33/720 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (12.35 to <50.00) | 39/723 | 0.60 (0.31–1.17) | 0.57 (0.29–1.12) | 0.59 (0.25–1.38) |
| Tertile 3 (≥50.00) | 44/805 | 0.62 (0.27–1.42) | 0.57 (0.25–1.30) | 0.61 (0.23–1.57) |
| Males | ||||
| Enterolactone | ||||
| Tertile 1 (<160.53) | 67/914 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (160.53 to <621.68) | 60/903 | 1.16 (0.67–1.99) | 1.08 (0.63–1.85) | 1.58 (0.86–2.91) |
| Tertile 3 (≥621.68) | 59/765 | 1.11 (0.65–1.88) | 0.93 (0.54–1.61) | 0.92 (0.46–1.83) |
| Enterodiol | ||||
| Tertile 1 (<21.52) | 63/962 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (21.52 to <70.27) | 70/881 | 0.99 (0.64–1.52) | 0.94 (0.61–1.46) | 0.92 (0.51–1.65) |
| Tertile 3 (≥70.27) | 53/739 | 0.88 (0.52–1.50) | 0.85 (0.51–1.42) | 0.92 (0.56–1.53) |
| Daidzein | ||||
| Tertile 1 (<24.22) | 62/903 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (24.22 to <108.62) | 55/864 | 1.10 (0.58–2.06) | 1.07 (0.58–1.98) | 0.88 (0.48–1.64) |
| Tertile 3 (≥108.62) | 69/815 | 1.02 (0.41–2.52) | 1.01 (0.42–2.45) | 1.05 (0.44–2.50) |
| O-Desmethylangolensin | ||||
| Tertile 1 (<1.04) | 57/927 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (1.04 to <9.06) | 61/860 | 1.17 (0.65–2.11) | 1.15 (0.65–2.04) | 1.05 (0.53–2.05) |
| Tertile 3 (≥9.06) | 68/795 | 1.57 (0.88–2.80) | 1.58 (0.89–2.80) | 1.58 (0.89–2.79) |
| Equol | ||||
| Tertile 1 (<3.75) | 53/944 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (3.75 to <9.73) | 72/860 | 1.12 (0.70–1.81) | 1.06 (0.65–1.73) | 0.79 (0.47–1.35) |
| Tertile 3 (≥9.73) | 61/778 | 0.91 (0.54–1.54) | 0.88 (0.52–1.48) | 0.86 (0.47–1.58) |
| Genistein | ||||
| Tertile 1 (<12.35) | 71/906 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (12.35 to <50.00) | 46/878 | 0.59 (0.36–0.97) * | 0.57 (0.35–0.93) * | 0.74 (0.41–1.33) |
| Tertile 3 (≥50.00) | 69/798 | 1.04 (0.52–2.10) | 0.96 (0.48–1.91) | 0.96 (0.42–2.20) |
| Phytoestrogen Concentrations (μg/g Creatinine) | Males | Females | ||
|---|---|---|---|---|
| Cases/Participants | Model 3 a | Cases/Participants | Model 3 a | |
| Age (18–39 years) | ||||
| Enterolactone | ||||
| Tertile 1 (<160.53) | 18/444 | 1.00 (reference) | 15/323 | 1.00 (reference) |
| Tertile 2 (160.53 to <621.68) | 13/353 | 1.65 (0.53–5.08) | 10/330 | 0.54 (0.14–2.14) |
| Tertile 3 (≥621.68) | 9/200 | 1.12 (0.27–4.69) | 6/267 | 0.46 (0.10–2.14) |
| Enterodiol | ||||
| Tertile 1 (<21.52) | 19/434 | 1.00 (reference) | 9/298 | 1.00 (reference) |
| Tertile 2 (21.52 to <70.27) | 12/328 | 0.90 (0.28–2.89) | 12/292 | 6.52 (1.69–25.23) ** |
| Tertile 3 (≥70.27) | 9/235 | 1.28 (0.36–4.59) | 10/330 | 1.84 (0.50–6.80) |
| Daidzein | ||||
| Tertile 1 (<24.22) | 12/381 | 1.00 (reference) | 4/298 | 1.00 (reference) |
| Tertile 2 (24.22 to <108.62) | 16/327 | 0.86 (0.38–1.93) | 16/334 | 2.35 (0.60–9.22) |
| Tertile 3 (≥108.62) | 12/289 | 0.45 (0.09–2.25) | 11/288 | 5.07 (0.52–49.84) |
| O-Desmethylangolensin | ||||
| Tertile 1 (<1.04) | 10/395 | 1.00 (reference) | 9/312 | 1.00 (reference) |
| Tertile 2 (1.04 to <9.06) | 12/311 | 1.28 (0.39–4.18) | 9/310 | 1.18 (0.37–3.72) |
| Tertile 3 (≥9.06) | 18/291 | 6.57 (2.06–20.99) ** | 13/298 | 0.74 (0.12–4.45) |
| Equol | ||||
| Tertile 1 (<3.75) | 15/396 | 1.00 (reference) | 12/298 | 1.00 (reference) |
| Tertile 2 (3.75 to <9.73) | 9/318 | 0.44 (0.15–1.33) | 13/313 | 1.37 (0.38–4.88) |
| Tertile 3 (≥9.73) | 16/283 | 0.74 (0.29–1.86) | 6/309 | 0.08 (0.01–0.84) * |
| Genistein | ||||
| Tertile 1 (<12.35) | 13/386 | 1.00 (reference) | 6/315 | 1.00 (reference) |
| Tertile 2 (12.35 to <50.00) | 15/337 | 1.60 (0.54–4.73) | 15/313 | 1.10 (0.29–4.10) |
| Tertile 3 (≥50.00) | 12/274 | 1.70 (0.37–7.85) | 10/292 | 0.59 (0.12–2.81) |
| Age (40–59 years) | ||||
| Enterolactone | ||||
| Tertile 1 (<160.53) | 25/304 | 1.00 (reference) | 14/229 | 1.00 (reference) |
| Tertile 2 (160.53 to <621.68) | 24/260 | 1.90 (0.74–4.88) | 13/208 | 0.50 (0.16–1.53) |
| Tertile 3 (≥621.68) | 17/209 | 1.15 (0.36–3.61) | 16/277 | 0.32 (0.10–1.03) |
| Enterodiol | ||||
| Tertile 1 (<21.52) | 23/272 | 1.00 (reference) | 7/198 | 1.00 (reference) |
| Tertile 2 (21.52 to <70.27) | 24/272 | 1.04 (0.32–3.42) | 13/243 | 3.02 (1.10–8.30) * |
| Tertile 3 (≥70.27) | 19/229 | 1.47 (0.43–5.00) | 23/273 | 13.66 (2.06–90.70) ** |
| Daidzein | ||||
| Tertile 1 (<24.22) | 25/281 | 1.00 (reference) | 11/240 | 1.00 (reference) |
| Tertile 2 (24.22 to <108.62) | 15/262 | 2.04 (0.45–9.16) | 15/207 | 1.65 (0.41–6.72) |
| Tertile 3 (≥108.62) | 26/230 | 2.94 (0.38–22.73) | 17/267 | 0.85 (0.13–5.75) |
| O-Desmethylangolensin | ||||
| Tertile 1 (<1.04) | 25/297 | 1.00 (reference) | 6/225 | 1.00 (reference) |
| Tertile 2 (1.04 to <9.06) | 18/252 | 0.56 (0.18–1.79) | 17/229 | 11.50 (2.14–61.72) ** |
| Tertile 3 (≥9.06) | 23/224 | 0.68 (0.21–2.20) | 20/260 | 15.14 (2.99–76.65) ** |
| Equol | ||||
| Tertile 1 (<3.75) | 21/316 | 1.00 (reference) | 11/218 | 1.00 (reference) |
| Tertile 2 (3.75 to <9.73) | 27/251 | 0.88 (0.27–2.86) | 13/236 | 0.95 (0.29–3.12) |
| Tertile 3 (≥9.73) | 18/206 | 1.28 (0.43–3.80) | 19/260 | 0.77 (0.26–2.31) |
| Genistein | ||||
| Tertile 1 (<12.35) | 30/285 | 1.00 (reference) | 17/241 | 1.00 (reference) |
| Tertile 2 (12.35 to <50.00) | 10/256 | 0.29 (0.05–1.61) | 9/218 | 0.20 (0.04–1.00) |
| Tertile 3 (≥50.00) | 26/232 | 0.43 (0.06–3.03) | 17/255 | 0.38 (0.07–2.05) |
| Age (≥60 years) | ||||
| Enterolactone | ||||
| Tertile 1 (<160.53) | 24/166 | 1.00 (reference) | 11/146 | 1.00 (reference) |
| Tertile 2 (160.53 to <621.68) | 23/290 | 1.59 (0.63–4.04) | 13/168 | 1.37 (0.43–4.40) |
| Tertile 3 (≥621.68) | 33/356 | 0.77 (0.34–1.75) | 18/300 | 1.47 (0.29–7.40) |
| Enterodiol | ||||
| Tertile 1 (<21.52) | 21/256 | 1.00 (reference) | 12/153 | 1.00 (reference) |
| Tertile 2 (21.52 to <70.27) | 34/281 | 0.98 (0.44–2.20) | 8/193 | 1.18 (0.26–5.28) |
| Tertile 3 (≥70.27) | 25/275 | 0.48 (0.20–1.16) | 22/268 | 2.00 (0.71–5.57) |
| Daidzein | ||||
| Tertile 1 (<24.22) | 25/241 | 1.00 (reference) | 6/170 | 1.00 (reference) |
| Tertile 2 (24.22 to <108.62) | 24/275 | 0.47 (0.16–1.39) | 20/204 | 13.45 (2.89–62.50) ** |
| Tertile 3 (≥108.62) | 31/296 | 0.80 (0.13–4.79) | 16/240 | 10.67 (1.88–60.44) ** |
| O-Desmethylangolensin | ||||
| Tertile 1 (<1.04) | 22/235 | 1.00 (reference) | 12/160 | 1.00 (reference) |
| Tertile 2 (1.04 to <9.06) | 31/297 | 1.16 (0.37–3.66) | 15/214 | 0.27 (0.06–1.19) |
| Tertile 3 (≥9.06) | 27/280 | 1.19 (0.33–4.37) | 15/240 | 0.25 (0.10–0.58) ** |
| Equol | ||||
| Tertile 1 (<3.75) | 17/232 | 1.00 (reference) | 8/153 | 1.00 (reference) |
| Tertile 2 (3.75 to <9.73) | 36/291 | 0.69 (0.23–2.08) | 21/203 | 2.05 (0.63–6.68) |
| Tertile 3 (≥9.73) | 27/289 | 0.72 (0.28–1.83) | 13/258 | 0.49 (0.12–1.97) |
| Genistein | ||||
| Tertile 1 (<12.35) | 28/235 | 1.00 (reference) | 10/164 | 1.00 (reference) |
| Tertile 2 (12.35 to <50.00) | 21/285 | 0.75 (0.22–2.53) | 15/192 | 0.71 (0.14–3.53) |
| Tertile 3 (≥50.00) | 31/292 | 1.29 (0.16–10.26) | 17/258 | 0.41 (0.06–2.82) |
| Phytoestrogen Concentrations (μg/g Creatinine) | Cases/Participants | Model 3 a |
|---|---|---|
| Mexican American | ||
| Enterolactone | ||
| Tertile 1 (<160.53) | 9/298 | 1.00 (reference) |
| Tertile 2 (160.53 to <621.68) | 11/347 | 2.24 (0.28–17.56) |
| Tertile 3 (≥621.68) | 19/308 | 2.47 (0.54–11.42) |
| Enterodiol | ||
| Tertile 1 (<21.52) | 9/366 | 1.00 (reference) |
| Tertile 2 (21.52 to <70.27) | 15/315 | 1.64 (0.85–3.17) |
| Tertile 3 (≥70.27) | 15/272 | 1.91 (0.56–6.50) |
| Daidzein | ||
| Tertile 1 (<24.22) | 10/359 | 1.00 (reference) |
| Tertile 2 (24.22 to <108.62) | 12/292 | 0.72 (0.14–3.83) |
| Tertile 3 (≥108.62) | 17/302 | 1.03 (0.11–9.46) |
| O-Desmethylangolensin | ||
| Tertile 1 (<1.04) | 7/401 | 1.00 (reference) |
| Tertile 2 (1.04 to <9.06) | 18/312 | 2.00 (0.53–7.61) |
| Tertile 3 (≥9.06) | 14/240 | 2.16 (0.73–6.39) |
| Equol | ||
| Tertile 1 (<3.75) | 13/421 | 1.00 (reference) |
| Tertile 2 (3.75 to <9.73) | 17/331 | 0.64 (0.17–2.45) |
| Tertile 3 (≥9.73) | 9/201 | 0.37 (0.09–1.48) |
| Genistein | ||
| Tertile 1 (<12.35) | 10/341 | 1.00 (reference) |
| Tertile 2 (12.35 to <50.00) | 13/314 | 2.26 (0.35–14.60) |
| Tertile 3 (≥50.00) | 16/298 | 2.00 (0.14–28.91) |
| Non-Hispanic White | ||
| Enterolactone | ||
| Tertile 1 (<160.53) | 57/673 | 1.00 (reference) |
| Tertile 2 (160.53 to <621.68) | 59/681 | 1.00 (0.60–1.67) |
| Tertile 3 (≥621.68) | 57/859 | 0.56 (0.33–0.95) * |
| Enterodiol | ||
| Tertile 1 (<21.52) | 43/606 | 1.00 (reference) |
| Tertile 2 (21.52 to <70.27) | 62/765 | 1.52 (0.88–2.63) |
| Tertile 3 (≥70.27) | 68/842 | 1.89 (1.13–3.13) * |
| Daidzein | ||
| Tertile 1 (<24.22) | 45/684 | 1.00 (reference) |
| Tertile 2 (24.22 to <108.62) | 62/771 | 1.12 (0.55–2.29) |
| Tertile 3 (≥108.62) | 66/758 | 0.95 (0.41–2.21) |
| O-Desmethylangolensin | ||
| Tertile 1 (<1.04) | 44/638 | 1.00 (reference) |
| Tertile 2 (1.04 to <9.06) | 60/798 | 1.48 (0.76–2.90) |
| Tertile 3 (≥9.06) | 69/777 | 2.16 (1.31–3.55) ** |
| Equol | ||
| Tertile 1 (<3.75) | 39/493 | 1.00 (reference) |
| Tertile 2 (3.75 to <9.73) | 67/729 | 0.97 (0.57–1.64) |
| Tertile 3 (≥9.73) | 67/991 | 0.76 (0.47–1.22) |
| Genistein | ||
| Tertile 1 (<12.35) | 60/696 | 1.00 (reference) |
| Tertile 2 (12.35 to <50.00) | 48/766 | 0.63 (0.34–1.16) |
| Tertile 3 (≥50.00) | 65/751 | 0.86 (0.42–1.76) |
| Non-Hispanic Black | ||
| Enterolactone | ||
| Tertile 1 (<160.53) | 20/388 | 1.00 (reference) |
| Tertile 2 (160.53 to <621.68) | 17/385 | 1.11 (0.48–2.55) |
| Tertile 3 (≥621.68) | 17/256 | 0.86 (0.26–2.89) |
| Enterodiol | ||
| Tertile 1 (<21.52) | 23/423 | 1.00 (reference) |
| Tertile 2 (21.52 to <70.27) | 16/338 | 0.81 (0.29–2.25) |
| Tertile 3 (≥70.27) | 15/268 | 0.79 (0.40–1.57) |
| Daidzein | ||
| Tertile 1 (<24.22) | 16/371 | 1.00 (reference) |
| Tertile 2 (24.22 to <108.62) | 24/331 | 2.05 (0.57–7.36) |
| Tertile 3 (≥108.62) | 14/327 | 0.91 (0.16–5.32) |
| O-Desmethylangolensin | ||
| Tertile 1 (<1.04) | 15/338 | 1.00 (reference) |
| Tertile 2 (1.04 to <9.06) | 17/331 | 2.53 (0.86–7.50) |
| Tertile 3 (≥9.06) | 22/360 | 2.23 (0.49–10.14) |
| Equol | ||
| Tertile 1 (<3.75) | 20/486 | 1.00 (reference) |
| Tertile 2 (3.75 to <9.73) | 23/327 | 0.99 (0.44–2.26) |
| Tertile 3 (≥9.73) | 11/216 | 0.54 (0.17–1.75) |
| Genistein | ||
| Tertile 1 (<12.35) | 24/411 | 1.00 (reference) |
| Tertile 2 (12.35 to <50.00) | 16/313 | 0.51 (0.20–1.28) |
| Tertile 3 (≥50.00) | 14/305 | 0.73 (0.18–3.02) |
| Other Hispanic | ||
| Enterolactone | ||
| Tertile 1 (<160.53) | 16/159 | 1.00 (reference) |
| Tertile 2 (160.53 to <621.68) | 8/135 | 1.34 (0.38–4.75) |
| Tertile 3 (≥621.68) | 4/119 | 0.45 (0.03–6.95) |
| Enterodiol | ||
| Tertile 1 (<21.52) | 13/159 | 1.00 (reference) |
| Tertile 2 (21.52 to <70.27) | 6/123 | 0.25 (0.05–1.31) |
| Tertile 3 (≥70.27) | 9/131 | 2.76 (0.49–15.59) |
| Daidzein | ||
| Tertile 1 (<24.22) | 9/147 | 1.00 (reference) |
| Tertile 2 (24.22 to <108.62) | 7/141 | 1.43 (0.21–9.83) |
| Tertile 3 (≥108.62) | 12/125 | 18.97 (0.50–719.28) |
| O-Desmethylangolensin | ||
| Tertile 1 (<1.04) | 15/171 | 1.00 (reference) |
| Tertile 2 (1.04 to <9.06) | 6/120 | 0.13 (0.02–0.86) * |
| Tertile 3 (≥9.06) | 7/122 | 0.10 (0.01–1.32) |
| Equol | ||
| Tertile 1 (<3.75) | 8/130 | 1.00 (reference) |
| Tertile 2 (3.75 to <9.73) | 8/137 | 0.46 (0.13–1.70) |
| Tertile 3 (≥9.73) | 12/146 | 3.22 (1.11–9.30) * |
| Genistein | ||
| Tertile 1 (<12.35) | 7/132 | 1.00 (reference) |
| Tertile 2 (12.35 to <50.00) | 6/144 | 0.58 (0.12–2.72) |
| Tertile 3 (≥50.00) | 15/137 | 0.30 (0.03–2.91) |
| Phytoestrogen Concentrations (μg/g Creatinine) | Model 3 a | ||
|---|---|---|---|
| Very Short Sleep (<5 h/Night) | Short Sleep (5–6 h/Night) | Long Sleep (≥9 h/Night) | |
| Enterolactone | |||
| Tertile 1 (<160.53) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (160.53 to <621.68) | 0.98 (0.64–1.50) | 1.15 (0.86–1.54) | 1.46 (0.93–2.28) |
| Tertile 3 (≥621.68) | 0.56 (0.36–0.86) ** | 0.94 (0.69–1.27) | 0.78 (0.45–1.33) |
| Enterodiol | |||
| Tertile 1 (<21.52) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (21.52 to <70.27) | 1.35 (0.76–2.42) | 1.09 (0.84–1.40) | 1.04 (0.65–1.67) |
| Tertile 3 (≥70.27) | 0.97 (0.54–1.76) | 1.04 (0.77–1.39) | 1.46 (0.88–2.40) |
| Daidzein | |||
| Tertile 1 (<24.22) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (24.22 to <108.62) | 1.02 (0.49–2.13) | 1.08 (0.85–1.37) | 1.10 (0.63–1.93) |
| Tertile 3 (≥108.62) | 1.49 (0.48–4.63) | 1.29 (0.83–2.03) | 1.15 (0.54–2.43) |
| O-Desmethylangolensin | |||
| Tertile 1 (<1.04) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (1.04 to <9.06) | 1.43 (0.83–2.47) | 1.01 (0.82–1.26) | 1.05 (0.67–1.64) |
| Tertile 3 (≥9.06) | 0.82 (0.36–1.88) | 0.86 (0.61–1.21) | 1.44 (0.80–2.56) |
| Equol | |||
| Tertile 1 (<3.75) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (3.75 to <9.73) | 1.01 (0.71–1.44) | 1.04 (0.81–1.33) | 1.06 (0.69–1.62) |
| Tertile 3 (≥9.73) | 0.89 (0.50–1.58) | 1.05 (0.84–1.31) | 0.92 (0.60–1.41) |
| Genistein | |||
| Tertile 1 (<12.35) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (12.35 to <50.00) | 0.94 (0.47–1.89) | 1.10 (0.85–1.43) | 0.62 (0.39–0.99) * |
| Tertile 3 (≥50.00) | 1.05 (0.44–2.53) | 0.95 (0.65–1.40) | 0.62 (0.33–1.15) |
| Phytoestrogen Concentrations (μg/g Creatinine) | Model 3 a | ||
|---|---|---|---|
| Very Short Sleep (<5 h/Night) | Short Sleep (5–6 h/Night) | Long Sleep (≥9 h/Night) | |
| Age (18–39 years) | |||
| Enterolactone | |||
| Tertile 1 (<160.53) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (160.53 to <621.68) | 0.69 (0.30–1.59) | 0.96 (0.62–1.48) | 2.21 (0.99–4.90) |
| Tertile 3 (≥621.68) | 0.24 (0.09–0.61) ** | 0.78 (0.45–1.36) | 1.33 (0.53–3.33) |
| Enterodiol | |||
| Tertile 1 (<21.52) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (21.52 to <70.27) | 2.73 (1.20–6.21) * | 1.20 (0.72–2.01) | 0.72 (0.33–1.58) |
| Tertile 3 (≥70.27) | 0.81 (0.25–2.63) | 1.00 (0.59–1.69) | 1.63 (0.71–3.77) |
| Daidzein | |||
| Tertile 1 (<24.22) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (24.22 to <108.62) | 1.24 (0.28–5.41) | 1.05 (0.72–1.52) | 0.58 (0.19–1.73) |
| Tertile 3 (≥108.62) | 1.94 (0.19–19.60) | 1.34 (0.65–2.74) | 1.09 (0.35–3.36) |
| O-Desmethylangolensin | |||
| Tertile 1 (<1.04) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (1.04 to <9.06) | 1.75 (0.54–5.73) | 1.03 (0.69–1.53) | 0.64 (0.27–1.52) |
| Tertile 3 (≥9.06) | 1.21 (0.24–6.20) | 0.84 (0.52–1.34) | 1.28 (0.54–3.08) |
| Equol | |||
| Tertile 1 (<3.75) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (3.75 to <9.73) | 1.67 (0.70–4.00) | 1.09 (0.71–1.67) | 0.88 (0.44–1.76) |
| Tertile 3 (≥9.73) | 0.69 (0.23–2.03) | 1.03 (0.67–1.59) | 0.68 (0.32–1.45) |
| Genistein | |||
| Tertile 1 (<12.35) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (12.35 to <50.00) | 1.52 (0.46–5.00) | 1.21 (0.79–1.83) | 1.35 (0.59–3.08) |
| Tertile 3 (≥50.00) | 1.61 (0.36–7.19) | 0.91 (0.46–1.79) | 0.92 (0.31–2.71) |
| Age (40-59 years) | |||
| Enterolactone | |||
| Tertile 1 (<160.53) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (160.53 to <621.68) | 1.32 (0.70–2.49) | 1.44 (0.87–2.38) | 1.98 (0.98–4.03) |
| Tertile 3 (≥621.68) | 0.87 (0.37–2.05) | 1.25 (0.80–1.94) | 0.55 (0.19–1.58) |
| Enterodiol | |||
| Tertile 1 (<21.52) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (21.52 to <70.27) | 0.95 (0.40–2.29) | 0.97 (0.65–1.44) | 0.69 (0.24–1.94) |
| Tertile 3 (≥70.27) | 0.59 (0.23–1.55) | 0.93 (0.65–1.33) | 1.45 (0.69–3.07) |
| Daidzein | |||
| Tertile 1 (<24.22) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (24.22 to <108.62) | 0.77 (0.26–2.27) | 1.12 (0.64–1.96) | 2.42 (0.96–6.12) |
| Tertile 3 (≥108.62) | 1.69 (0.39–7.36) | 1.45 (0.62–3.42) | 3.92 (0.55–27.81) |
| O-Desmethylangolensin | |||
| Tertile 1 (<1.04) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (1.04 to <9.06) | 1.37 (0.59–3.17) | 0.87 (0.59–1.26) | 1.54 (0.58–4.11) |
| Tertile 3 (≥9.06) | 0.43 (0.12–1.60) | 0.71 (0.35–1.44) | 1.41 (0.56–3.58) |
| Equol | |||
| Tertile 1 (<3.75) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (3.75 to <9.73) | 0.41 (0.14–1.22) | 0.98 (0.65–1.48) | 1.42 (0.68–2.96) |
| Tertile 3 (≥9.73) | 0.65 (0.25–1.73) | 0.95 (0.67–1.36) | 1.14 (0.37–3.55) |
| Genistein | |||
| Tertile 1 (<12.35) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (12.35 to <50.00) | 0.73 (0.27–2.01) | 1.09 (0.68–1.76) | 0.14 (0.05–0.40) *** |
| Tertile 3 (≥50.00) | 0.85 (0.23–3.17) | 1.00 (0.53–1.86) | 0.10 (0.02–0.56) * |
| Age (≥60 years) | |||
| Enterolactone | |||
| Tertile 1 (<160.53) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (160.53 to <621.68) | 1.75 (0.62–4.94) | 1.07 (0.61–1.87) | 0.61 (0.29–1.30) |
| Tertile 3 (≥621.68) | 1.27 (0.52–3.10) | 0.81 (0.52–1.27) | 0.49 (0.29–0.84) * |
| Enterodiol | |||
| Tertile 1 (<21.52) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (21.52 to <70.27) | 0.45 (0.16–1.30) | 1.09 (0.65–1.84) | 1.56 (0.82–2.96) |
| Tertile 3 (≥70.27) | 1.38 (0.63–3.02) | 1.11 (0.66–1.88) | 1.64 (0.92–2.93) |
| Daidzein | |||
| Tertile 1 (<24.22) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (24.22 to <108.62) | 0.94 (0.11–7.95) | 1.07 (0.61–1.88) | 1.35 (0.68–2.68) |
| Tertile 3 (≥108.62) | 0.62 (0.04–10.32) | 1.08 (0.46–2.55) | 0.62 (0.26–1.48) |
| O-Desmethylangolensin | |||
| Tertile 1 (<1.04) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (1.04 to <9.06) | 1.21 (0.47–3.14) | 1.53 (0.92–2.53) | 1.33 (0.72–2.45) |
| Tertile 3 (≥9.06) | 0.75 (0.17–3.22) | 1.21 (0.64–2.27) | 1.38 (0.66–2.89) |
| Equol | |||
| Tertile 1 (<3.75) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (3.75 to <9.73) | 1.26 (0.52–3.06) | 0.95 (0.54–1.67) | 1.07 (0.48–2.40) |
| Tertile 3 (≥9.73) | 1.93 (0.81–4.62) | 1.10 (0.66–1.86) | 1.16 (0.59–2.26) |
| Genistein | |||
| Tertile 1 (<12.35) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (12.35 to <50.00) | 0.89 (0.14–5.68) | 0.84 (0.47–1.51) | 0.50 (0.23–1.09) |
| Tertile 3 (≥50.00) | 1.77 (0.15–21.09) | 0.73 (0.37–1.42) | 1.32 (0.59–2.94) |
| Phytoestrogen Concentrations (μg/g Creatinine) | Model 3 a | ||
|---|---|---|---|
| Very Short Sleep (<5 h/Night) | Short Sleep (5–6 h/Night) | Long Sleep (≥9 h/Night) | |
| Females | |||
| Enterolactone | |||
| Tertile 1 (<160.53) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (160.53 to <621.68) | 0.69 (0.38–1.27) | 0.99 (0.63–1.55) | 1.55 (0.83–2.87) |
| Tertile 3 (≥621.68) | 0.61 (0.31–1.20) | 0.91 (0.62–1.35) | 0.74 (0.35–1.54) |
| Enterodiol | |||
| Tertile 1 (<21.52) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (21.52 to <70.27) | 1.39 (0.70–2.77) | 0.77 (0.53–1.13) | 1.03 (0.56–1.90) |
| Tertile 3 (≥70.27) | 0.91 (0.42–1.99) | 0.99 (0.61–1.59) | 2.14 (1.11–4.13) * |
| Daidzein | |||
| Tertile 1 (<24.22) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (24.22 to <108.62) | 1.50 (0.58–3.89) | 0.85 (0.57–1.28) | 0.79 (0.34–1.86) |
| Tertile 3 (≥108.62) | 1.69 (0.35–8.12) | 0.72 (0.41–1.28) | 0.75 (0.27–2.10) |
| O-Desmethylangolensin | |||
| Tertile 1 (<1.04) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (1.04 to <9.06) | 1.33 (0.63–2.80) | 1.25 (0.83–1.87) | 1.19 (0.64–2.20) |
| Tertile 3 (≥9.06) | 0.87 (0.32–2.39) | 1.23 (0.77–1.97) | 2.23 (0.95–5.26) |
| Equol | |||
| Tertile 1 (<3.75) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (3.75 to <9.73) | 0.90 (0.44–1.85) | 0.95 (0.71–1.27) | 1.12 (0.64–1.97) |
| Tertile 3 (≥9.73) | 0.54 (0.25–1.16) | 0.76 (0.53–1.09) | 0.74 (0.39–1.40) |
| Genistein | |||
| Tertile 1 (<12.35) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (12.35 to <50.00) | 0.58 (0.26–1.31) | 1.50 (0.93–2.41) | 0.70 (0.34–1.45) |
| Tertile 3 (≥50.00) | 0.70 (0.17–2.85) | 1.37 (0.76–2.46) | 0.70 (0.29–1.69) |
| Males | |||
| Enterolactone | |||
| Tertile 1 (<160.53) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (160.53 to <621.68) | 1.48 (0.70–3.13) | 1.29 (0.89–1.88) | 1.20 (0.65–2.23) |
| Tertile 3 (≥621.68) | 0.50 (0.23–1.13) | 0.94 (0.62–1.42) | 0.89 (0.44–1.82) |
| Enterodiol | |||
| Tertile 1 (<21.52) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (21.52 to <70.27) | 1.31 (0.60–2.87) | 1.40 (0.99–1.99) | 1.05 (0.56–1.98) |
| Tertile 3 (≥70.27) | 0.95 (0.39–2.28) | 1.06 (0.73–1.54) | 0.93 (0.47–1.84) |
| Daidzein | |||
| Tertile 1 (<24.22) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (24.22 to <108.62) | 0.63 (0.23–1.76) | 1.28 (0.87–1.88) | 1.72 (0.82–3.59) |
| Tertile 3 (≥108.62) | 1.14 (0.24–5.40) | 1.87 (0.95–3.65) | 1.79 (0.68–4.72) |
| O-Desmethylangolensin | |||
| Tertile 1 (<1.04) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (1.04 to <9.06) | 1.59 (0.65–3.87) | 0.90 (0.69–1.16) | 0.94 (0.50–1.76) |
| Tertile 3 (≥9.06) | 0.75 (0.21–2.68) | 0.70 (0.46–1.04) | 0.83 (0.38–1.82) |
| Equol | |||
| Tertile 1 (<3.75) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (3.75 to <9.73) | 1.07 (0.64–1.79) | 1.06 (0.74–1.51) | 0.95 (0.49–1.82) |
| Tertile 3 (≥9.73) | 1.22 (0.66–2.26) | 1.29 (0.92–1.81) | 1.18 (0.62–2.27) |
| Genistein | |||
| Tertile 1 (<12.35) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Tertile 2 (12.35 to <50.00) | 1.55 (0.61–3.93) | 0.90 (0.63–1.29) | 0.51 (0.30–0.87) * |
| Tertile 3 (≥50.00) | 1.78 (0.68–4.62) | 0.81 (0.51–1.27) | 0.51 (0.25–1.02) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Jiang, H.; Wang, W.; Dong, X.; Zhang, D. Associations of Urinary Phytoestrogen Concentrations with Sleep Disorders and Sleep Duration among Adults. Nutrients 2020, 12, 2103. https://doi.org/10.3390/nu12072103
Sun J, Jiang H, Wang W, Dong X, Zhang D. Associations of Urinary Phytoestrogen Concentrations with Sleep Disorders and Sleep Duration among Adults. Nutrients. 2020; 12(7):2103. https://doi.org/10.3390/nu12072103
Chicago/Turabian StyleSun, Jing, Hong Jiang, Weijing Wang, Xue Dong, and Dongfeng Zhang. 2020. "Associations of Urinary Phytoestrogen Concentrations with Sleep Disorders and Sleep Duration among Adults" Nutrients 12, no. 7: 2103. https://doi.org/10.3390/nu12072103
APA StyleSun, J., Jiang, H., Wang, W., Dong, X., & Zhang, D. (2020). Associations of Urinary Phytoestrogen Concentrations with Sleep Disorders and Sleep Duration among Adults. Nutrients, 12(7), 2103. https://doi.org/10.3390/nu12072103






