Dietary Antioxidant Intake and Human Papillomavirus Infection: Evidence from a Cross-Sectional Study in Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Assessment of Socio-Demographic and Behavioral Information
2.3. Assessment of Dietary Antioxidant Intake
2.4. Statistical Analyses
3. Results
3.1. Dietary Intake of Antioxidants According to HPV Status
3.2. Population Characteristics According to the Composite Dietary Antioxidant Index
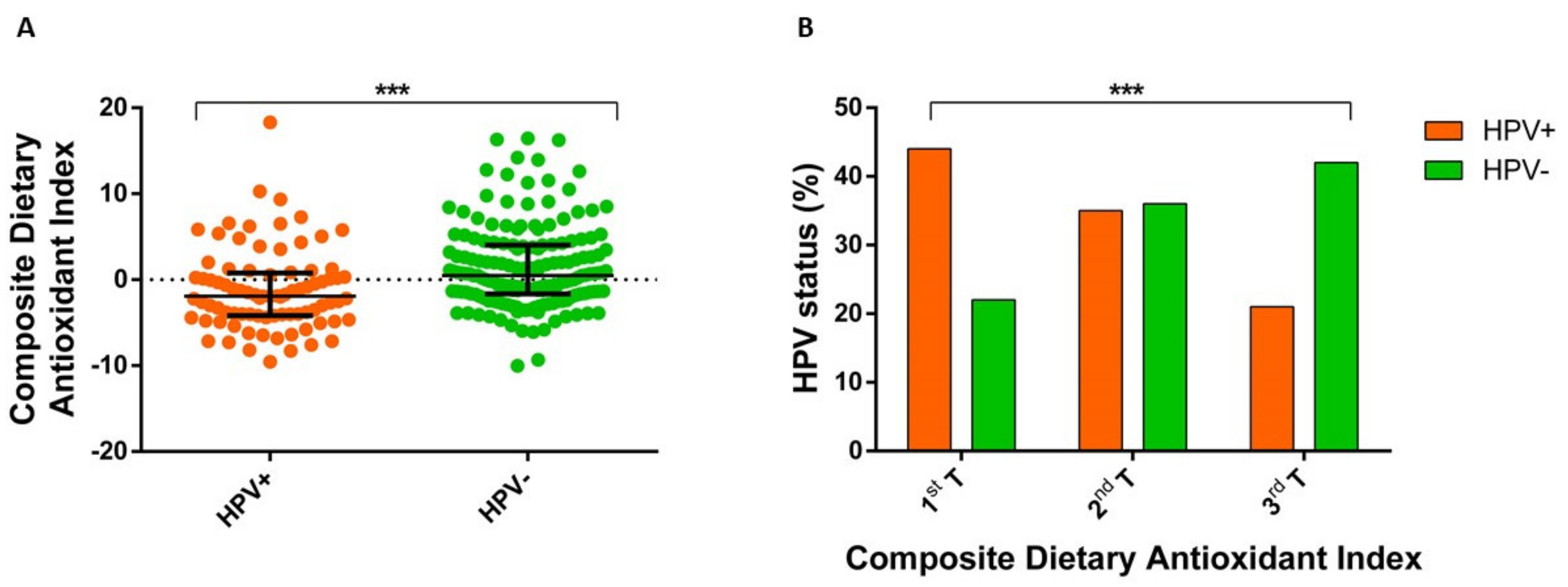
3.3. Association of Composite Dietary Antioxidant Index with HPV Status
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rodríguez, A.C.; Schiffman, M.; Herrero, R.; Wacholder, S.; Hildesheim, A.; Castle, P.E.; Solomon, D.; Burk, R.; Proyecto Epidemiológico Guanacaste Group. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J. Natl. Cancer Inst. 2008, 100, 513–517. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Plummer, M.; de Martel, C.; Vignat, J.; Ferlay, J.; Bray, F.; Franceschi, S. Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob. Health 2016, 4, e609–e616. [Google Scholar] [CrossRef]
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; de Sanjosé, S.; Fakhry, C.; Monk, B.J.; Stanley, M.A.; Franceschi, S. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Primers 2016, 2, 16086. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Quattrocchi, A.; Agrifoglio, O.; Scalisi, A.; Agodi, A. The Association of Dietary Patterns with High-Risk Human Papillomavirus Infection and Cervical Cancer: A Cross-Sectional Study in Italy. Nutrients 2018, 10, 469. [Google Scholar] [CrossRef]
- Zhou, X.; Meng, Y. Association between serum folate level and cervical cancer: A meta-analysis. Arch. Gynecol. Obstet. 2016, 293, 871–877. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, B.; Zhang, B.; Wang, Z. Vitamin A and risk of cervical cancer: A meta-analysis. Gynecol. Oncol. 2012, 124, 366–373. [Google Scholar] [CrossRef]
- Cao, D.; Shen, K.; Li, Z.; Xu, Y.; Wu, D. Association between vitamin C Intake and the risk of cervical neoplasia: A meta-analysis. Nutr. Cancer 2016, 68, 48–57. [Google Scholar] [CrossRef]
- Hwang, J.H.; Lee, J.K.; Kim, T.J.; Kim, M.K. The association between fruit and vegetable consumption and HPV viral load in high-risk HPV-positive women with cervical intraepithelial neoplasia. Cancer Causes Control. 2010, 21, 51–59. [Google Scholar] [CrossRef]
- Siegel, E.M.; Salemi, J.L.; Villa, L.L.; Ferenczy, A.; Franco, E.L.; Giuliano, A.R. Dietary consumption of antioxidant nutrients and risk of incident cervical intraepithelial neoplasia. Gynecol. Oncol. 2010, 118, 289–294. [Google Scholar] [CrossRef]
- Guo, L.; Zhu, H.; Lin, C.; Che, J.; Tian, X.; Han, S.; Zhao, H.; Zhu, Y.; Mao, D. Associations between antioxidant vitamins and the risk of invasive cervical cancer in Chinese women: A case-control study. Sci. Rep. 2015, 5, 13607. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.K.; Lee, J.K.; Kim, J.H.; Son, S.K.; Song, E.S.; Lee, K.B.; Lee, J.P.; Lee, J.M.; Yun, Y.M. Intakes of vitamin A, C, and E, and beta-carotene are associated with risk of cervical cancer: A case-control study in Korea. Nutr. Cancer 2010, 62, 181–189. [Google Scholar] [CrossRef]
- Tomita, L.Y.; Longatto Filho, A.; Costa, M.C.; Andreoli, M.A.; Villa, L.L.; Franco, E.L.; Cardoso, M.A.; Brazilian Investigation into Nutrition and Cervical Cancer Prevention (BRINCA) Study Team. Diet and serum micronutrients in relation to cervical neoplasia and cancer among low-income Brazilian women. Int. J. Cancer 2010, 126, 703–714. [Google Scholar] [CrossRef]
- Piyathilake, C.J.; Henao, O.L.; Macaluso, M.; Cornwell, P.E.; Meleth, S.; Heimburger, D.C.; Partridge, E.E. Folate is associated with the natural history of high-risk human papillomaviruses. Cancer Res. 2004, 64, 8788–8793. [Google Scholar] [CrossRef]
- Peterhans, E. Oxidants and antioxidants in viral diseases: Disease mechanisms and metabolic regulation. J. Nutr. 1997, 127, 962S–965S. [Google Scholar] [CrossRef]
- De Marco, F.; Bucaj, E.; Foppoli, C.; Fiorini, A.; Blarzino, C.; Filipi, K.; Giorgi, A.; Schininà, M.E.; Di Domenico, F.; Coccia, R.; et al. Oxidative stress in HPV-driven viral carcinogenesis: Redox proteomics analysis of HPV-16 dysplastic and neoplastic tissues. PLoS ONE 2012, 7, e34366. [Google Scholar] [CrossRef]
- De Marco, F. Oxidative stress and HPV carcinogenesis. Viruses 2013, 5, 708–731. [Google Scholar] [CrossRef]
- Basu, J.; Palan, P.R.; Vermund, S.H.; Goldberg, G.L.; Burk, R.D.; Romney, S.L. Plasma ascorbic acid and beta-carotene levels in women evaluated for HPV infection, smoking, and cervix dysplasia. Cancer Detect. Prev. 1991, 15, 165–170. [Google Scholar]
- Potischman, N.; Herrero, R.; Brinton, L.A.; Reeves, W.C.; Stacewicz-Sapuntzakis, M.; Jones, C.J.; Brenes, M.M.; Tenorio, F.; de Britton, R.C.; Gaitan, E. A case-control study of nutrient status and invasive cervical cancer. II. Serologic indicators. Am. J. Epidemiol. 1991, 134, 1347–1355. [Google Scholar] [CrossRef]
- Potischman, N.; Hoover, R.N.; Brinton, L.A.; Swanson, C.A.; Herrero, R.; Tenorio, F.; de Britton, R.C.; Gaitan, E.; Reeves, W.C. The relations between cervical cancer and serological markers of nutritional status. Nutr. Cancer 1994, 21, 193–201. [Google Scholar] [CrossRef]
- Peterson, C.E.; Sedjo, R.L.; Davis, F.G.; Beam, C.A.; Giuliano, A.R. Combined antioxidant carotenoids and the risk of persistent human papillomavirus infection. Nutr. Cancer 2010, 62, 728–733. [Google Scholar] [CrossRef]
- Goodman, M.T.; Shvetsov, Y.B.; McDuffie, K.; Wilkens, L.R.; Zhu, X.; Franke, A.A.; Bertram, C.C.; Kessel, B.; Bernice, M.; Sunoo, C.; et al. Hawaii cohort study of serum micronutrient concentrations and clearance of incident oncogenic human papillomavirus infection of the cervix. Cancer Res. 2007, 67, 5987–5996. [Google Scholar] [CrossRef][Green Version]
- Siegel, E.M.; Craft, N.E.; Duarte-Franco, E.; Villa, L.L.; Franco, E.L.; Giuliano, A.R. Associations between serum carotenoids and tocopherols and type-specific HPV persistence: The Ludwig-McGill cohort study. Int. J. Cancer 2007, 120, 672–680. [Google Scholar] [CrossRef]
- Giuliano, A.R.; Papenfuss, M.; Nour, M.; Canfield, L.M.; Schneider, A.; Hatch, K. Antioxidant nutrients: Associations with persistent human papillomavirus infection. Cancer Epidemiol. Biomarkers Prev. 1997, 6, 917–923. [Google Scholar]
- García-Closas, R.; Castellsagué, X.; Bosch, X.; González, C.A. The role of diet and nutrition in cervical carcinogenesis: A review of recent evidence. Int. J. Cancer 2005, 117, 629–637. [Google Scholar] [CrossRef]
- Keefe, K.A.; Schell, M.J.; Brewer, C.; McHale, M.; Brewster, W.; Chapman, J.A.; Rose, G.S.; McMeeken, D.S.; Lagerberg, W.; Peng, Y.M.; et al. A randomized, double blind, Phase III trial using oral beta-carotene supplementation for women with high-grade cervical intraepithelial neoplasia. Cancer Epidemiol. Biomarkers Prev. 2001, 10, 1029–1035. [Google Scholar]
- Romney, S.L.; Ho, G.Y.; Palan, P.R.; Basu, J.; Kadish, A.S.; Klein, S.; Mikhail, M.; Hagan, R.J.; Chang, C.J.; Burk, R.D. Effects of beta-carotene and other factors on outcome of cervical dysplasia and human papillomavirus infection. Gynecol. Oncol. 1997, 65, 483–492. [Google Scholar] [CrossRef]
- Goodman, M.T.; Kiviat, N.; McDuffie, K.; Hankin, J.H.; Hernandez, B.; Wilkens, L.R.; Franke, A.; Kuypers, J.; Kolonel, L.N.; Nakamura, J.; et al. The association of plasma micronutrients with the risk of cervical dysplasia in Hawaii. Cancer Epidemiol. Biomarkers Prev. 1998, 7, 537–544. [Google Scholar]
- Mackerras, D.; Irwig, L.; Simpson, J.M.; Weisberg, E.; Cardona, M.; Webster, F.; Walton, L.; Ghersi, D. Randomized double-blind trial of beta-carotene and vitamin C in women with minor cervical abnormalities. Br. J. Cancer 1999, 79, 1448–1453. [Google Scholar] [CrossRef]
- Le Gal, K.; Ibrahim, M.X.; Wiel, C.; Sayin, V.I.; Akula, M.K.; Karlsson, C.; Dalin, M.G.; Akyürek, L.M.; Lindahl, P.; Nilsson, J.; et al. Antioxidants can increase melanoma metastasis in mice. Sci. Transl. Med. 2015, 7, 308re308. [Google Scholar] [CrossRef]
- Sayin, V.I.; Ibrahim, M.X.; Larsson, E.; Nilsson, J.A.; Lindahl, P.; Bergo, M.O. Antioxidants accelerate lung cancer progression in mice. Sci. Transl. Med. 2014, 6, 221ra215. [Google Scholar] [CrossRef]
- Piskounova, E.; Agathocleous, M.; Murphy, M.M.; Hu, Z.; Huddlestun, S.E.; Zhao, Z.; Leitch, A.M.; Johnson, T.M.; DeBerardinis, R.J.; Morrison, S.J. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 2015, 527, 186–191. [Google Scholar] [CrossRef]
- Lawenda, B.D.; Kelly, K.M.; Ladas, E.J.; Sagar, S.M.; Vickers, A.; Blumberg, J.B. Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? J. Natl. Cancer Inst. 2008, 100, 773–783. [Google Scholar] [CrossRef]
- Wright, M.E.; Mayne, S.T.; Stolzenberg-Solomon, R.Z.; Li, Z.; Pietinen, P.; Taylor, P.R.; Virtamo, J.; Albanes, D. Development of a comprehensive dietary antioxidant index and application to lung cancer risk in a cohort of male smokers. Am. J. Epidemiol. 2004, 160, 68–76. [Google Scholar] [CrossRef]
- Barchitta, M.; Quattrocchi, A.; Maugeri, A.; Canto, C.; La Rosa, N.; Cantarella, M.A.; Spampinato, G.; Scalisi, A.; Agodi, A. LINE-1 hypermethylation in white blood cell DNA is associated with high-grade cervical intraepithelial neoplasia. BMC Cancer 2017, 17, 601. [Google Scholar] [CrossRef]
- Physical status: The use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ. Tech. Rep. Ser. 1995, 854, 1–452. [Google Scholar]
- Agodi, A.; Barchitta, M.; Quattrocchi, A.; Maugeri, A.; Canto, C.; Marchese, A.E.; Vinciguerra, M. Low fruit consumption and folate deficiency are associated with LINE-1 hypomethylation in women of a cancer-free population. Genes Nutr. 2015, 10, 480. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Quattrocchi, A.; Barone, G.; Mazzoleni, P.; Catalfo, A.; De Guidi, G.; Iemmolo, M.G.; Crimi, N.; Agodi, A. Mediterranean Diet and Particulate Matter Exposure Are Associated With LINE-1 Methylation: Results From a Cross-Sectional Study in Women. Front. Genet. 2018, 9, 514. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Magnano San Lio, R.; Favara, G.; La Rosa, M.C.; La Mastra, C.; Quattrocchi, A.; Agodi, A. Dietary Patterns are Associated with Leukocyte LINE-1 Methylation in Women: A Cross-Sectional Study in Southern Italy. Nutrients 2019, 11, 1843. [Google Scholar] [CrossRef]
- Maugeri, A.; Barchitta, M.; Fiore, V.; Rosta, G.; Favara, G.; La Mastra, C.; La Rosa, M.C.; Magnano San Lio, R.; Agodi, A. Determinants of Adherence to the Mediterranean Diet: Findings from a Cross-Sectional Study in Women from Southern Italy. Int. J. Environ. Res. Public Health 2019, 16, 2963. [Google Scholar] [CrossRef]
- Willett, W. Nutritional Epidemiology; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Si, C.J.; Shu, L.; Zheng, P.F.; Zhang, X.Y.; Yu, X.L.; Gao, W.; Zhang, L. Dietary patterns and endometrial cancer: A meta-analysis. Eur. J. Cancer Prev. 2017, 26, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Brennan, S.F.; Cantwell, M.M.; Cardwell, C.R.; Velentzis, L.S.; Woodside, J.V. Dietary patterns and breast cancer risk: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 91, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Chih, H.J.; Lee, A.H.; Colville, L.; Binns, C.W.; Xu, D. A review of dietary prevention of human papillomavirus-related infection of the cervix and cervical intraepithelial neoplasia. Nutr. Cancer 2013, 65, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.R.; Siegel, E.M.; Roe, D.J.; Ferreira, S.; Baggio, M.L.; Galan, L.; Duarte-Franco, E.; Villa, L.L.; Rohan, T.E.; Marshall, J.R.; et al. Dietary intake and risk of persistent human papillomavirus (HPV) infection: The Ludwig-McGill HPV Natural History Study. J. Infect. Dis. 2003, 188, 1508–1516. [Google Scholar] [CrossRef]
- Sedjo, R.L.; Roe, D.J.; Abrahamsen, M.; Harris, R.B.; Craft, N.; Baldwin, S.; Giuliano, A.R. Vitamin A, carotenoids, and risk of persistent oncogenic human papillomavirus infection. Cancer Epidemiol. Biomarkers Prev. 2002, 11, 876–884. [Google Scholar]
- Haase, H.; Rink, L. The immune system and the impact of zinc during aging. Immun. Ageing 2009, 6, 9. [Google Scholar] [CrossRef]
- Bao, B.; Prasad, A.S.; Beck, F.W.; Fitzgerald, J.T.; Snell, D.; Bao, G.W.; Singh, T.; Cardozo, L.J. Zinc decreases C-reactive protein, lipid peroxidation, and inflammatory cytokines in elderly subjects: A potential implication of zinc as an atheroprotective agent. Am. J. Clin. Nutr. 2010, 91, 1634–1641. [Google Scholar] [CrossRef]
- Prasad, A.S. Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp. Gerontol. 2008, 43, 370–377. [Google Scholar] [CrossRef]
- Maywald, M.; Wessels, I.; Rink, L. Zinc Signals and Immunity. Int. J. Mol. Sci. 2017, 18, 2222. [Google Scholar] [CrossRef]
- Rink, L.; Haase, H. Zinc homeostasis and immunity. Trends Immunol. 2007, 28, 1–4. [Google Scholar] [CrossRef]
- Kim, J.H.; Bae, S.N.; Lee, C.W.; Song, M.J.; Lee, S.J.; Yoon, J.H.; Lee, K.H.; Hur, S.Y.; Park, T.C.; Park, J.S. A pilot study to investigate the treatment of cervical human papillomavirus infection with zinc-citrate compound (CIZAR®). Gynecol. Oncol. 2011, 122, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Hruskova, J.; Jakubik, J.; Kunzova, S.; Sochor, O.; Barchitta, M.; Agodi, A.; Bauerova, H.; Medina-Inojosa, J.R.; Vinciguerra, M. Dietary antioxidant intake decreases carotid intima media thickness in women but not in men: A cross-sectional assessment in the Kardiovize study. Free Radic. Biol. Med. 2019, 131, 274–281. [Google Scholar] [CrossRef] [PubMed]
| Dietary Intake | HPV-Negative (n = 167) | HPV-Positive (n = 84) | p-Value |
|---|---|---|---|
| Total energy intake, kcal | 2080 (703) | 1747 (722) | <0.001 |
| Zinc, mg | 9.21 (2.93) | 7.60 (3.80) | <0.001 |
| Selenium, μg | 319.14 (485.53) | 311.63 (461.46) | 0.272 |
| Manganese, mg | 314.64 (93.03) | 266.33 (101.19) | <0.001 |
| Vitamin A, IU | 1097.59 (538.14) | 827.32 (586.10) | 0.002 |
| Vitamin C, mg | 116.71 (107.55) | 82.21 (84.34) | 0.001 |
| Vitamin E, mg | 37.97 (23.44) | 34.08 (20.49) | 0.158 |
| Carotenoids, μg | 9267.17 (7369.62) | 7749.60 (6973.24) | 0.052 |
| Flavonoids, μg | 1624.20 (6850.78) | 819.81 (4964.17) | 0.163 |
| Characteristics | Composite Dietary Antioxidant Index | p-Value | ||
|---|---|---|---|---|
| First Tertile (n = 73) | Second Tertile (n = 89) | Third Tertile (n = 89) | ||
| Age, years | 31.0 (8.0) | 41.0 (7.0) | 52.0 (9.0) | <0.001 |
| Current smokers (%) | 40.3% | 38.2% | 25.8% | 0.102 |
| BMI, kg/m2 | 20.8 (4.2) | 23.4 (4.8) | 23.7 (5.4) | <0.001 |
| BMI Categories (%) | ||||
| Underweight | 12.3% | 6.8% | 1.1% | <0.001 |
| Normal weight | 74.0% | 61.4% | 55.7% | |
| Overweight | 6.8% | 18.2% | 31.8% | |
| Obese | 6.8% | 13.6% | 11.4% | |
| Living in a couple (%) | 39.7% | 61.8% | 74.2% | <0.001 |
| Employed (%) | 46.6% | 47.2% | 40.4% | 0.613 |
| Low educational level (%) | 26.0% | 39.3% | 47.2% | 0.022 |
| Having children (%) | 50.7% | 78.7% | 91.0% | <0.001 |
| Use of oral contraceptive (%) | 16.4% | 9.0% | 4.5% | 0.036 |
| Use of multivitamin supplements (%) | 8.2% | 12.4% | 16.9% | 0.258 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barchitta, M.; Maugeri, A.; La Mastra, C.; La Rosa, M.C.; Favara, G.; Magnano San Lio, R.; Agodi, A. Dietary Antioxidant Intake and Human Papillomavirus Infection: Evidence from a Cross-Sectional Study in Italy. Nutrients 2020, 12, 1384. https://doi.org/10.3390/nu12051384
Barchitta M, Maugeri A, La Mastra C, La Rosa MC, Favara G, Magnano San Lio R, Agodi A. Dietary Antioxidant Intake and Human Papillomavirus Infection: Evidence from a Cross-Sectional Study in Italy. Nutrients. 2020; 12(5):1384. https://doi.org/10.3390/nu12051384
Chicago/Turabian StyleBarchitta, Martina, Andrea Maugeri, Claudia La Mastra, Maria Clara La Rosa, Giuliana Favara, Roberta Magnano San Lio, and Antonella Agodi. 2020. "Dietary Antioxidant Intake and Human Papillomavirus Infection: Evidence from a Cross-Sectional Study in Italy" Nutrients 12, no. 5: 1384. https://doi.org/10.3390/nu12051384
APA StyleBarchitta, M., Maugeri, A., La Mastra, C., La Rosa, M. C., Favara, G., Magnano San Lio, R., & Agodi, A. (2020). Dietary Antioxidant Intake and Human Papillomavirus Infection: Evidence from a Cross-Sectional Study in Italy. Nutrients, 12(5), 1384. https://doi.org/10.3390/nu12051384







