Effects of Arginine Supplementation on Athletic Performance Based on Energy Metabolism: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Literature Search Strategies
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection
2.4. Outcome Measures
2.5. Publication Bias
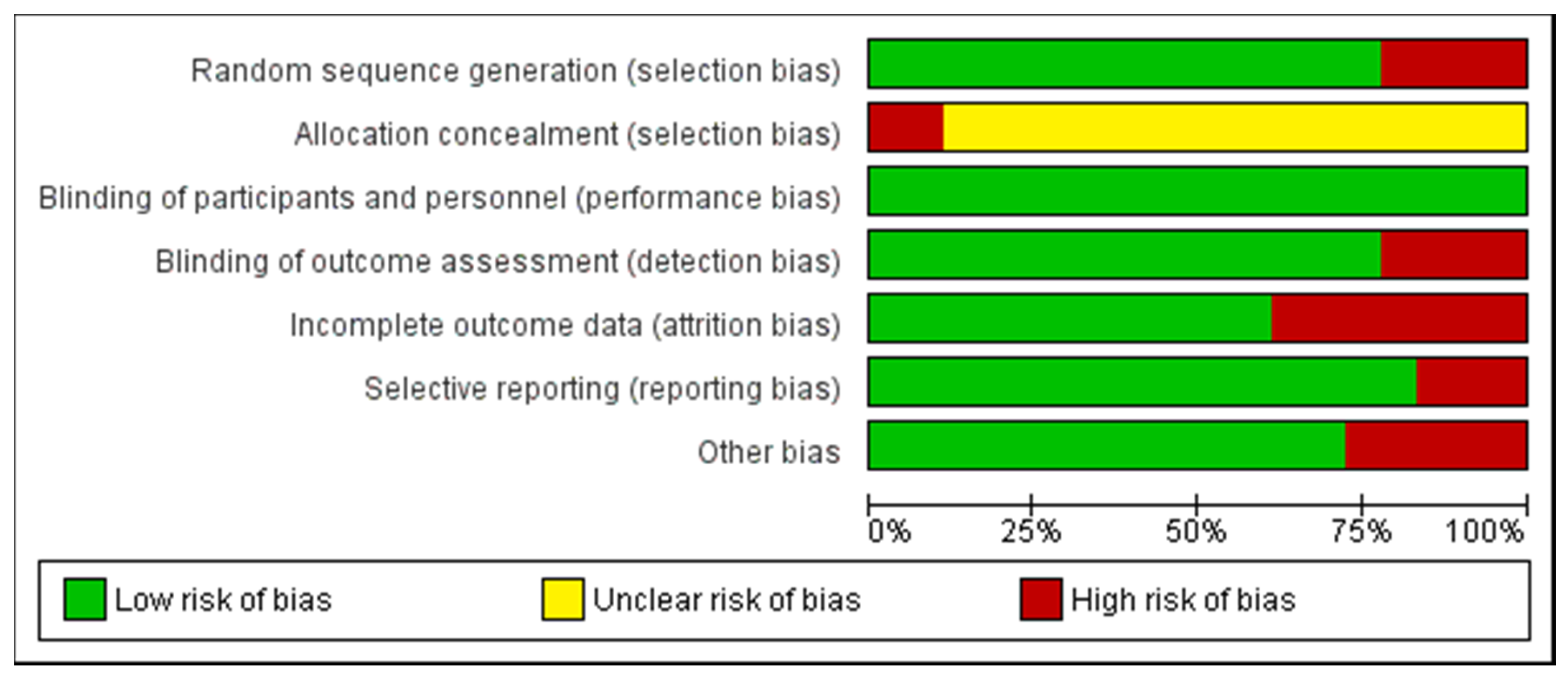
2.6. Quality Assessment of the Experiments
2.7. Statistical Analysis
3. Results
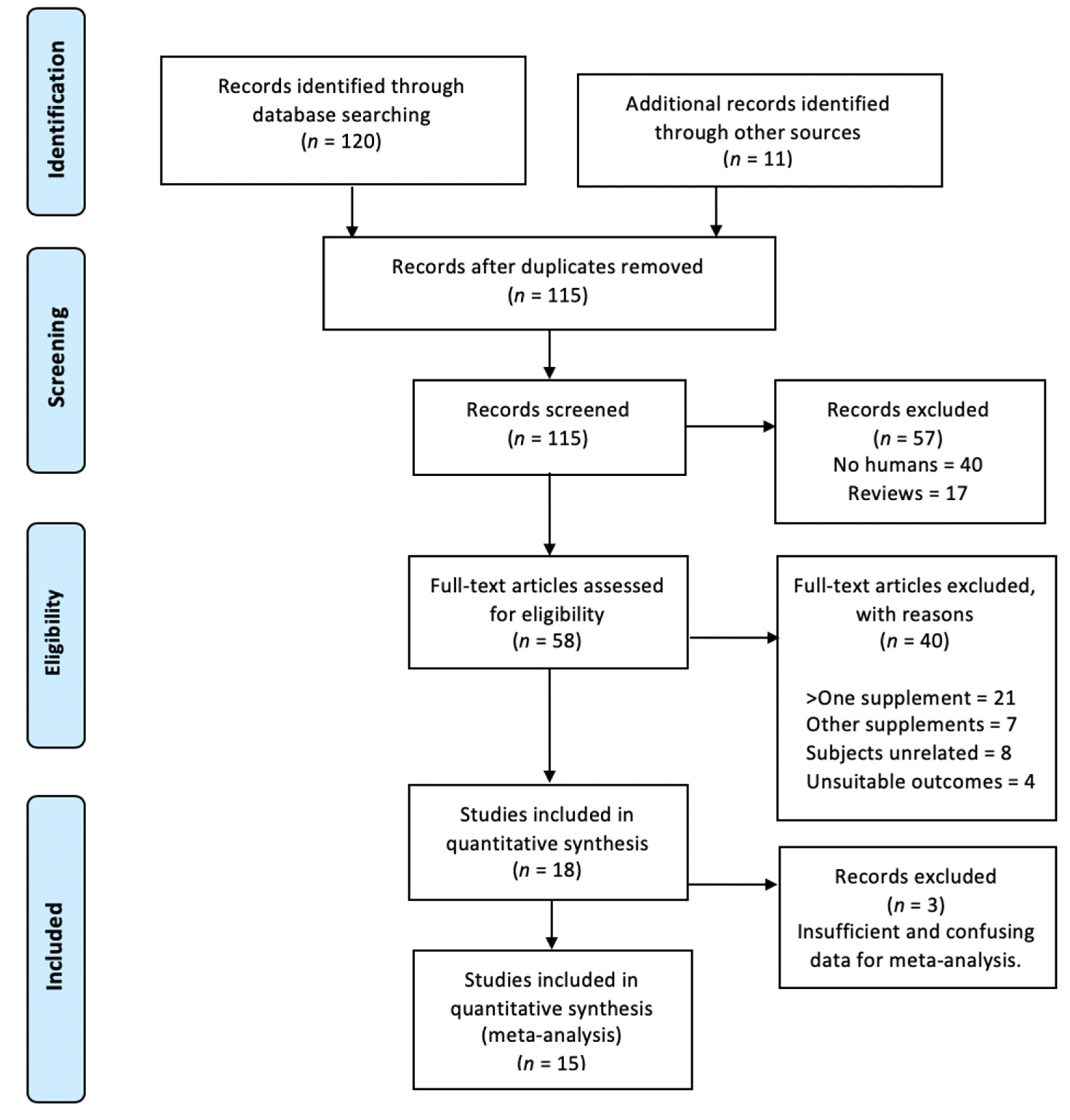
3.1. Main Search
3.2. Arginine Supplementation
3.3. Effect of Arginine on Anaerobic Performance (>VO2max)
3.4. Effect of Arginine on Aerobic Performance (≤VO2max)
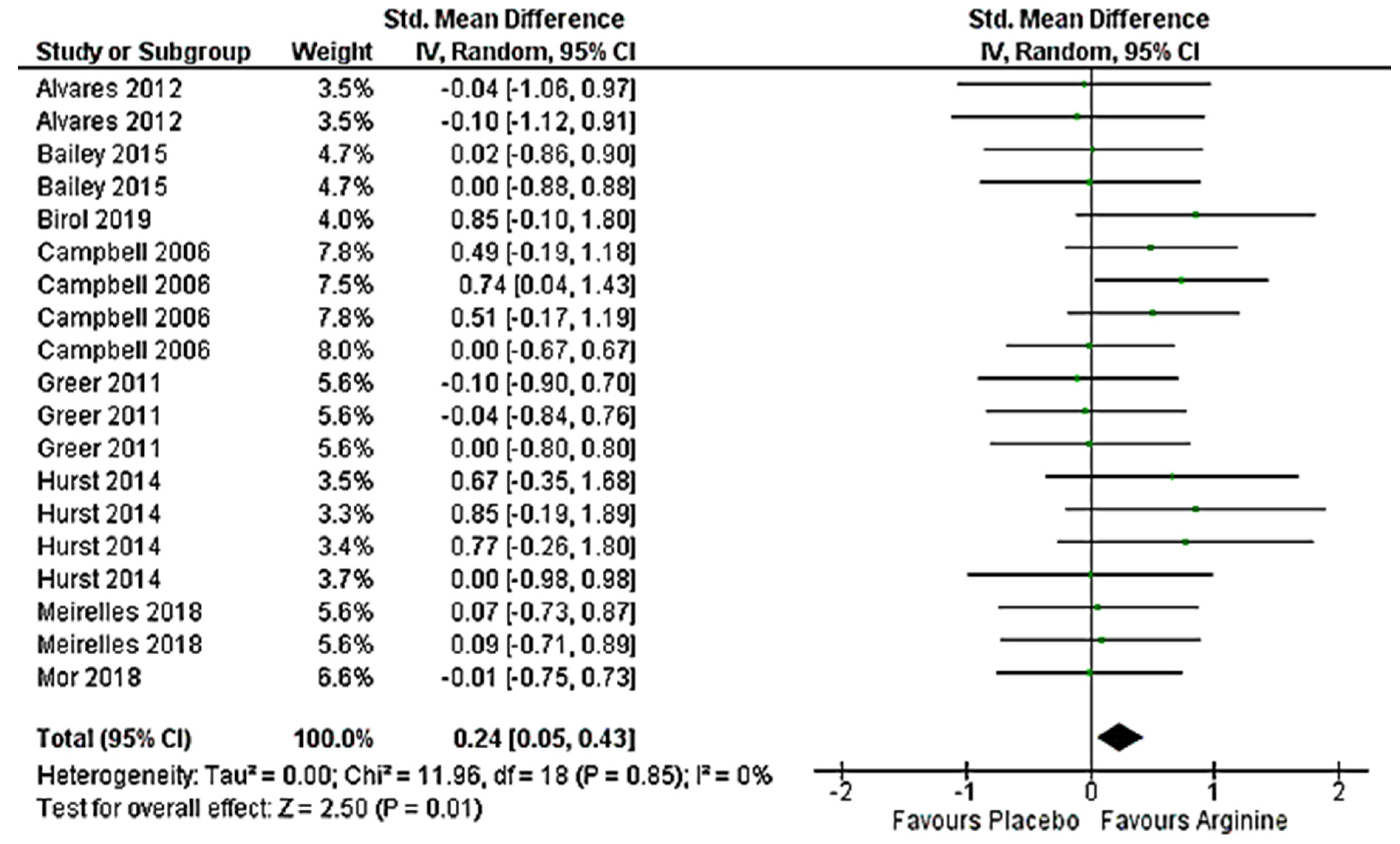
3.5. Effect on Anaerobic Performance (>VO2max) Meta-Analysis
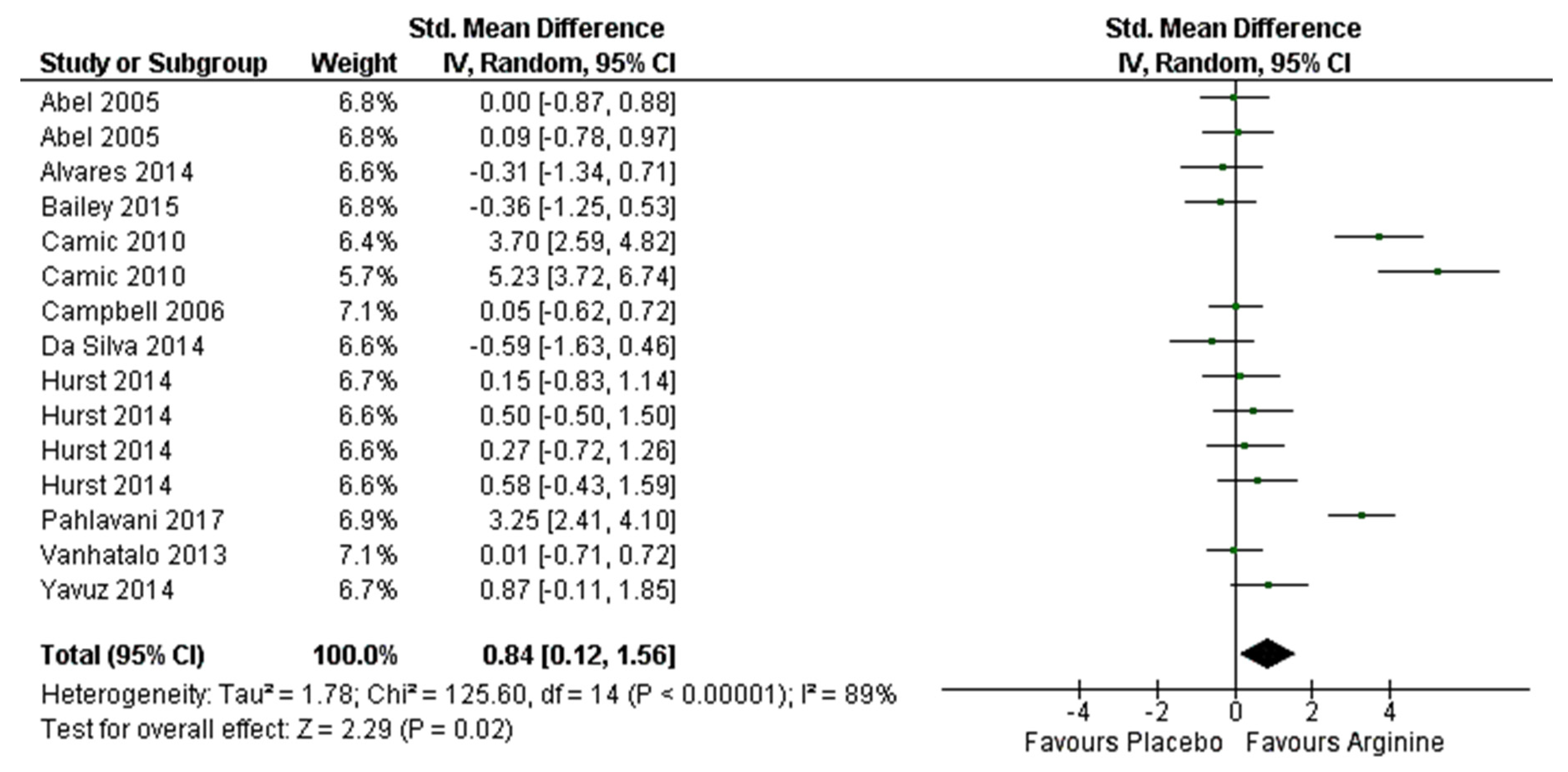
3.6. Effect on Aerobic Performance (≤VO2max) Meta-Analysis
4. Discussion
4.1. Effect on Anaerobic Performance (>VO2max)
4.2. Effect on Aerobic Performance (≤VO2max)
4.3. Strength, Limitations, and Future Lines of Research
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Garthe, I.; Maughan, R.J. Athletes and Supplements: Prevalence and Perspectives. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 126–138. [Google Scholar] [CrossRef]
- Baltazar-Martins, G.; Brito de Souza, D.; Aguilar-Navarro, M.; Munoz-Guerra, J.; Plata, M.D.M.; Del Coso, J. Prevalence and patterns of dietary supplement use in elite Spanish athletes. J. Int. Soc. Sports Nutr. 2019, 16, 30. [Google Scholar] [CrossRef]
- Australian Institute of Sport. ABCD Classification System. Australian Institute of Sport. 2017 [2018; 2018] Disponible en. Available online: https://ais.gov.au/nutrition/supplements#group_a (accessed on 18 April 2020).
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.; Kleiner, S.M.; Jager, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E.; et al. ISSN exercise & sports nutrition review update: Research & recommendations. J. Int. Soc. Sports Nutr. 2018, 15, 38. [Google Scholar] [PubMed]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC consensus statement: Dietary supplements and the high-performance athlete. Br. J. Sports Med. 2018, 52, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, R.; Cuenca, E.; Mate-Munoz, J.L.; Garcia-Fernandez, P.; Serra-Paya, N.; Estevan, M.C.L.; Herreros, P.V.; Garnacho-Castano, M.V. Effects of Beetroot Juice Supplementation on Cardiorespiratory Endurance in Athletes. A Systematic Review. Nutrients 2017, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Poortmans, J.R.; Gualano, B.; Carpentier, A. Nitrate supplementation and human exercise performance: Too much of a good thing? Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Morris, S.M.J. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336, 1–17. [Google Scholar] [CrossRef]
- Pahlavani, N.; Entezari, M.H.; Nasiri, M.; Miri, A.; Rezaie, M.; Bagheri-Bidakhavidi, M.; Sadeghi, O. The effect of L-arginine supplementation on body composition and performance in male athletes: A double-blinded randomized clinical trial. Eur. J. Clin. Nutr. 2017, 71, 544–548. [Google Scholar] [CrossRef]
- Yavuz, H.U.; Turnagol, H.; Demirel, A.H. Pre-exercise arginine supplementation increases time to exhaustion in elite male wrestlers. Biol. Sport 2014, 31, 187–191. [Google Scholar] [CrossRef]
- Hurst, H.; Hurst, H.T.; Sinclair, J.; Beenham, M. Influence of absolute versus relative L-arginine dosage on 1 km and 16.1 km time trial performance in trained cyclists. J. Sci. Cycl. 2014, 3, 2–8. [Google Scholar]
- Camic, C.L.; Housh, T.J.; Zuniga, J.M.; Hendrix, R.C.; Mielke, M.; Johnson, G.O.; Schmidt, R.J. Effects of arginine-based supplements on the physical working capacity at the fatigue threshold. J. Strength Cond. Res. 2010, 24, 1306–1312. [Google Scholar] [CrossRef] [PubMed]
- Curis, E.; Nicolis, I.; Moinard, C.; Osowska, S.; Zerrouk, N.; Benazeth, S.; Cynober, L. Almost all about citrulline in mammals. Amino Acids 2005, 29, 177–205. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.M.J. Arginine: Beyond protein. Am. J. Clin. Nutr. 2006, 83, 508S–512S. [Google Scholar] [CrossRef] [PubMed]
- Huerta Ojeda, Á.; Domínguez, A.; Barahona-Fuentes, G.D.F. Efecto de la suplementación de L-arginina y L-citrulina sobre el rendimiento físico: Una revisión sistemática. Nutr. Hosp. Organo of. La Soc. Esp. Nutr. Parenter. Y Enter. 2019, 36, 389–1402. [Google Scholar]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Jones, A.M. Dietary nitrate supplementation and exercise performance. Sports Med. 2014, 44, S35–S45. [Google Scholar] [CrossRef]
- Kanaley, J.A. Growth hormone, arginine and exercise. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 50–54. [Google Scholar] [CrossRef]
- Forbes, S.C.; Harber, V.; Bell, G.J. Oral L-arginine before resistance exercise blunts growth hormone in strength trained males. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 236–244. [Google Scholar] [CrossRef]
- Campbell, B.; Roberts, M.; Kerksick, C.; Wilborn, C.; Marcello, B.; Taylor, L.; Nassar, E.; Leutholtz, B.; Bowden, R.; Rasmussen, C.; et al. Pharmacokinetics, safety, and effects on exercise performance of L-arginine alpha-ketoglutarate in trained adult men. Nutrition 2006, 22, 872–881. [Google Scholar] [CrossRef]
- Borsheim, E.; Bui, Q.-U.T.; Tissier, S.; Kobayashi, H.; Ferrando, A.A.; Wolfe, R.R. Effect of amino acid supplementation on muscle mass, strength and physical function in elderly. Clin. Nutr. 2008, 27, 189–195. [Google Scholar] [CrossRef]
- Tang, J.E.; Lysecki, P.J.; Manolakos, J.J.; MacDonald, M.J.; Tarnopolsky, M.A.; Phillips, S.M. Bolus arginine supplementation affects neither muscle blood flow nor muscle protein synthesis in young men at rest or after resistance exercise. J. Nutr. 2011, 141, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, A.; Piquard, F.; Geny, B.; Doutreleau, S.; Lampert, E.; Mettauer, B.; Lonsdorfer, J. L-arginine reduces exercise-induced increase in plasma lactate and ammonia. Int. J. Sports Med. 2002, 23, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.-H.; Tang, T.-K.; Juang, C.-L.; Chen, K.W.-C.; Chi, C.-A.; Hsu, M.-C. Effects of arginine supplementation on post-exercise metabolic responses. Chin. J. Physiol. 2009, 52, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.C.; Harber, V.; Bell, G.J. The acute effects of L-arginine on hormonal and metabolic responses during submaximal exercise in trained cyclists. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 369–377. [Google Scholar] [CrossRef]
- Rowlands, D.S.; Clarke, J.; Green, J.G.; Shi, X. L-Arginine but not L-glutamine likely increases exogenous carbohydrate oxidation during endurance exercise. Eur. J. Appl. Physiol. 2012, 112, 2443–2453. [Google Scholar] [CrossRef]
- Bassett, D.R.J.; Howley, E.T. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med. Sci. Sports Exerc. 2000, 32, 70–84. [Google Scholar] [CrossRef]
- Joyner, M.J.; Coyle, E.F. Endurance exercise performance: The physiology of champions. J. Physiol. 2008, 586, 35–44. [Google Scholar] [CrossRef]
- Jones, A.M.; Burnley, M. Oxygen uptake kinetics: An underappreciated determinant of exercise performance. Int. J. Sports Physiol. Perform. 2009, 4, 524–532. [Google Scholar] [CrossRef]
- Bailey, S.J.; Winyard, P.G.; Vanhatalo, A.; Blackwell, J.R.; DiMenna, F.J.; Wilkerson, D.P.; Jones, A.M. Acute L-arginine supplementation reduces the O2 cost of moderate-intensity exercise and enhances high-intensity exercise tolerance. J. Appl. Physiol. 2010, 109, 1394–1403. [Google Scholar] [CrossRef]
- Alvares, T.S.; Conte-Junior, C.A.; Silva, J.T.; Paschoalin, V.M.F. L-arginine does not improve biochemical and hormonal response in trained runners after 4 weeks of supplementation. Nutr. Res. 2014, 34, 31–39. [Google Scholar] [CrossRef]
- Bailey, S.J.; Blackwell, J.R.; Lord, T.; Vanhatalo, A.; Winyard, P.G.; Jones, A.M. L-Citrulline supplementation improves O2 uptake kinetics and high-intensity exercise performance in humans. J. Appl. Physiol. 2015, 119, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Chamari, K.; Padulo, J. “Aerobic” and “Anaerobic” terms used in exercise physiology: A critical terminology reflection. Sport. Med. Open 2015, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Gaitanos, G.C.; Williams, C.; Boobis, L.H.; Brooks, S. Human muscle metabolism during intermittent maximal exercise. J. Appl. Physiol. 1993, 75, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Gastin, P.B. Energy system interaction and relative contribution during maximal exercise. Sports Med. 2001, 31, 725–741. [Google Scholar] [CrossRef] [PubMed]
- Sahlin, K. Muscle energetics during explosive activities and potential effects of nutrition and training. Sports Med. 2014, 44, S167–S173. [Google Scholar] [CrossRef]
- Ghigo, E.; Arvat, E.; Valente, F.; Nicolosi, M.; Boffano, G.M.; Procopio, M.; Bellone, J.; Maccario, M.; Mazza, E.; Camanni, F. Arginine reinstates the somatotrope responsiveness to intermittent growth hormone-releasing hormone administration in normal adults. Neuroendocrinology 1991, 54, 291–294. [Google Scholar] [CrossRef]
- Zajac, A.; Poprzecki, S.; Zebrowska, A.; Chalimoniuk, M.; Langfort, J. Arginine and ornithine supplementation increases growth hormone and insulin-like growth factor-1 serum levels after heavy-resistance exercise in strength-trained athletes. J. Strength Cond. Res. 2010, 24, 1082–1090. [Google Scholar] [CrossRef]
- Greer, B.K.; Jones, B.T. Acute arginine supplementation fails to improve muscle endurance or affect blood pressure responses to resistance training. J. Strength Cond. Res. 2011, 25, 1789–1794. [Google Scholar] [CrossRef]
- Meirelles, C.M.; Matsuura, C. Acute supplementation of L-arginine affects neither strength performance nor nitric oxide production. J. Sports Med. Phys. Fitness 2018, 58, 216–220. [Google Scholar]
- Mor, A.; Atan, T.; Agaoglu, S.A.; Ayyildiz, M. Effect of arginine supplementation on footballers’ anaerobic performance and recovery. Prog. Nutr. 2018, 20, 104–112. [Google Scholar]
- Schneider, K.L.; Yahia, N. Effectiveness of Arginine Supplementation on Wound Healing in Older Adults in Acute and Chronic Settings: A Systematic Review. Adv. Skin Wound Care 2019, 32, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Krause, J.; Krause, M.; Rocha, I.M.G.D.; Umpierre, D.; Fayh, A.P.T. Association of l-Arginine Supplementation with Markers of Endothelial Function in Patients with Cardiovascular or Metabolic Disorders: A Systematic Review and Meta-Analysis. Nutrients 2018, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.; Green, S.; Higgins, J.P. Defining the Review Question and Developing Criteria for Including Studies. In Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series; Cochrane: London, UK, 2008; pp. 81–94. [Google Scholar]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Berthon, P.; Fellmann, N.; Bedu, M.; Beaune, B.; Dabonneville, M.; Coudert, J.; Chamoux, A. A 5-min running field test as a measurement of maximal aerobic velocity. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 75, 233–238. [Google Scholar] [CrossRef]
- Tong, T.K.; Fu, F.H.; Chow, B.C. Reliability of a 5-min running field test and its accuracy in VO2max evaluation. J. Sports Med. Phys. Fit. 2001, 41, 318–323. [Google Scholar]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Green, S.; Higgins, J. Cochrane Handbook for Systematic Reviews of Interventions; Version 5.0.2; Cochrane Libr: London, UK; Online Libr: Chichester, UK, 2009. [Google Scholar]
- Hedges, L. V Distribution theory for Glass’s estimator of effect size and related estimators. J. Educ. Stat. 1981, 6, 107–128. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 2013; ISBN 1483276481. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 1119964377. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Olek, R.A.; Ziemann, E.; Grzywacz, T.; Kujach, S.; Luszczyk, M.; Antosiewicz, J.; Laskowski, R. A single oral intake of arginine does not affect performance during repeated Wingate anaerobic test. J Sport. Med. Phys. Fit. 2010, 50, 52–56. [Google Scholar]
- Liu, T.H.; Wu, C.L.; Chiang, C.W.; Lo, Y.W.; Tseng, H.F.; Chang, C.K. No effect of short-term arginine supplementation on nitric oxide production, metabolism and performance in intermittent exercise in athletes. J. Nutr. Biochem. 2009, 20, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Álvares, T.S.; Conte, C.A.; Paschoalin, V.M.F.; Silva, J.T.; de Meirelles, C.M.; Bhambhani, Y.N.; Gomes, P.S.C. Acute L-arginine supplementation increases muscle blood volume but not strength performance. Appl. Physiol. Nutr. Metab. 2012, 37, 115–126. [Google Scholar]
- Birol, A.; Kılınç, F.N.; Deliceoğlu, G.; Keskin, E.D. The effect of acute L-arginine supplementation on repeated sprint ability performance. Prog. Nutr. 2019, 21, 5–11. [Google Scholar]
- Abel, T.; Knechtle, B.; Perret, C.; Eser, P.; Von Arx, P.; Knecht, H. Influence of chronic supplementation of arginine aspartate in endurance athletes on performance and substrate metabolism: A randomized, double-blind, placebo-controlled study. Int. J. Sports Med. 2005, 26, 344–349. [Google Scholar] [CrossRef]
- Vanhatalo, A.; Bailey, S.J.; Dimenna, F.J.; Blackwell, J.R.; Wallis, G.A.; Jones, A.M. No effect of acute L-arginine supplementation on O2 cost or exercise tolerance. Eur. J. Appl. Physiol. 2013, 113, 1805–1819. [Google Scholar] [CrossRef]
- Da Silva, D.V.T.; Conte, C.A.; Paschoalin, V.M.F.; Alvares, T.D.S. Hormonal response to L-arginine supplementation in physically active individuals. Food Nutr. Res. 2014, 58, 1–6. [Google Scholar]
- Alvares, T.S.; Meirelles, C.M.; Bhambhani, Y.N.; Paschoalin, V.M.F.; Gomes, P.S.C. L-Arginine as a potential ergogenic aid in healthy subjects. Sports Med. 2011, 41, 233–248. [Google Scholar] [CrossRef]
- Bonilla Ocampo, D.A.; Paipilla, A.F.; Marin, E.; Vargas-Molina, S.; Petro, J.L.; Perez-Idarraga, A. Dietary Nitrate from Beetroot Juice for Hypertension: A Systematic Review. Biomolecules 2018, 8, 134. [Google Scholar] [CrossRef]
- Bode-Boger, S.M.; Boger, R.H.; Galland, A.; Tsikas, D.; Frolich, J.C. L-arginine-induced vasodilation in healthy humans: Pharmacokinetic-pharmacodynamic relationship. Br. J. Clin. Pharmacol. 1998, 46, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Jeukendrup, A.E.; Jones, A.M.; Mooses, M. Contemporary Nutrition Strategies to Optimize Performance in Distance Runners and Race Walkers. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Maté-Muñoz, J.L.; Cuenca, E.; García-Fernández, P.; Mata-Ordoñez, F.; Lozano-Estevan, M.C.; Veiga-Herreros, P.; da Silva, S.F.; Garnacho-Castaño, M.V. Effects of beetroot juice supplementation on intermittent high-intensity exercise efforts. J. Int. Soc. Sports Nutr. 2018, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Ferguson, S.K.; Bailey, S.J.; Vanhatalo, A.; Poole, D.C. Fiber Type-Specific Effects of Dietary Nitrate. Exerc. Sport Sci. Rev. 2016, 44, 53–60. [Google Scholar] [CrossRef]
- Vandewalle, H.; Pérès, G.; Monod, H. Standard anaerobic exercise tests. Sports Med. 1987, 4, 268–289. [Google Scholar] [CrossRef]
- Driss, T.; Vandewalle, H. The measurement of maximal (anaerobic) power output on a cycle ergometer: A critical review. Biomed Res. Int. 2013, 2013, 589361. [Google Scholar] [CrossRef]
- Meckel, Y.; Machnai, O.; Eliakim, A. Relationship among repeated sprint tests, aerobic fitness, and anaerobic fitness in elite adolescent soccer players. J. Strength Cond. Res. 2009, 23, 163–169. [Google Scholar] [CrossRef]
- San-Millan, I.; Brooks, G.A. Assessment of Metabolic Flexibility by Means of Measuring Blood Lactate, Fat, and Carbohydrate Oxidation Responses to Exercise in Professional Endurance Athletes and Less-Fit Individuals. Sports Med. 2018, 48, 467–479. [Google Scholar] [CrossRef]
- Moran, C.N.; Pitsiladis, Y.P. Tour de France Champions born or made: Where do we take the genetics of performance? J. Sports Sci. 2017, 35, 1411–1419. [Google Scholar] [CrossRef]
- Bescos, R.; Sureda, A.; Tur, J.A.; Pons, A. The effect of nitric-oxide-related supplements on human performance. Sports Med. 2012, 42, 99–117. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed]

 indicate low risk of bias;
indicate low risk of bias;  indicate unknown risk of bias;
indicate unknown risk of bias;  indicate high risk of bias.
indicate high risk of bias.
 indicate low risk of bias;
indicate low risk of bias;  indicate unknown risk of bias;
indicate unknown risk of bias;  indicate high risk of bias.
indicate high risk of bias.| Random Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding of Participants and Personnel (Performance Bias) | Blinding of Outcome Assessment (Detection Bias) | Incomplete Outcome Data (Attrition Bias) | Selective Reporting (Reporting Bias) | Other Bias | |
|---|---|---|---|---|---|---|---|
| Abel et al., 2005 |  |  |  |  |  |  |  |
| Alvares et al., 2012 |  |  |  |  |  |  |  |
| Alvares et al., 2014 |  |  |  |  |  |  |  |
| Bailey et al., 2015 |  |  |  |  |  |  |  |
| Birol et al., 2019 |  |  |  |  |  |  |  |
| Camic et al., 2010 |  |  |  |  |  |  |  |
| Campbell et al., 2006 |  |  |  |  |  |  |  |
| Da silva et al., 2014 |  |  |  |  |  |  |  |
| Forbes et al., 2013 |  |  |  |  |  |  |  |
| Greer & Jones, 2011 |  |  |  |  |  |  |  |
| Hurst et al., 2014 |  |  |  |  |  |  |  |
| Liu et al., 2009 |  |  |  |  |  |  |  |
| Meirelles & Matsuura, 2018 |  |  |  |  |  |  |  |
| Mor et al., 2018 |  |  |  |  |  |  |  |
| Olek et al., 2010 |  |  |  |  |  |  |  |
| Pahlavani et al., 2017 |  |  |  |  |  |  |  |
| Vanhatalo et al., 2013 |  |  |  |  |  |  |  |
| Yavuz et al., 2014 |  |  |  |  |  |  |  |
| General Characteristic | Intervention Characteristic | Studies Included | |
|---|---|---|---|
| Study Design | Randomized, double-blind and placebo-controlled | 8 studies [9,11,12,20,31,59,60,61] | |
| Randomized, double-blind, cross-over and placebo-controlled | 7 studies [10,25,39,40,57,58,62] | ||
| Randomized and placebo-controlled | 3 studies [32,41,63] | ||
| Conflict of Interests | None | 18 studies [9,10,11,12,20,25,31,32,39,40,41,57,58,59,60,61,62,63] | |
| Subjects Characteristics | Endurance-trained | 6 studies [11,25,31,40,59,61] | |
| Fight sports athletes | Judo—1 study [58] | ||
| Wrestlers—1 study [10] | |||
| Soccer athletes | 3 studies [9,41,60] | ||
| Resistance-trained | 1 study [20] | ||
| Active | 6 studies [12,32,39,57,62,63] | ||
| Type of Arginine Supplement | L-Arginine | 15 studies [9,10,11,12,25,31,32,40,41,57,58,59,60,62,63] | |
| Arginine Aspartate | 1 study [61] | ||
| Arginine Alpha-Ketoglutarate | 2 studies [20,39] | ||
| Type of Arginine Administration | Absolute | 14 studies [9,12,20,31,32,39,40,41,57,58,59,61,62,63] | |
| Based on individual’s body mass | 3 studies [3,10,13] | ||
| Both | 1 study [4] | ||
| Dose Used | 12 g/day | 1 study [20] | |
| 6 g/day | 8 studies [31,32,40,41,58,59,62,63] | ||
| 3.7 g/day | 1 study [39] | ||
| 2 g/day | 2 studies [9,57] | ||
| 5.7 d/day (group 1) and 2.85 g/day (group 2) | 1 study [61] | ||
| 1.5 d/day (group 1) and 3 g/day (group 2) | 1 study [12] | ||
| 0.075 g·kg−1 body mass | 1 study [25] | ||
| 0.15 g·kg−1 body mass | 2 studies [10,60] | ||
| 6 g/day (group 1) and 0.15 g·kg−1 body mass (group 2) | 1 study [11] | ||
| Time of Ingestion | Acute | 60 min before test | 5 studies [10,25,57,58,60] |
| 90 min before test | 2 studies [11,62] | ||
| 80 min before test | 1 study [59] | ||
| 4 h + 30 min before test | 1 study [39] | ||
| 60 min + 30 min before test | 1 study [40] | ||
| 30 min before test | 1 study [63] | ||
| Chronic | 56 days or 8 weeks | 1 study [20] | |
| 45 days | 1 study [9] | ||
| 28 days or 4 weeks | 3 studies [12,31,61] | ||
| 14 days | 1 study [41] | ||
| 7 days | 1 study [32] | ||
| Author/s | Population | Intervention | Test | Outcomes | Main Conclusion |
|---|---|---|---|---|---|
| Alvares, T.S. et al., 2012 | 15 healthy male volunteers with previous resistance training experience. Arg group 26.3 ± 4.9 years vs. PLA 24.7 ± 1.8 years. | Randomized, double-blind, placebo-controlled. 6 g/ of L-Arg (80 min before test). | Dominant elbow flexion and extension exercise with an isokinetic dynamometer. 3 sets of 10 maximal voluntary contractions. | • Peak Torque | ↔ |
| • Total Work | ↔ | ||||
| Bailey S.J., et al., 2015 | 10 healthy, recreationally active men (19 ± 1 years). | Randomized, double-blind, placebo-controlled. 6 g/d of L-Arg (7 days). | Day 6: 1 min all out cycle sprint. | • Peak Power | ↔ |
| • Total Work | ↔ | ||||
| Birol A. et al., 2019 | 20 volunteer healthy male football players (18.30 ± 0.48 years). | Randomized, double-blind, placebo-controlled. L-Arg 0.15 g/kg/day (60 min before test). | RSAT: 12 × 20 m with 30 s rest. | • Total sprint time | ↔ |
| Campbell B., et al., 2006 | 35 resistance-trained adult men (39.8 ± 5.8 years). | Randomized, double-blind, placebo-controlled. 12 g/d (4 g × 3) Arg Alpha-Ketoglutarate (8 weeks). | Upper body flat bench 1RM.Wingate test Isokinetic leg extension 50 rep. | • Upper body 1RM | ↑ |
| • Peak power | ↑ | ||||
| • Time to Peak power | ↑ | ||||
| • Rate to Fatigue | ↑ | ||||
| • Isokinetic leg extension | ↔ | ||||
| Greer B.K. et al., 2011. | 12 trained college-aged men (22.6 ± 3.9 years). | Randomized, double-blind, placebo-controlled, cross-over. 3.7 g Arg Alpha-Ketoglutarate (4 h + 30 min pre-test). | 3 sets of chin-ups, reverse chin-ups and push-ups to exhaustion. | • Chin-ups | ↑ |
| • Reverse Chin-ups | ↔ | ||||
| • Push-ups | ↔ | ||||
| Hurst H.T., et al., 2014. | 8 healthy, trained male cyclists (21.00 ± 1.41 years). | Randomized, double-blind, placebo-controlled. Group 1: 6 g L-Arg, Group 2: 0.15 g·kg−1 body mass (90 min before test). | 1 km TT in cycloergometer. | • Time to complete Group 1 | ↔ |
| • Power output Group 1 | ↔ | ||||
| • Time to complete Group 2 | ↔ | ||||
| • Power output Group 2 | ↔ | ||||
| Liu T.H., et al., 2009. | 10 elite male college judo athletes (20.2 ± 0.6 years) | Randomized, cross-over, placebo-controlled. 6 g/d L-Arg (2 days, 60 min before test). | 13 × All out test: 20 s with 15 s rest. Cycloergometer. | • Total power | ↔ |
| Meirelles C.M., et al., 2018 | 12 healthy university students, resistance trained males. (27 ± 3 years). | Randomized, double-blind, cross-over, placebo-controlled. 6 g L-Arg (3 g 60 min before test + 3 g 30 min before test). | Bench press in a Smith Machine and unilateral knee extension of the right leg. | • Bench press repetitions | ↔ |
| • Knee extension repetitions | ↔ | ||||
| Mor A., et al., 2018 | 28 amateur male soccer players (18–30 years). | Randomized, placebo-controlled. 6 g/d L-Arg (14 days). | Running Anaerobic Sprint Test (RAST): 6 × 32 m with 10 s rest. | • Mean power | ↔ |
| Olek R.A., et al., 2010. | 6 healthy, active, but not highly trained volunteers (23.2 ± 0.5 year). | Randomized, double-blind, cross-over, placebo-controlled. 2 g L-Arg (60 min before test). | 3 × All out 30 s Wingate Tet in Cycloergometer with 4 min rest. | • Power output | ↔ |
| Author/s | Population | Intervention | Test | Outcomes | Main Conclusion |
|---|---|---|---|---|---|
| Abel, T. et al., 2005 | 30 male endurance-trained athletes (Group 1: 38.5 ± 10 years; Group 2: 34.4 ± 8.6 years) | Randomized, double-blind, placebo-controlled. Group 1: 5.7 g/d Arg-Aspartate; Group 2: 2.85 g/d Arg-Aspartate. (4 weeks). | Incremental cycloergometer test | Time to exhaustion Group1 | ↔ |
| Time to exhaustion Group 2 | ↔ | ||||
| Alvares, T.S. et al., 2014 | 15 healthy experienced runners (11 males and 4 females) (36.8 ± 7.1 years). | Randomized, double-blind, placebo-controlled. 6 g/d of encapsulated L-Arg hydrochloride (4 weeks). | 2 × 5 Km TT running with 10 min recovery | Total running time. | ↔ |
| Bailey S.J., et al., 2015 | 10 healthy, recreationally active men (19 ± 1 years). | Randomized, double-blind, placebo-controlled. 6 g/d of L-Arg (7 days). | Day 7: Time to exhaustion test in cycloergometer | Time to exhaustion. | ↔ |
| Camic, C.L. et al., 2010 | 50 college-aged men (23.9 ± 3.0 years). | Randomized, double-blind, placebo-controlled.3 groups: (a) placebo (n = 19); (b) 1.5 g/d Arg (n = 14); or (c) 3.0 g/d Arg (n = 17) (4 weeks). | Incremental test to exhaustion in cycloergometer | PWCFT Group 1 | ↑ |
| PWCFT Group 2 | ↑ | ||||
| Campbell B., et al., 2006 | 35 resistance-trained adult men (39.8 ± 5.8 years). | Randomized, double-blind, placebo-controlled. 12 g/d (4 g × 3) Arg Alpa-Ketoglutarate (8 weeks). | Bruce protocol: Incremental test running | Time to exhaustion | ↔ |
| Da Silva D.V., 2014 | 15 physically active and healthy volunteers (11 males and 4 females). Arg group: 36.8 ± 7.1 years; PLA: 30.6 ± 9.5 years). | Randomized, placebo-controlled. 6 g/d of L-Arg (30 min before test). | 2 × 5 km TT running with 10 min recovery | Total running time. | ↔ |
| Forbes S.C., et al., 2013 | 15 aerobically trained men (age: 28 ± 5 years) | Randomized, double-blind, placebo-controlled, cross-over. L-Arg 0.075 g·kg−1 body mass (60 min before test). | 60 min at 80% of VT incycloergometer | Average power output at ventilatory threshold. | ↔ |
| Hurst H.T., et al., 2014 | 8 healthy, trained male cyclists (21.00 ± 1.41 years). | Randomized, double-blind, placebo-controlled. Group 1: 6 g L-Arg, Group 2: 0.15 g·kg−1 body mass (90 min before test). | 16.1 km TT in cycloergometer | Time to complete Group 1 | ↔ |
| Power output Group 1 | ↔ | ||||
| Time to complete Group 2 | ↔ | ||||
| Power output Group 2 | ↑ | ||||
| Pahlavani N., et al., 2017 | 56 male soccer players (20.85 ± 4.29 years). | Randomized, double-blind, placebo-controlled. 2 g/d L-Arg (45 days). | Harvard Step Test | Performance Score | ↑ |
| Vanhatalo A., et al., 2013 | 18 healthy, recreationally active male students (22 ± 3 year). | Randomized, double-blind, cross-over placebo-controlled. 6 g/d of L-Arg (90 min before test). | 2 × 6 min running moderate test + 1 × running test until exhaustion | Time to exhaustion | ↔ |
| Yavuz, H.U., et al., 2014 | 9 volunteer elite male wrestlers (24.7 ± 3.8 years) | Randomized, placebo-controlled, cross-over. L-Arg 0.15 g·kg−1 body mass (60 min before test). | Incremental test to exhaustion in cycloergometer | Time to exhaustion | ↑ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viribay, A.; Burgos, J.; Fernández-Landa, J.; Seco-Calvo, J.; Mielgo-Ayuso, J. Effects of Arginine Supplementation on Athletic Performance Based on Energy Metabolism: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1300. https://doi.org/10.3390/nu12051300
Viribay A, Burgos J, Fernández-Landa J, Seco-Calvo J, Mielgo-Ayuso J. Effects of Arginine Supplementation on Athletic Performance Based on Energy Metabolism: A Systematic Review and Meta-Analysis. Nutrients. 2020; 12(5):1300. https://doi.org/10.3390/nu12051300
Chicago/Turabian StyleViribay, Aitor, José Burgos, Julen Fernández-Landa, Jesús Seco-Calvo, and Juan Mielgo-Ayuso. 2020. "Effects of Arginine Supplementation on Athletic Performance Based on Energy Metabolism: A Systematic Review and Meta-Analysis" Nutrients 12, no. 5: 1300. https://doi.org/10.3390/nu12051300
APA StyleViribay, A., Burgos, J., Fernández-Landa, J., Seco-Calvo, J., & Mielgo-Ayuso, J. (2020). Effects of Arginine Supplementation on Athletic Performance Based on Energy Metabolism: A Systematic Review and Meta-Analysis. Nutrients, 12(5), 1300. https://doi.org/10.3390/nu12051300







