Leucine Supplementation: A Novel Strategy for Modulating Lipid Metabolism and Energy Homeostasis
Abstract
1. Introduction
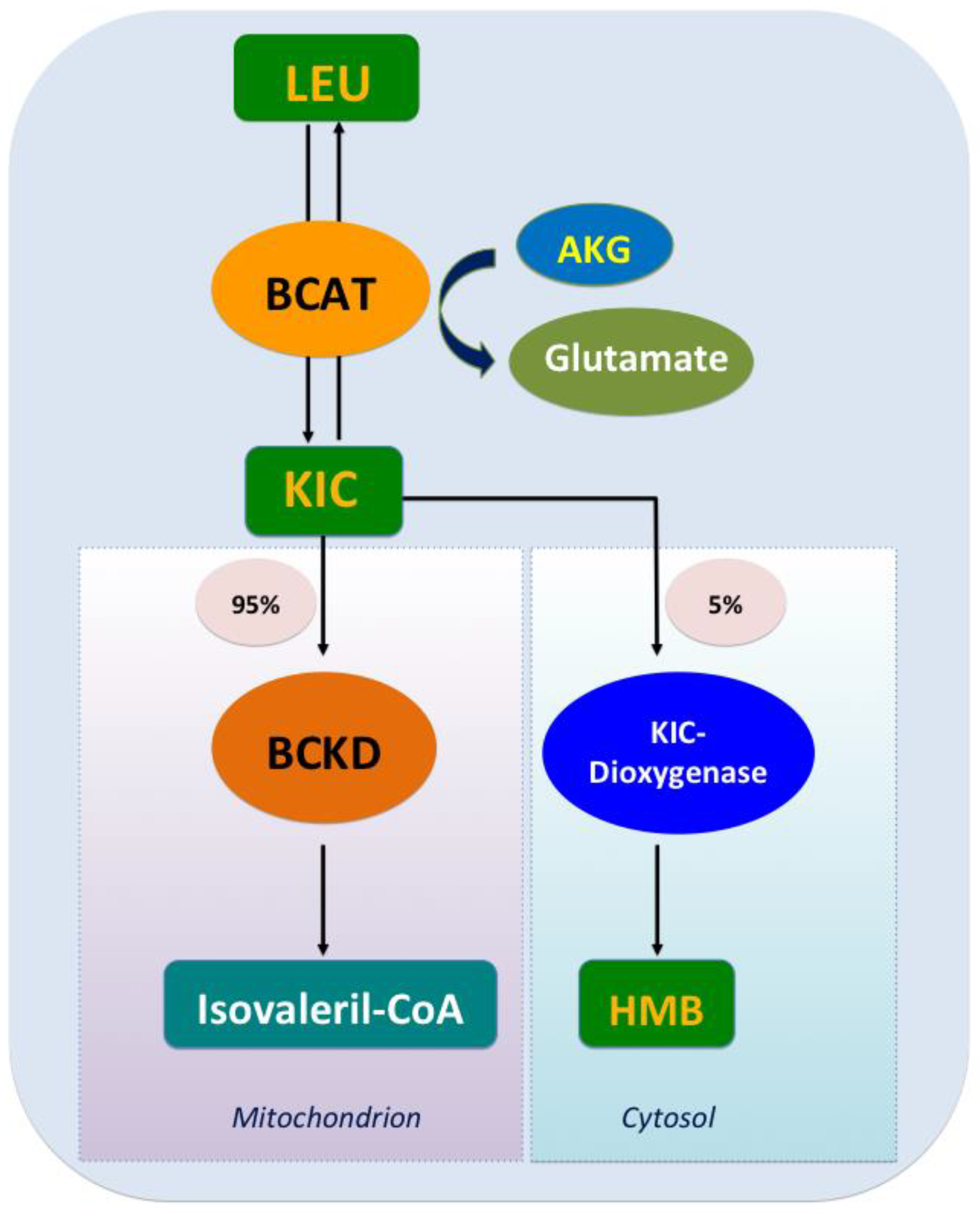
2. Leu Metabolism
2.1. The Decomposing Process of Leu Oxidation
2.2. Metabolites
2.2.1. KIC
2.2.2. HMB
3. Leu and Lipid Metabolism in Adipose Tissue
3.1. Leu and Fatty Acid Oxidation in Adipose Tissue
3.2. Leu Promotes Browning
3.3. Leu Modulates Lipid Metabolism Via Mitochondria
4. Leu and Lipid Metabolism in Skeletal Muscle
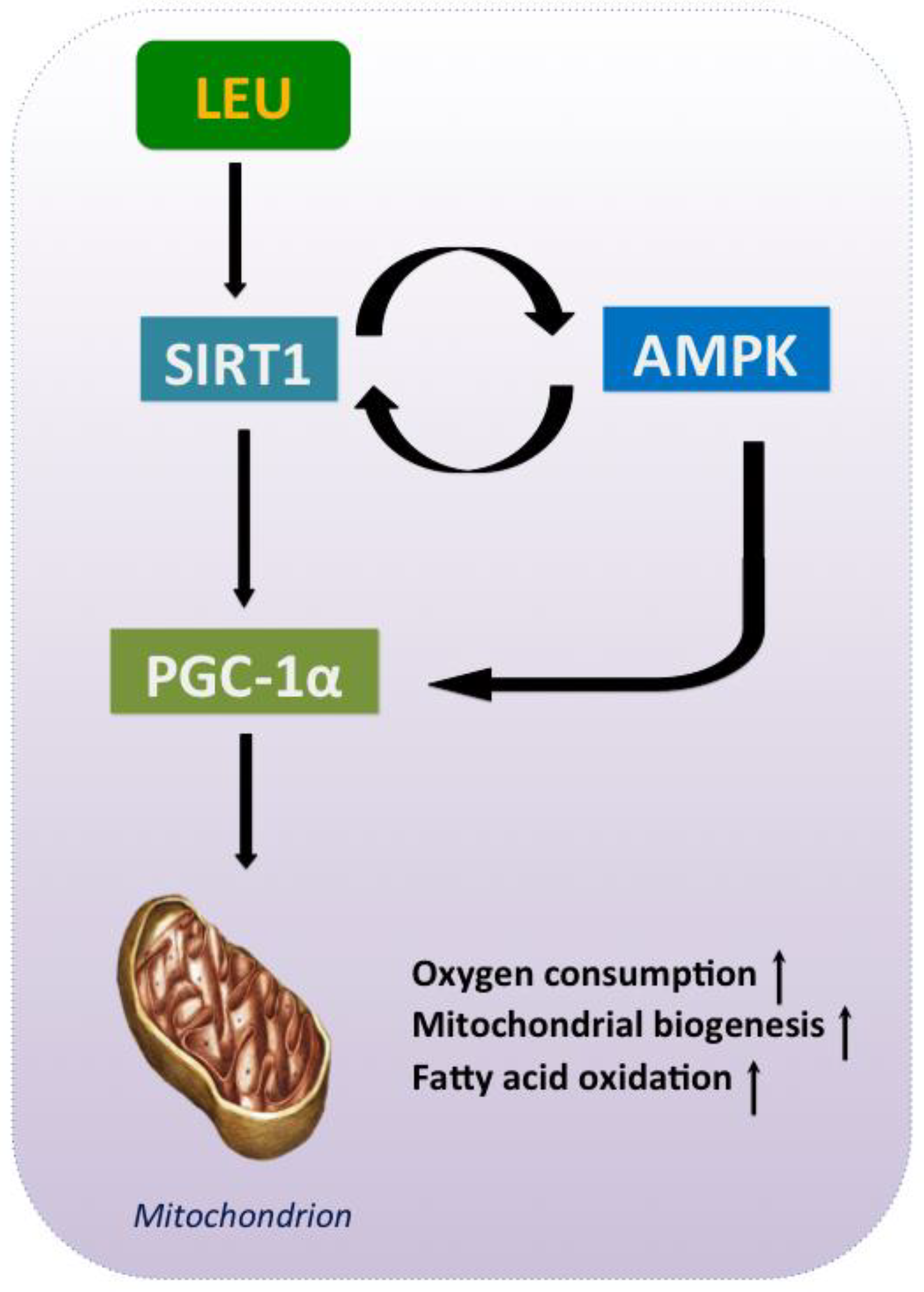
5. Leu and the Energy Axis, AMPK/SIRT1/PGC-1α
6. Leu and Adipokines/Myokines
7. Cross-Talk Between Leu and Intestinal Lipid Metabolism
7.1. Dietary Leu and Intestinal Metabolism
7.2. Leu and Microbiota
8. Application in Livestock Production
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACADVL | acylcoenzyme A dehydrogenase very long-chain |
| AMP | adenosine monophosphate |
| AMPK | (AMP)-activated protein kinase |
| ATP | adenosine triphosphate |
| BAT | brown adipose tissue |
| BCAA | branched chain amino acid |
| BCAT | BCAA transferase |
| CAS | Chinese Academy of Sciences |
| CoA | coenzyme A |
| CPT | carnitine O-palmitoyltransferase |
| BCKD | branched chain a-keto acid dehydrogenase |
| BDK | branched chain a-keto acid dehydrogenase kinase |
| FABP1 | fatty acid-binding protein liver |
| FAO | fatty acid oxidation |
| GATOR2 | GAP activity toward Rags 2 |
| GLUT-4 | glucose transporter-4 |
| HADHA | tifunctional enzyme subunit alpha |
| HFD | high fat diet |
| HMB | β-hydroxy-β-methylbutyrate |
| IL | interleukin |
| ISA | Institute of Subtropical Agriculture |
| Leu | leucine |
| LKB1 | liver kinase B1 |
| KIC | α-ketoisocaproate |
| mRNA | messenger ribonucleicacid |
| mTOR | mammalian target of rapamycin |
| mTORC1 | mammalian target of rapamycin complex 1 |
| NAD | nicotinamide adenine dinucleotide |
| p-AMPK | phosphorylated AMPK |
| PGC-1α | peroxisome proliferator-activated receptor γ coactivator-1α |
| PPARγ | peroxisome proliferator-activated receptor γ |
| PPM1K | protein phosphatase 1K |
| SCFA | short-chain fatty acid |
| SIRT1 | silent information regulator of transcription 1 |
| STS | Science and Technology Service |
| S6K1 | protein S6 kinase 1 |
| TNFα | tumor necrosis factor alpha |
| UCP1 | uncoupling protein 1 |
| WAT | white adipose tissue |
| 4EBP1 | translational inhibitor 4E-binding protein-1 |
References
- Block, R.J. Some Amino Acids, Peptides and Amines in Milk, Concentrated Milks and Cheese. J. Dairy Sci. 1951, 34, 1–10. [Google Scholar] [CrossRef]
- Gruhn, K. Effect of the set of amino acids contained in casein, extracted soy bean meal, horse beans and wheat gluten on the content of leucine, isoleucine, valine, phenylalanine, tyrosine, glutamic acid, aspartic acid, alanine, glycine and proline in the deproteinized blood plasma of laying hens. Arch Tierernahr. 1973, 23, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Madeira, M.S.; Alfaia, C.M.; Costa, P.; Lopes, P.A.; Lemos, J.P.C.; Bessa, R.J.B.; Prates, J.A.M. The combination of arginine and leucine supplementation of reduced crude protein diets for boars increases eating quality of pork. J. Anim. Sci. 2014, 92, 2030–2040. [Google Scholar] [CrossRef] [PubMed]
- Salles, J.; Chanet, A.; Berry, A.; Giraudet, C.; Patrac, V.; Domingues-Faria, C.; Rocher, C.; Guillet, C.; Denis, P.; Pouyet, C.; et al. Fast digestive, leucine-rich, soluble milk proteins improve muscle protein anabolism, and mitochondrial function in undernourished old rats. Mol. Nutr. Food Res. 2017, 61, 1700287. [Google Scholar] [CrossRef] [PubMed]
- Kamruzzaman, M.; Torita, A.; Sako, Y.; Al-Mamun, M.; Sano, H. Effects of feeding garlic stem and leaf silage on rates of plasma leucine turnover, whole body protein synthesis and degradation in sheep. Small Rumin. Res. 2011, 99, 37–43. [Google Scholar] [CrossRef]
- Miralles-Arnau, S.; Nacher, A.; Jimenez, A.; Jimenez-Torres, N.V.; Merino-Sanjuan, M. Impact of nutritional status on the oral bioavailability of leucine administered to rats as part of a standard enteral diet. Clin. Nutr. 2011, 30, 517–523. [Google Scholar] [CrossRef]
- Anthony, J.C.; Yoshizawa, F.; Anthony, T.G.; Vary, T.C.; Jefferson, L.S.; Kimball, S.R. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J. Nutr. 2000, 130, 2413–2419. [Google Scholar] [CrossRef]
- Atherton, P.J.; Smith, K.; Etheridge, T.; Rankin, D.; Rennie, M.J. Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids 2010, 38, 1533–1539. [Google Scholar] [CrossRef]
- Schroeder, G.F.; Titgemeyer, E.C.; Mooret, E.S. Effects of energy supply on leucine utilization by growing steers at two body weights. J. Anim. Sci. 2007, 85, 3348–3354. [Google Scholar] [CrossRef]
- Liu, T.; Zuo, B.; Wang, W.; Wang, S.L.; Wang, J.J. Dietary Supplementation of Leucine in Premating Diet Improves the Within-Litter Birth Weight Uniformity, Antioxidative Capability, and Immune Function of Primiparous SD Rats. Biomed. Res. Int. 2018. [Google Scholar] [CrossRef]
- Han, G.F.; Yang, H.; Bungo, T.; Ikeda, H.; Wang, Y.H.; Nguyen, L.T.N.; Eltahan, H.M.; Furuse, M.; Chowdhury, V.S. In ovo L-leucine administration stimulates lipid metabolisms in heat-exposed male, but not female, chicks to afford thermotolerance. J. Therm. Biol. 2018, 71, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Harper, A.E.; Benevenga, N.J.; Wohlhueter, R.M. Effects of Ingestion of Disproportionate Amounts of Amino Acids. Physiol. Rev. 1970, 50, 428–558. [Google Scholar] [CrossRef]
- Swain, L.M.; Shiota, T.; Walser, M. Utilization for Protein-Synthesis of Leucine and Valine Compared with Their Keto Analogs. Am. J. Clin. Nutr. 1990, 51, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, J.; Bruckbauer, A.; Zemel, M.B. Activation of the AMPK/Sirt1 pathway by a leucine-metformin combination increases insulin sensitivity in skeletal muscle, and stimulates glucose and lipid metabolism and increases life span in Caenorhabditis elegans. Metabolism 2016, 65, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Reimer, R.A. Dairy protein and leucine alter GLP-1 release and mRNA of genes involved in intestinal lipid metabolism in vitro. Nutrition 2009, 25, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Li, F.N.; Yin, Y.L.; Tan, B.; Kong, X.F.; Wu, G.Y. Leucine nutrition in animals and humans: mTOR signaling and beyond. Amino Acids 2011, 41, 1185–1193. [Google Scholar] [CrossRef]
- Liu, R.; Li, H.; Fan, W.J.; Jin, Q.; Chao, T.T.; Wu, Y.J.; Huang, J.M.; Hao, L.P.; Yang, X.F. Leucine Supplementation Differently Modulates Branched-Chain Amino Acid Catabolism, Mitochondrial Function and Metabolic Profiles at the Different Stage of Insulin Resistance in Rats on High-Fat Diet. Nutrients 2017, 9, 565. [Google Scholar]
- Wilkinson, D.J.; Hossain, T.; Hill, D.S.; Phillips, B.E.; Crossland, H.; Williams, J.; Loughna, P.; Churchward-Venne, T.A.; Breen, L.; Phillips, S.M.; et al. Effects of leucine and its metabolite -hydroxy--methylbutyrate on human skeletal muscle protein metabolism. J. Physiol. London 2013, 591, 2911–2923. [Google Scholar] [CrossRef]
- Karaivanova, V.K.; Ivanov, S.X.; Chelibonova-Lorer, H. Pattern of sialoglycoproteins obtained by chromatofocusing of chicken liver and hepatoma Mc-29 microsomal preparations labelled in vivo with 3H-leucine and N-acetyl-14C-mannosamine. Cancer Biochem. Biophys. 1992, 12, 275–282. [Google Scholar]
- Chaves, D.F.; Zanchii, N.E.; Nicastro, H.; Lancha, A.H. Effects Of Leucine Supplementation In The Protein Synthesis Signalling Pathways Of Soleus And Edl Muscle In Young And Old Rats. Med. Sci. Sport Exer. 2011, 43, 135. [Google Scholar]
- Xu, G.; Kwon, G.; Cruz, W.S.; Marshall, C.A.; McDaniel, M.L. Metabolic regulation by leucine of translation initiation through the mTOR-signaling pathway by pancreatic beta-cells. Diabetes 2001, 50, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Bruckbauer, A.; Zemel, M.B. Effects of dairy consumption on SIRT1 and mitochondrial biogenesis in adipocytes and muscle cells. Nutr. Metab. 2011, 8, 91. [Google Scholar] [CrossRef]
- Zhou, Y.S.; Jetton, T.; She, P.X. Essential Role of Transamination for alpha-Ketoisocaproate (KIC) but Not Leucine (Leu) To Stimulate Insulin Secretion. Diabetes 2009, 58, A417. [Google Scholar]
- Zhong, Y.; Song, B.; Zheng, C.; Li, F.; Kong, X.; Duan, Y.; Deng, J. alpha-Ketoisocaproate and beta-hydroxy-beta-methyl butyrate regulate fatty acid composition and lipid metabolism in skeletal muscle of growing pigs. J. Anim. Physiol. Anim. Nutr. 2019, 103, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.E.; Rentsch, K.; Furst, P.; Cefalu, W.T.; Matthews, D.E. Splanchnic bed utilization of [alpha]-ketoisocaproate (KIC) as a dietary supplement given to humans to spare leucine (Leu). Faseb J. 2002, 16, A746. [Google Scholar]
- Duan, Y.H.; Zhang, L.Y.; Li, F.N.; Guo, Q.P.; Long, C.M.; Yin, Y.L.; Kong, X.F.; Peng, M.J.; Wang, W.C. beta-Hydroxy--methylbutyrate modulates lipid metabolism in adipose tissues of growing pigs. Food Funct. 2018, 9, 4836–4846. [Google Scholar] [CrossRef] [PubMed]
- Durkalec-Michalski, K.; Jeszka, J. The efficacy of a beta-hydroxy-beta-methylbutyrate supplementation on physical capacity, body composition and biochemical markers in elite rowers: A randomised, double-blind, placebo-controlled crossover study. J. Int. Soc. Sports Nutr. 2015, 12, 31. [Google Scholar] [CrossRef]
- Panton, L.B.; Rathmacher, J.A.; Baier, S.; Nissen, S. Nutritional supplementation of the leucine metabolite beta-hydroxy-beta-methylbutyrate (hmb) during resistance training. Nutrition 2000, 16, 734–739. [Google Scholar] [CrossRef]
- Durkalec-Michalski, K.; Jeszka, J.; Podgorski, T. The Effect of a 12-Week Beta-hydroxy-beta-methylbutyrate (HMB) Supplementation on Highly-Trained Combat Sports Athletes: A Randomised, Double-Blind, Placebo-Controlled Crossover Study. Nutrients 2017, 9, 753. [Google Scholar] [CrossRef]
- Bruckbauer, A.; Zemel, M.B.; Thorpe, T.; Akula, M.R.; Stuckey, A.C.; Osborne, D.; Martin, E.B.; Kennel, S.; Wall, J.S. Synergistic effects of leucine and resveratrol on insulin sensitivity and fat metabolism in adipocytes and mice. Nutr. Metab. 2012, 9, 77. [Google Scholar] [CrossRef]
- Bruckbauer, A.; Baggett, B.; Zemel, M.B. Synergistic effects of polyphenols with leucine and beta-hydroxy- beta-methylbutyrate (HMB) on energy metabolism. FASEB J. 2013, 27. [Google Scholar] [CrossRef]
- Bruckbauer, A.; Zemel, M.B. Synergistic Effects of Polyphenols and Methylxanthines with Leucine on AMPK/Sirtuin-Mediated Metabolism in Muscle Cells and Adipocytes. PLoS ONE 2014, 9, e89166. [Google Scholar] [CrossRef]
- Duan, Y.H.H.; Zeng, L.M.M.; Li, F.N.N.; Kong, X.F.F.; Xu, K.; Guo, Q.P.P.; Wang, W.L.L.; Zhang, L.Y.Y. beta-hydroxy—methyl butyrate promotes leucine metabolism and improves muscle fibre composition in growing pigs. J. Anim. Physiol. N. 2018, 102, 1328–1339. [Google Scholar] [CrossRef]
- Duan, Y.H.; Li, F.N.; Guo, Q.P.; Wang, W.L.; Zhang, L.Y.; Wen, C.Y.; Yin, Y.L. Branched-chain amino acid ratios modulate lipid metabolism in adipose tissues of growing pigs. J. Funct. Foods 2018, 40, 614–624. [Google Scholar] [CrossRef]
- Jelenik, T.; Roden, M. Mitochondrial Plasticity in Obesity and Diabetes Mellitus. Antioxid Redox Sign 2013, 19, 258–268. [Google Scholar] [CrossRef]
- Zhong, Y.Z.; Zeng, L.M.; Deng, J.P.; Duan, Y.H.; Li, F.N. beta-hydroxy-beta-methylbutyrate (HMB) improves mitochondrial function in myocytes through pathways involving PPAR beta/delta and CDK4. Nutrition 2019, 60, 217–226. [Google Scholar] [CrossRef]
- Duan, Y.H.; Li, F.N.; Song, B.; Zheng, C.B.; Zhong, Y.Z.; Xu, K.; Kong, X.F.; Yin, Y.L.; Wang, W.; Shu, G. beta-hydroxy-beta-methyl butyrate, but not alpha-ketoisocaproate and excess leucine, stimulates skeletal muscle protein metabolism in growing pigs fed low-protein diets. J. Funct. Foods 2019, 52, 34–42. [Google Scholar] [CrossRef]
- Liang, H.L.; Mokrani, A.; Chisomo-Kasiya, H.; Ji, K.; Ge, X.P.; Ren, M.C.; Liu, B.; Xi, B.W.; Sun, A. Dietary leucine affects glucose metabolism and lipogenesis involved in TOR/PI3K/Akt signaling pathway for juvenile blunt snout bream Megalobrama amblycephala. Fish Physiol. Biochem. 2019, 45, 719–732. [Google Scholar] [CrossRef]
- Koh, P.L.; Ho, J.P.; Pang, C.; Tan, H.C.; Kovalik, J.P. The role of leucine in stimulation of adipocyte lipolysis. Diabetes Res. Clin. Pr. 2016, 120, S181–S182. [Google Scholar] [CrossRef]
- Yuan, X.W.; Han, S.F.; Zhang, J.W.; Xu, J.Y.; Qin, L.Q. Leucine supplementation improves leptin sensitivity in high-fat diet fed rats. Food Nutr. Res. 2015, 59, 27373. [Google Scholar] [CrossRef]
- Manders, R.J.F.; Praet, S.F.E.; Meex, R.C.R.; Koopman, R.; de Roos, A.L.; Wagenmakers, A.J.M.; Saris, W.H.M.; van Loon, L.J.C. Protein hydrolysate/leucine co-ingestion reduces the prevalence of hyperglycemia in type 2 diabetic patients. Diabetes Care 2006, 29, 2721–2722. [Google Scholar] [CrossRef]
- Arazi, H.; Taati, B.; Suzuki, K. A Review of the Effects of Leucine Metabolite (beta-Hydroxy-beta-methylbutyrate) Supplementation and Resistance Training on Inflammatory Markers: A New Approach to Oxidative Stress and Cardiovascular Risk Factors. Antioxidants 2018, 7, 148. [Google Scholar] [CrossRef]
- Nissen, S.; Sharp, R.L.; Panton, L.; Vukovich, M.; Trappe, S.; Fuller, J.C. beta-hydroxy-beta-methylbutyrate (HMB) supplementation in humans is safe and may decrease cardiovascular risk factors. J. Nutr. 2000, 130, 1937–1945. [Google Scholar] [CrossRef]
- Jiao, J.; Han, S.F.; Zhang, W.; Xu, J.Y.; Tong, X.; Yin, X.B.; Yuan, L.X.; Qin, L.Q. Chronic leucine supplementation improves lipid metabolism in C57BL/6J mice fed with a high-fat/cholesterol diet. Food Nutr. Res. 2016, 60, 31304. [Google Scholar] [CrossRef]
- Lynch, C.; Fujii, H.; Halle, B.; Vary, T.; Wallin, R.; Hutson, S. Leucine regulation of adipose tissue branched chain keto acid dehydrogenase complex and the mTOR signaling pathway in vivo. Diabetes 2003, 52, A316. [Google Scholar]
- Yan, G.K.; Li, X.Z.; Peng, Y.; Long, B.S.; Fan, Q.W.; Wang, Z.C.; Shi, M.; Xie, C.L.; Zhao, L.; Yan, X.H. The Fatty Acid beta-Oxidation Pathway is Activated by Leucine Deprivation in HepG2 Cells: A Comparative Proteomics Study. Sci. Rep. 2017, 7, 1–11. [Google Scholar]
- Bartelt, A.; Heeren, J. Adipose tissue browning and metabolic health. Nat. Rev. Endocrinol. 2014, 10, 24–36. [Google Scholar] [CrossRef]
- Fernandez-Veledo, S.; Vazquez-Carballo, A.; Vila-Bedmar, R.; Ceperuelo-Mallafre, V.; Vendrell, J. Role of energy- and nutrient-sensing kinases AMP-activated Protein Kinase (AMPK) and Mammalian Target of Rapamycin (mTOR) in Adipocyte Differentiation. Iubmb Life 2013, 65, 572–583. [Google Scholar] [CrossRef]
- Garcia-Guerra, L.; Nieto-Vazquez, I.; Vila-Bedmar, R.; Jurado-Pueyo, M.; Zalba, G.; Diez, J.; Murga, C.; Fernandez-Veledo, S.; Mayor, F.; Lorenzo, M. G Protein-Coupled Receptor Kinase 2 Plays a Relevant Role in Insulin Resistance and Obesity. Diabetes 2010, 59, 2407–2417. [Google Scholar] [CrossRef]
- Wada, S.; Arany, Z. Adipose tissue browning: mTOR branches out. Cell Cycle 2017, 16, 493–494. [Google Scholar] [CrossRef][Green Version]
- Moreno-Navarrete, J.M.; Fernandez-Real, J.M. The gut microbiota modulates both browning of white adipose tissue and the activity of brown adipose tissue. Rev. Endocr. Metab. Dis. 2019, 20, 387–397. [Google Scholar] [CrossRef]
- Ye, Y.Q.; Liu, H.L.; Zhang, F.; Hu, F. mTOR signaling in Brown and Beige adipocytes: Implications for thermogenesis and obesity. Nutr. Metab. 2019, 16, 74. [Google Scholar] [CrossRef]
- Yao, K.; Duan, Y.H.; Li, F.N.; Tan, B.; Hou, Y.Q.; Wu, G.Y.; Yin, Y.L. Leucine in Obesity: Therapeutic Prospects. Trends Pharmacol. Sci. 2016, 37, 714–727. [Google Scholar]
- Ortega-Senovilla, H.; de Oya, M.; Garces, C. Relationship of NEFA concentrations to RBP4 and to RBP4/retinol in prepubertal children with and without obesity. J. Clin. Lipidol. 2019, 13, 301–307. [Google Scholar] [CrossRef]
- Johnson, M.A.; Gannon, N.P.; Schnuck, J.K.; Lyon, E.S.; Sunderland, K.L.; Vaughan, R.A. Leucine, Palmitate, or Leucine/Palmitate Cotreatment Enhances Myotube Lipid Content and Oxidative Preference. Lipids 2018, 53, 1043–1057. [Google Scholar]
- Bradley, J.; Swann, K. Mitochondria and lipid metabolism in mammalian oocytes and early embryos. Int. J. Dev. Biol. 2019, 63, 93–103. [Google Scholar] [CrossRef]
- Cui, L.J.; Mirza, A.H.; Zhang, S.Y.; Liang, B.; Liu, P.S. Lipid droplets and mitochondria are anchored during brown adipocyte differentiation. Protein Cell 2019, 10, 921–926. [Google Scholar] [CrossRef]
- Freyre, C.A.C.; Rauher, P.C.; Ejsing, C.S.; Klemm, R.W. MIGA2 Links Mitochondria, the ER, and Lipid Droplets and Promotes De Novo Lipogenesis in Adipocytes. Mol. Cell 2019, 76, 811–825. [Google Scholar]
- Treberg, J.R.; Braun, K.; Selseleh, P. Mitochondria can act as energy-sensing regulators of hydrogen peroxide availability. Redox Biol. 2019, 20, 483–488. [Google Scholar]
- Wang, Y.B.; Li, C.H.; Cheng, K.; Zhang, R.; Narsinh, K.; Li, S.; Li, X.J.; Qin, X.; Zhang, R.Q.; Li, C.Y.; et al. Activation of Liver X Receptor Improves Viability of Adipose-Derived Mesenchymal Stem Cells to Attenuate Myocardial Ischemia Injury Through TLR4/NF-kappa B and Keap-1/Nrf-2 Signaling Pathways. Antioxid Redox Sign 2014, 21, 2543–2557. [Google Scholar] [CrossRef]
- Snarr, C.; Redmond, E.; Morais, J.A.; Wykes, L.J.; Chevalier, S. Leucine balance, metabolic and satiety responses to a leucine-rich meal in healthy elderly women. FASEB J. 2012, 26. [Google Scholar] [CrossRef]
- Boehmer, B.H.; Brown, L.D.; Wesolowski, S.R.; Hay, W.W.; Rozance, P.J. Leucine and Isoleucine Potentiate Glucose Stimulated Insulin Secretion in Fetal Sheep. Reprod Sci. 2018, 25, 281a. [Google Scholar]
- Su, W.P.; Xu, W.; Zhang, H.; Ying, Z.X.; Zhou, L.; Zhang, L.L.; Wang, T. Effects of dietary leucine supplementation on the hepatic mitochondrial biogenesis and energy metabolism in normal birth weight and intrauterine growth-retarded weanling piglets. Nutr. Res. Pract. 2017, 11, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, R.L.; Chantranupong, L.; Saxton, R.A.; Shen, K.; Scaria, S.M.; Cantor, J.R.; Sabatini, D.M. METABOLISM Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 2016, 351, 43–48. [Google Scholar] [CrossRef]
- Cheng, Y.; Meng, Q.S.; Wang, C.X.; Li, H.K.; Huang, Z.Y.; Chen, S.H.; Xiao, F.; Guo, F.F. Leucine Deprivation Decreases Fat Mass by Stimulation of Lipolysis in White Adipose Tissue and Upregulation of Uncoupling Protein 1 (UCP1) in Brown Adipose Tissue. Diabetes 2010, 59, 17–25. [Google Scholar] [CrossRef]
- Vianna, D.; Resende, G.F.T.; Torres-Leal, F.L.; Pantaleao, L.C.; Donato, J.; Tirapegui, J. Long-term leucine supplementation reduces fat mass gain without changing body protein status of aging rats. Nutrition 2012, 28, 182–189. [Google Scholar] [CrossRef]
- Kleinert, M.; Sylow, L.; Fazakerley, D.J.; Krycer, J.R.; Thomas, K.C.; Oxboll, A.J.; Jordy, A.B.; Jensen, T.E.; Yang, G.; Schjerling, P.; et al. Acute mTOR inhibition induces insulin resistance and alters substrate utilization in vivo. Mol. Metab. 2014, 3, 630–641. [Google Scholar] [CrossRef]
- Fox, H.L.; Pham, P.T.; Kimball, S.R.; Jefferson, L.S.; Lynch, C.J. Amino acid effects on translational repressor 4E-BP1 are mediated primarily by L-leucine in isolated adipocytes. Am. J. Physiol. 1998, 275, C1232–C1238. [Google Scholar] [CrossRef]
- Mao, X.B.; Zeng, X.F.; Wang, J.J.; Qiao, S.Y. Leucine promotes leptin receptor expression in mouse C2C12 myotubes through the mTOR pathway. Mol. Biol. Rep. 2011, 38, 3201–3206. [Google Scholar] [CrossRef]
- Bianchi, S.; Giovannini, L. Inhibition of mTOR/S6K1/4E-BP1 Signaling by Nutraceutical SIRT1 Modulators. Nutr. Cancer 2018, 70, 490–501. [Google Scholar]
- Albersheim, J.; Sathianathen, N.J.; Zabell, J.; Renier, J.; Bailey, T.; Hanna, P.; Konety, B.R.; Weight, C.J. Skeletal Muscle and Fat Mass Indexes Predict Discharge Disposition after Radical Cystectomy. J. Urol. 2019, 202, 1143–1149. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Tunison, K.; Mitsche, M.A.; McDonald, J.G.; Garg, A. Insights into lipid accumulation in skeletal muscle in dysferlin-deficient mice. J. Lipid Res. 2019, 60, 2057–2073. [Google Scholar]
- Jarmuszkiewicz, W.; Szewczyk, A. Energy-dissipating hub in muscle mitochondria: Potassium channels and uncoupling proteins. Arch Biochem. Biophys. 2019, 664, 102–109. [Google Scholar] [CrossRef]
- Kim, E.J.; Lee, M.; Kim, D.; Kim, K.I.; Yi, J.Y. Mechanisms of Energy Metabolism in Skeletal Muscle Mitochondria Following Radiation Exposure. Cells 2019, 8, 950. [Google Scholar] [CrossRef]
- Vargas-Ortiz, K.; Perez-Vazquez, V.; Macias-Cervantes, M.H. Exercise and Sirtuins: A Way to Mitochondrial Health in Skeletal Muscle. Int. J. Mol. Sci. 2019, 20, 2717. [Google Scholar] [CrossRef]
- Debashree, B.; Kumar, M.; Prasad, T.S.K.; Natarajan, A.; Christopher, R.; Nalini, A.; Bindu, P.S.; Gayathri, N.; Bharath, M.M.S. Mitochondrial dysfunction in human skeletal muscle biopsies of lipid storage disorder. J. Neurochem. 2018, 145, 323–341. [Google Scholar] [CrossRef]
- Koves, T.R.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.; Stevens, R.; Dyck, J.R.; Newgard, C.B.; et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008, 7, 45–56. [Google Scholar] [CrossRef]
- Wicks, S.E.; Vandanmagsar, B.; Haynie, K.R.; Fuller, S.E.; Warfel, J.D.; Stephens, J.M.; Wang, M.; Han, X.; Zhang, J.; Noland, R.C.; et al. Impaired mitochondrial fat oxidation induces adaptive remodeling of muscle metabolism. Proc. Natl. Acad. Sci. USA 2015, 112, E3300–E3309. [Google Scholar] [CrossRef]
- Zemel, M.B.; Bruckbauer, A. Leucine-Metformin Synergy Activates the AMPK/Sirt1 Pathway to Increase Insulin Sensitivity in Skeletal Muscle and Glucose and Lipid Metabolism and Lifespan in C. elegans. Diabetes 2015, 64, A320. [Google Scholar]
- Zhang, L.; Zhou, Y.; Wu, W.J.; Hou, L.M.; Chen, H.X.; Zuo, B.; Xiong, Y.Z.; Yang, J.Z. Skeletal Muscle-Specific Overexpression of PGC-1 alpha Induces Fiber-Type Conversion through Enhanced Mitochondrial Respiration and Fatty Acid Oxidation in Mice and Pigs. Int. J. Biol. Sci. 2017, 13, 1152–1162. [Google Scholar] [CrossRef]
- Simao, A.L.; Afonso, M.B.; Rodrigues, P.M.; Gama-Carvalho, M.; Machado, M.V.; Cortez-Pinto, H.; Rodrigues, C.M.P.; Castro, R.E. Activation of the miR-34a/SIRT1: AMPK axis contributes for insulin resistance and mitochondrial dysfunction in the NAFLD muscle. J. Hepatol. 2019, 70, E7. [Google Scholar] [CrossRef]
- Chen, X.F.; Wang, L.; Wu, Y.Z.; Song, S.Y.; Min, H.Y.; Yang, Y.; He, X.; Liang, Q.; Yi, L.; Wang, Y.; et al. Effect of puerarin in promoting fatty acid oxidation by increasing mitochondrial oxidative capacity and biogenesis in skeletal muscle in diabetic rats. Nutr. Diabetes 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Brunetta, H.S.; de Paula, G.C.; Fritzen, M.; Cecchini, M.S.; dos Santos, G.J.; Nazari, E.M.; Rafacho, A.; de Bem, A.F.; Nunes, E.A. Leucine increases muscle mitochondrial respiration and attenuates glucose intolerance in diet-induced obesity in Swiss mice. J. Funct. Foods 2019, 62, 103544. [Google Scholar] [CrossRef]
- Sun, X.C.; Zemel, M.B. Leucine and calcium regulate fat metabolism and energy partitioning in murine adipocytes and muscle cells. Lipids 2007, 42, 297–305. [Google Scholar] [CrossRef]
- Rachdi, L.; Aiello, V.; Duvillie, B.; Scharfmann, R. L-Leucine Alters Pancreatic beta-Cell Differentiation and Function via the mTor Signaling Pathway. Diabetes 2012, 61, 409–417. [Google Scholar] [CrossRef]
- Liang, C.Z.; Zemel, M.B. Leucine modulation of AMPK and mitochondrial biogenesis in C2C12 myotubes is Sirt1 dependent. Faseb J. 2013, 27. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Guo, K.Y.; LeBlanc, R.E.; Loh, D.; Schwartz, G.J.; Yu, Y.H. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes 2007, 56, 1647–1654. [Google Scholar] [CrossRef]
- Donato, J.; Pedrosa, R.G.; Cruzat, V.F.; Pires, I.S.D.; Tirapegui, J. Effects of leucine supplementation on the body composition and protein status of rats submitted to food restriction. Nutrition 2006, 22, 520–527. [Google Scholar] [CrossRef]
- Vaughan, R.A.; Garcia-Smith, R.; Gannon, N.P.; Bisoffi, M.; Trujillo, K.A.; Conn, C.A. Leucine treatment enhances oxidative capacity through complete carbohydrate oxidation and increased mitochondrial density in skeletal muscle cells. Amino Acids 2013, 45, 901–911. [Google Scholar] [CrossRef]
- Gowans, G.J.; Hawley, S.A.; Ross, F.A.; Hardie, D.G. AMP Is a True Physiological Regulator of AMP-Activated Protein Kinase by Both Allosteric Activation and Enhancing Net Phosphorylation. Cell Metab. 2013, 18, 556–566. [Google Scholar] [CrossRef]
- Fu, L.Z.; Bruckbauer, A.; Li, F.F.; Cao, Q.; Cui, X.; Wu, R.; Shi, H.; Zemel, M.B.; Xue, B.Z. Interaction between metformin and leucine in reducing hyperlipidemia and hepatic lipid accumulation in diet-induced obese mice. Metabolism 2015, 64, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, F.; Ge, X.J.; Yan, T.T.; Chen, X.M.; Shi, X.L.; Zhai, Q.W. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007, 6, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.N.; Dong, W.; Wang, R.; Li, Y.; Xu, B.L.; Zhang, J.S.; Zhao, Z.W.; Wang, Y.L. Effect of caloric restriction on the SIRT1/mTOR signaling pathways in senile mice. Brain Res. Bull. 2015, 116, 67–72. [Google Scholar] [CrossRef]
- Xu, X.Z.; Tu, L.; Feng, W.J.; Ma, B.; Li, R.; Zheng, C.L.; Li, G.; Wang, D.W. CYP2J3 Gene Delivery Up-Regulated Adiponectin Expression via Reduced Endoplasmic Reticulum Stress in Adipocytes. Endocrinology 2013, 154, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.C.; Zemel, M.B. Leucine modulation of mitochondrial mass and oxygen consumption in skeletal muscle cells and adipocytes. Nutr. Metab. 2009, 6, 26. [Google Scholar] [CrossRef]
- Lin, J.D.; Handschin, C.; Spiegelman, B.M. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005, 1, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.T.; Rodgers, J.T.; Arlow, D.H.; Vazquez, F.; Mootha, V.K.; Puigserver, P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1 alpha transcriptional complex. Nature 2007, 450, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Tanada, Y.; Shioi, T.; Kato, T.; Kawamoto, A.; Okuda, J.; Kimura, T. Branched-chain amino acids ameliorate heart failure with cardiac cachexia in rats. Life Sci. 2015, 137, 20–27. [Google Scholar] [CrossRef]
- Giovannini, L.; Bianchi, S. Role of nutraceutical SIRT1 modulators in AMPK and mTOR pathway: Evidence of a synergistic effect. Nutrition 2017, 34, 82–96. [Google Scholar] [CrossRef]
- Liang, C.Z.; Bruckbauer, A.; Zemel, M.B. Leucine modulation of sirtuins and AMPK in adipocytes and myotubes. FASEB J. 2012, 26. [Google Scholar] [CrossRef]
- Qiang, L.; Wang, L.H.; Kon, N.; Zhao, W.H.; Lee, S.; Zhang, Y.Y.; Rosenbaum, M.; Zhao, Y.M.; Gu, W.; Farmer, S.R.; et al. Brown Remodeling of White Adipose Tissue by SirT1-Dependent Deacetylation of Ppar gamma. Cell 2012, 150, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yang, H.; Duan, Y.; Yin, Y. Myostatin regulates preadipocyte differentiation and lipid metabolism of adipocyte via ERK1/2. Cell Biol. Int. 2011, 35, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gil, A.M.; Peschard-Franco, M.; Castillo, E.C.; Gutierrez-DelBosque, G.; Trevino, V.; Silva-Platas, C.; Perez-Villarreal, L.; Garcia-Rivas, G.; Elizondo-Montemayor, L. Myokine-adipokine cross-talk: Potential mechanisms for the association between plasma irisin and adipokines and cardiometabolic risk factors in Mexican children with obesity and the metabolic syndrome. Diabetol. Metab. Syndr. 2019, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Mantzoros, C.S.; Magkos, F.; Brinkoetter, M.; Sienkiewicz, E.; Dardeno, T.A.; Kim, S.Y.; Hamnvik, O.P.R.; Koniaris, A. Leptin in human physiology and pathophysiology. Am. J. Physiol. Endoc. M 2011, 301, E567–E584. [Google Scholar]
- Zhang, Q.; Liu, B.; Cheng, Y.; Meng, Q.S.; Xia, T.T.; Jiang, L.; Chen, S.H.; Liu, Y.; Guo, F.F. Leptin Signaling Is Required for Leucine Deprivation-enhanced Energy Expenditure. J. Biol. Chem. 2014, 289, 1779–1787. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.J.; Gern, B.; Lloyd, C.; Hutson, S.M.; Eicher, R.; Vary, T.C. Leucine in food mediates some of the postprandial rise in plasma leptin concentrations. Am. J. Physiol. Endoc. M 2006, 291, E621–E630. [Google Scholar] [CrossRef]
- Galic, S.; Oakhill, J.S.; Steinberg, G.R. Adipose tissue as an endocrine organ. Mol. Cell. Endocrinol. 2010, 316, 129–139. [Google Scholar] [CrossRef]
- You, M.; Rogers, C.Q. Adiponectin: A Key Adipokine in Alcoholic Fatty Liver. Exp. Biol. Med. 2009, 234, 850–859. [Google Scholar] [CrossRef]
- Ying, W.; Riopel, M.; Bandyopadhyay, G.; Dong, Y.; Birmingham, A.; Seo, J.B.; Ofrecio, J.M.; Wollam, J.; Hernandez-Carretero, A.; Fu, W.X.; et al. Adipose Tissue Macrophage-Derived Exosomal miRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity. Cell 2017, 171, 372–384. [Google Scholar] [CrossRef]
- Pan, Y.; Hui, X.; Hoo, R.L.C.; Ye, D.; Chan, C.Y.C.; Feng, T.; Wang, Y.; Lam, K.S.L.; Xu, A. Adipocyte-secreted exosomal microRNA-34a inhibits M2 macrophage polarization to promote obesity-induced adipose inflammation. J. Clin. Investig. 2019, 129, 834–849. [Google Scholar] [CrossRef]
- Barile, L.; Lionetti, V.; Cervio, E.; Matteucci, M.; Gherghiceanu, M.; Popescu, L.M.; Torre, T.; Siclari, F.; Moccetti, T.; Vassalli, G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc. Res. 2014, 103, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.M.; Wang, Y.; Cooper, G.J.S.; Lam, K.S.L. Regulation of adiponectin production by adipocytes: Role in the progression of ethanol-induced liver injury. Alcohol. Clin. Exp. Res. 2006, 30, 71a. [Google Scholar]
- Macotela, Y.; Emanuelli, B.; Bang, A.M.; Espinoza, D.O.; Boucher, J.; Beebe, K.; Gall, W.; Kahn, C.R. Dietary Leucine - An Environmental Modifier of Insulin Resistance Acting on Multiple Levels of Metabolism. PLoS ONE 2011, 6, e21187. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.C.; Zemel, M.B. Adiponectin mediates leucine-induced adipocyte-muscle cross-talk. FASEB J. 2010, 24. [Google Scholar] [CrossRef]
- Li, F.N.; Li, Y.H.; Duan, Y.H.; Hu, C.A.A.; Tang, Y.L.; Yin, Y.L. Myokines and adipokines: Involvement in the crosstalk between skeletal muscle and adipose tissue. Cytokine Growth Factor Rev. 2017, 33, 73–82. [Google Scholar]
- Ding, L.; Kang, Y.; Dai, H.B.; Wang, F.Z.; Zhou, H.; Gao, Q.; Xiong, X.Q.; Zhang, F.; Song, T.R.; Yuan, Y.; et al. Adipose afferent reflex is enhanced by TNF alpha in paraventricular nucleus through NADPH oxidase-dependent ROS generation in obesity-related hypertensive rats. J. Transl. Med. 2019, 17, 256. [Google Scholar] [CrossRef]
- Bradley, E.W.; Carpio, L.R.; Newton, A.C.; Westendorf, J.J. Deletion of the PH-domain and Leucine-rich Repeat Protein Phosphatase 1 (Phlpp1) Increases Fibroblast Growth Factor (Fgf) 18 Expression and Promotes Chondrocyte Proliferation. J. Biol. Chem. 2015, 290, 16272–16280. [Google Scholar]
- Baggett, B.; Bruckbauer, A.; Zemel, M. Synergistic Effects of Leucine and its Metabolites with Polyphenols on Irisin in Myotubes and Diet-induced Obese Mice. FASEB J. 2013, 27. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, S.Y. Fermentative production of branched chain amino acids: A focus on metabolic engineering. Appl. Microbiol. Biot. 2010, 85, 491–506. [Google Scholar]
- Zhang, S.H.; Ren, M.; Zeng, X.F.; He, P.L.; Ma, X.; Qiao, S.Y. Leucine stimulates ASCT2 amino acid transporter expression in porcine jejunal epithelial cell line (IPEC-J2) through PI3K/Akt/mTOR and ERK signaling pathways. Amino Acids 2014, 46, 2633–2642. [Google Scholar]
- Mao, X.; Ren, M.; Chen, D.; Yu, B.; Che, L.; He, J.; Luo, J.; Luo, Y.; Wang, J.; Sun, H. Leucine modulates the IPEC-J2 cell proteome associated with cell proliferation, metabolism and phagocytosis. Anim. Nutr. 2018, 4, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Nie, Y.; Chen, S.; Xie, C.; Fan, Q.; Wang, Z.; Long, B.; Yan, G.; Zhong, Q.; Yan, X. Leucine reduces reactive oxygen species levels via an energy metabolism switch by activation of the mTOR-HIF-1alpha pathway in porcine intestinal epithelial cells. Int. J. Biochem. Cell Biol. 2017, 89, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Minegishi, Y.; Komine, Y.; Mori, T.; Matsumoto, I.; Abe, K.; Tokimitsu, I.; Hase, T.; Murase, T. Differential regulation of intestinal lipid metabolism-related genes in obesity-resistant A/J vs. obesity-prone C57BL/6J mice. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E1092–E1099. [Google Scholar] [CrossRef] [PubMed]
- Goichon, A.; Chan, P.; Lecleire, S.; Coquard, A.; Cailleux, A.F.; Walrand, S.; Lerebours, E.; Vaudry, D.; Dechelotte, P.; Coeffier, M. An enteral leucine supply modulates human duodenal mucosal proteome and decreases the expression of enzymes involved in fatty acid beta-oxidation. J. Proteom. 2013, 78, 535–544. [Google Scholar] [CrossRef]
- Buffie, C.G.; Pamer, E.G. Microbiota-mediated colonization resistance against intestinal pathogens. Nat. Rev. Immunol. 2013, 13, 790–801. [Google Scholar] [CrossRef]
- Meng, X.L.; Li, S.; Qin, C.B.; Zhu, Z.X.; Hu, W.P.; Yang, L.P.; Lu, R.H.; Li, W.J.; Nie, G.X. Intestinal microbiota and lipid metabolism responses in the common carp (Cyprinus carpio L.) following copper exposure. Ecotox Environ. Safe 2018, 160, 257–264. [Google Scholar] [CrossRef]
- Busnelli, M.; Bruneau, A.; Manzini, S.; Boukadiri, A.; Philippe, C.; Parolini, C.; Chiesa, G.; Gerard, P. Effect of Different Microbiota on Lipid Metabolism, Liver Steatosis and Intestinal Homeostasis in Mice Fed a Low-Protein Diet. Atherosclerosis 2017, 263, E6–E7. [Google Scholar] [CrossRef]
- Everard, A.; Lazarevic, V.; Derrien, M.; Girard, M.; Muccioli, G.G.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; Francois, P.; de Vos, W.M.; et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice (vol 60, pg 2775, 2011). Diabetes 2011, 60, 3307. [Google Scholar]
- Ashaolu, T.J.; Saibandith, B.; Yupanqui, C.T.; Wichienchot, S. Human colonic microbiota modulation and branched chain fatty acids production affected by soy protein hydrolysate. Int. J. Food Sci. Tech. 2019, 54, 141–148. [Google Scholar]
- Tiihonen, K.; Ouwehand, A.C.; Rautonen, N. Human intestinal microbiota and healthy ageing. Ageing Res. Rev. 2010, 9, 107–116. [Google Scholar] [CrossRef]
- Wang, F.L.; Wan, Y.; Yin, K.H.; Wei, Y.G.; Wang, B.B.; Yu, X.M.; Ni, Y.; Zheng, J.S.; Huang, T.; Song, M.Y.; et al. Lower Circulating Branched-Chain Amino Acid Concentrations Among Vegetarians are Associated with Changes in Gut Microbial Composition and Function. Mol. Nutr. Food Res. 2019, 63, 1900612. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, S.; Macfarlane, G.T. Regulation of short-chain fatty acid production. Proc. Nutr. Soc. 2003, 62, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Prodhan, M.A.I.; Yuan, F.; Yin, X.; Lorkiewicz, P.K.; Wei, X.; Feng, W.; McClain, C.; Zhang, X. Simultaneous quantification of straight-chain and branched-chain short chain fatty acids by gas chromatography mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1092, 359–367. [Google Scholar] [CrossRef]
- Backhed, F.; Manchester, J.K.; Semenkovich, C.F.; Gordon, J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA 2007, 104, 979–984. [Google Scholar] [CrossRef]
- Milo, L.A.; Reardon, K.A.; Tappenden, K.A. Effects of short-chain fatty acid-supplemented total parenteral nutrition on intestinal pro-inflammatory cytokine abundance. Dig. Dis. Sci. 2002, 47, 2049–2055. [Google Scholar] [CrossRef]
- Cao, W.; Chin, Y.; Chen, X.; Mi, Y.; Xue, C.; Wang, Y.; Tang, Q. The role of gut microbiota in the resistance to obesity in mice fed a high fat diet. Int. J. Food Sci. Nutr. 2019, 1–11. [Google Scholar] [CrossRef]
- Yin, J.; Li, Y.Y.; Han, H.; Chen, S.; Gao, J.; Liu, G.; Wu, X.; Deng, J.P.; Yu, Q.F.; Huang, X.G.; et al. Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in high-fat diet-fed mice. J. Pineal Res. 2018, 65, e12524. [Google Scholar] [CrossRef]
- Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Cheng, J.; Duncan, A.E.; Kau, A.L.; Griffin, N.W.; Lombard, V.; Henrissat, B.; Bain, J.R.; et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013, 341, 1241214. [Google Scholar] [CrossRef]
- Hu, C.J.; Li, F.N.; Duan, Y.H.; Yin, Y.L.; Kong, X.F. Dietary Supplementation With Leucine or in Combination With Arginine Decreases Body Fat Weight and Alters Gut Microbiota Composition in Finishing Pigs. Front. Microbiol. 2019, 10, 1767. [Google Scholar] [CrossRef]
- Zhang, Z.; Mocanu, V.; Cai, C.; Dang, J.; Slater, L.; Deehan, E.C.; Walter, J.; Madsen, K.L. Impact of Fecal Microbiota Transplantation on Obesity and Metabolic Syndrome-A Systematic Review. Nutrients 2019, 11, 2291. [Google Scholar] [CrossRef]
- Kang, C.; Wang, B.; Kaliannan, K.; Wang, X.; Lang, H.; Hui, S.; Huang, L.; Zhang, Y.; Zhou, M.; Chen, M.; et al. Erratum for Kang et al., “Gut Microbiota Mediates the Protective Effects of Dietary Capsaicin against Chronic Low-Grade Inflammation and Associated Obesity Induced by High-Fat Diet”. Mbio 2017, 8, e00470-17. [Google Scholar] [CrossRef]
- Bai, J.; Greene, E.; Li, W.F.; Kidd, M.T.; Dridi, S. Branched-chain amino acids modulate the expression of hepatic fatty acid metabolism-related genes in female broiler chickens. Mol. Nutr. Food Res. 2015, 59, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.P.; Jiang, W.D.; Liu, Y.; Qu, B.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Wu, P.; Zhang, Y.A.; et al. Dietary leucine improves flesh quality and alters mRNA expressions of Nrf2-mediated antioxidant enzymes in the muscle of grass carp (Ctenopharyngodon idella). Aquaculture 2016, 452, 380–387. [Google Scholar] [CrossRef]
- Cervantes-Ramirez, M.; Mendez-Trujillo, V.; Araiza-Pina, B.A.; Barrera-Silva, M.A.; Gonzalez-Mendoza, D.; Morales-Trejo, A. Supplemental leucine and isoleucine affect expression of cationic amino acid transporters and myosin, serum concentration of amino acids, and growth performance of pigs. Genet. Mol. Res. 2013, 12, 115–126. [Google Scholar]
- Fan, Q.W.; Long, B.S.; Yan, G.K.; Wang, Z.C.; Shi, M.; Bao, X.Y.; Hu, J.; Li, X.Z.; Chen, C.Q.; Zheng, Z.L.; et al. Dietary leucine supplementation alters energy metabolism and induces slow-to-fast transitions in longissimus dorsi muscle of weanling piglets. Brit. J. Nutr. 2017, 117, 1222–1234. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Li, F.; Guo, Q.; Duan, Y.; Wang, W.; Zhong, Y.; Yang, Y.; Yin, Y. Leucine Supplementation: A Novel Strategy for Modulating Lipid Metabolism and Energy Homeostasis. Nutrients 2020, 12, 1299. https://doi.org/10.3390/nu12051299
Zhang L, Li F, Guo Q, Duan Y, Wang W, Zhong Y, Yang Y, Yin Y. Leucine Supplementation: A Novel Strategy for Modulating Lipid Metabolism and Energy Homeostasis. Nutrients. 2020; 12(5):1299. https://doi.org/10.3390/nu12051299
Chicago/Turabian StyleZhang, Lingyu, Fengna Li, Qiuping Guo, Yehui Duan, Wenlong Wang, Yinzhao Zhong, Yuhuan Yang, and Yulong Yin. 2020. "Leucine Supplementation: A Novel Strategy for Modulating Lipid Metabolism and Energy Homeostasis" Nutrients 12, no. 5: 1299. https://doi.org/10.3390/nu12051299
APA StyleZhang, L., Li, F., Guo, Q., Duan, Y., Wang, W., Zhong, Y., Yang, Y., & Yin, Y. (2020). Leucine Supplementation: A Novel Strategy for Modulating Lipid Metabolism and Energy Homeostasis. Nutrients, 12(5), 1299. https://doi.org/10.3390/nu12051299





