Long-Term Coffee Consumption is Associated with Fecal Microbial Composition in Humans
Abstract
1. Introduction
2. Subjects and Methods
2.1. Nutritional Assessment
2.2. Blood Biochemical Analyses
2.3. Fecal Collection and Microbial Analysis
2.4. Statistical Analyses
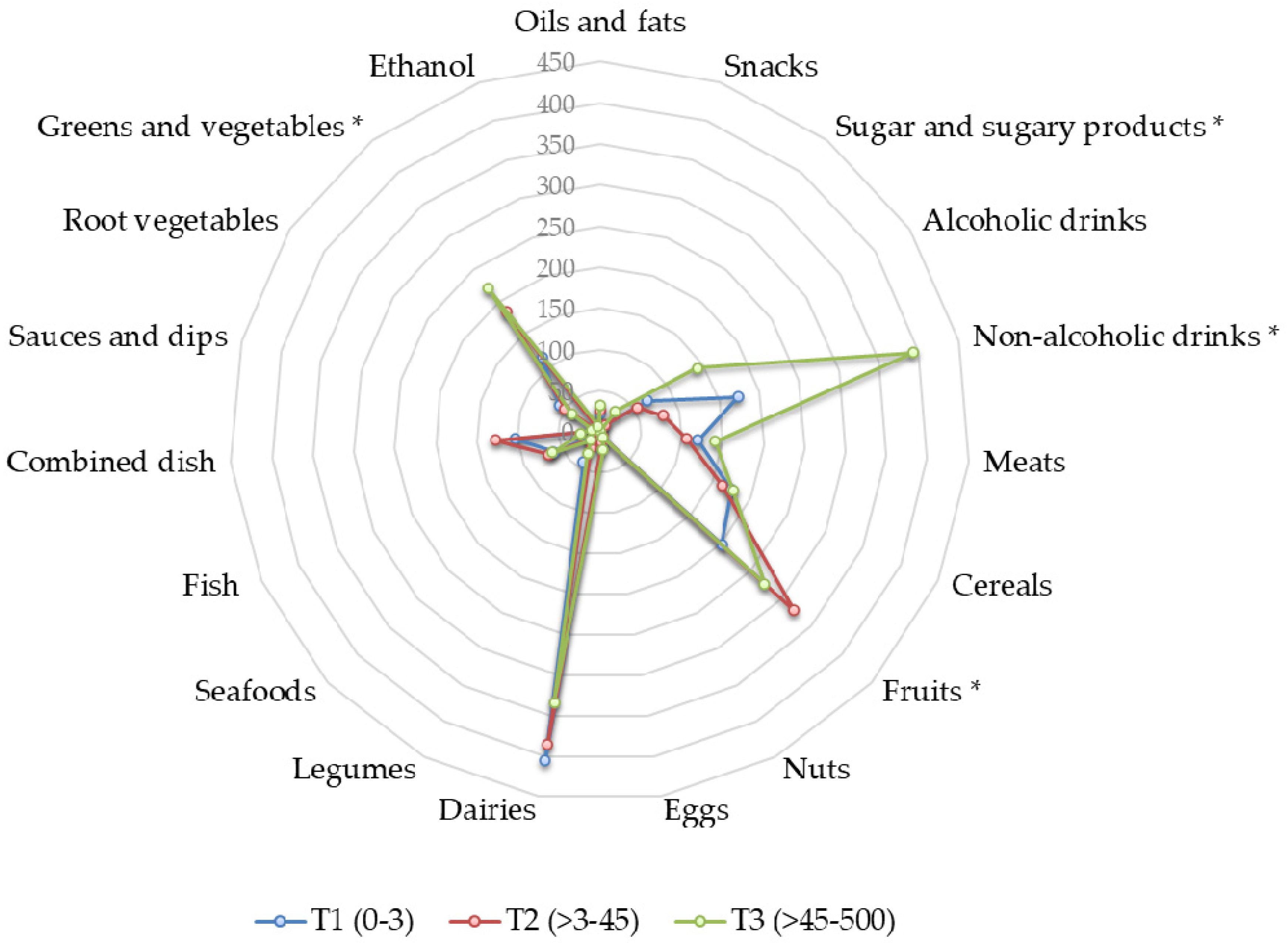
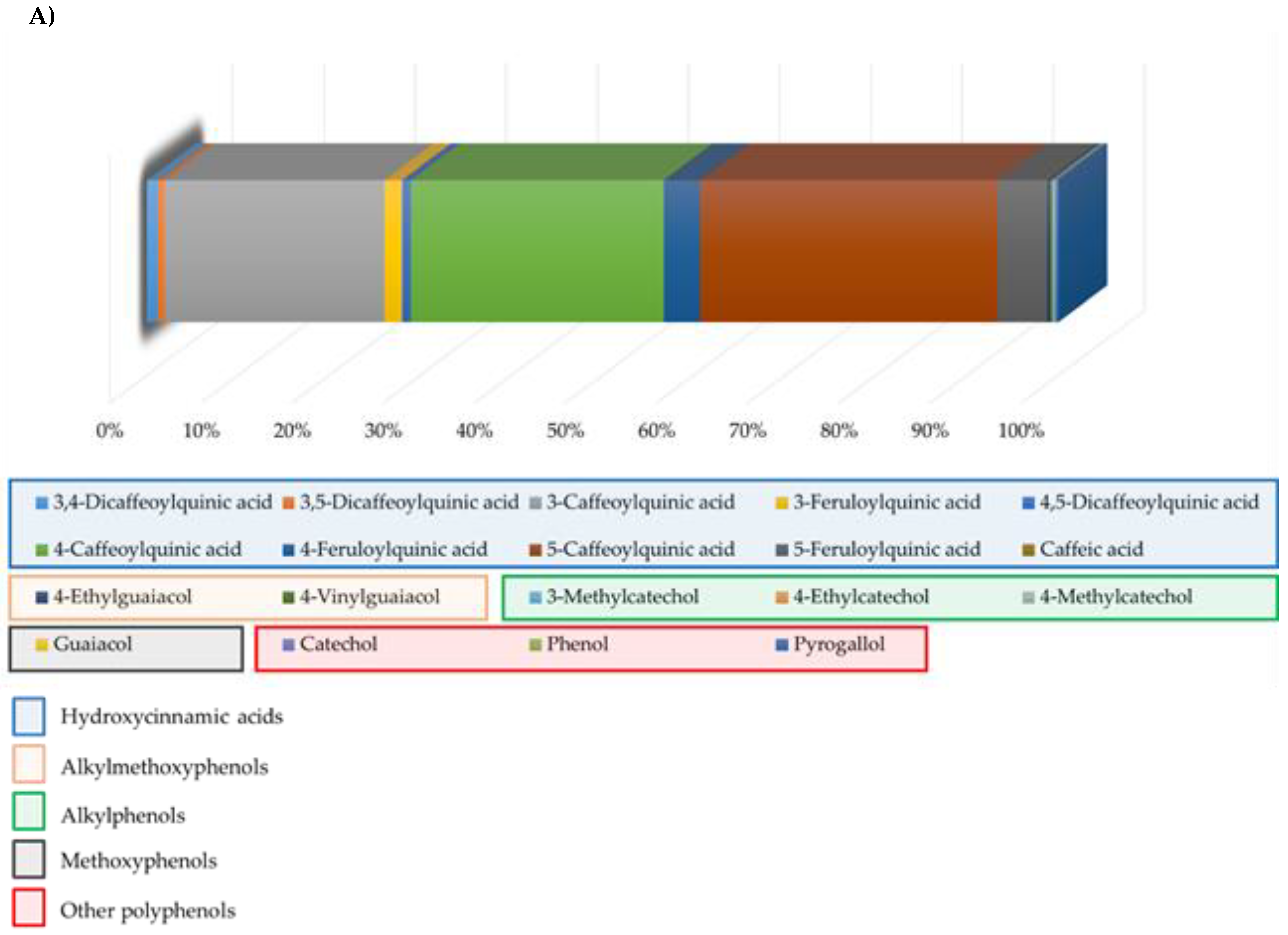
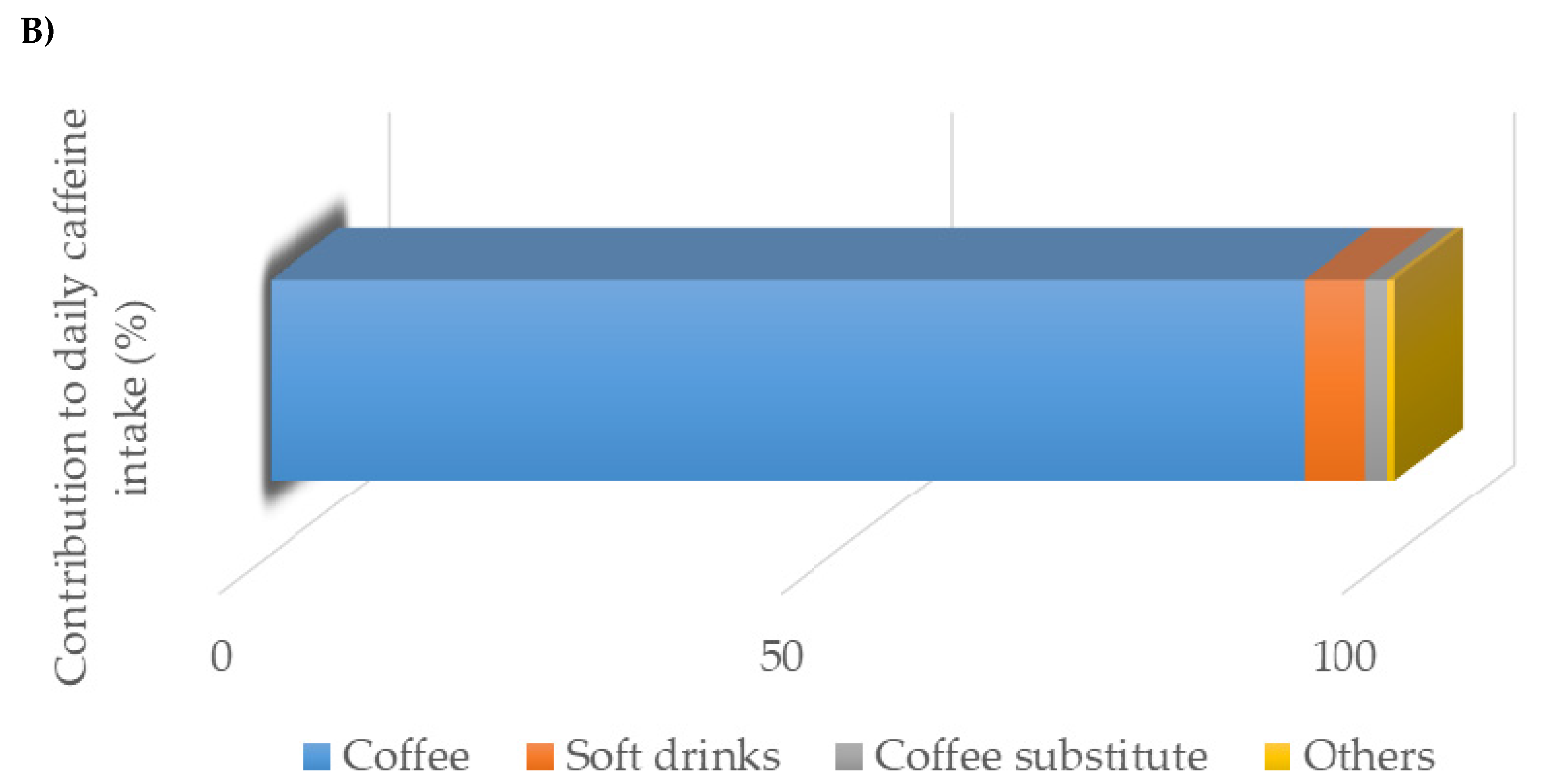
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mubarak, A.; Bondonno, C.P.; Liu, A.H.; Considine, M.J.; Rich, L.; Mas, E.; Croft, K.D.; Hodgson, J.M. Acute effects of chlorogenic acid on nitric oxide status, endothelial function, and blood pressure in healthy volunteers: A randomized trial. J. Agric. Food Chem. 2012, 60, 9130–9136. [Google Scholar] [CrossRef] [PubMed]
- Farias-Pereira, R.; Park, C.-S.; Park, Y. Mechanisms of action of coffee bioactive components on lipid metabolism. Food Sci. Biotechnol. 2019, 28, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.H.; DiNicolantonio, J.J.; Lavie, C.J. Coffee for cardioprotection and longevity. Prog. Cardiovasc. Dis. 2018, 61, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Godos, J.; Galvano, F.; Giovannucci, E.L. Coffee, Caffeine, and health outcomes: An umbrella review. Annu. Rev. Nutr. 2017, 37, 131–156. [Google Scholar] [CrossRef] [PubMed]
- Dórea, J.G.; da Costa, T.H.M. Is coffee a functional food? Br. J. Nutr. 2005, 93, 773–782. [Google Scholar] [CrossRef]
- Higdon, J.V.; Frei, B. Coffee and Health: A review of recent human research. Crit. Rev. Food Sci. Nutr. 2006, 46, 101–123. [Google Scholar] [CrossRef]
- Poole, R.; Kennedy, O.J.; Roderick, P.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. BMJ 2017, 359, j5024. [Google Scholar] [CrossRef]
- Brown, S.R.; Cann, P.A.; Read, N.W. Effect of coffee on distal colon function. Gut 1990, 31, 450–453. [Google Scholar] [CrossRef]
- Jaquet, M.; Rochat, I.; Moulin, J.; Cavin, C.; Bibiloni, R. Impact of coffee consumption on the gut microbiota: A human volunteer study. Int. J. Food Microbiol. 2009, 130, 117–121. [Google Scholar] [CrossRef]
- Mills, C.E.; Tzounis, X.; Oruna-Concha, M.-J.; Mottram, D.S.; Gibson, G.R.; Spencer, J.P.E. In vitro colonic metabolism of coffee and chlorogenic acid results in selective changes in human faecal microbiota growth. Br. J. Nutr. 2015, 113, 1220–1227. [Google Scholar] [CrossRef]
- Salvucci, E. The human-microbiome superorganism and its modulation to restore health. Int. J. Food Sci. Nutr. 2019, 70, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, C.B.; Shannon, K.M.; Kordower, J.H.; Voigt, R.M.; Shaikh, M.; Jaglin, J.A.; Estes, J.D.; Dodiya, H.B.; Keshavarzian, A. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS ONE 2011, 6, e28032. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Oishi, K. Influence of coffee ( Coffea arabica ) and galacto-oligosaccharide consumption on intestinal microbiota and the host responses. FEMS Microbiol. Lett. 2013, 343, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Khokhlova, E.V.; Smeianov, V.V.; Efimov, B.A.; Kafarskaia, L.I.; Pavlova, S.I.; Shkoporov, A.N. Anti-inflammatory properties of intestinal Bifidobacterium strains isolated from healthy infants. Microbiol. Immunol. 2012, 56, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Gniechwitz, D.; Reichardt, N.; Blaut, M.; Steinhart, H.; Bunzel, M. Dietary fiber from coffee beverage: Degradation by human fecal microbiota. J. Agric. Food Chem. 2007, 55, 6989–6996. [Google Scholar] [CrossRef]
- Faust, K.; Sathirapongsasuti, J.F.; Izard, J.; Segata, N.; Gevers, D.; Raes, J.; Huttenhower, C. Microbial co-occurrence relationships in the human microbiome. PLoS Comput. Biol. 2012, 8, e1002606. [Google Scholar] [CrossRef]
- Kleber Silveira, A.; Moresco, K.S.; Mautone Gomes, H.; da Silva Morrone, M.; Kich Grun, L.; Pens Gelain, D.; de Mattos Pereira, L.; Giongo, A.; Rodrigues De Oliveira, R.; Fonseca Moreira, J.C. Guarana (Paullinia cupana Mart.) alters gut microbiota and modulates redox status, partially via caffeine in Wistar rats. Phyther. Res. 2018, 32, 2466–2474. [Google Scholar] [CrossRef]
- Yamamoto, T.; Takahashi, H.; Suzuki, K.; Hirano, A.; Kamei, M.; Goto, T.; Takahashi, N.; Kawada, T. Theobromine enhances absorption of cacao polyphenol in rats. Biosci. Biotechnol. Biochem. 2014, 78, 2059–2063. [Google Scholar] [CrossRef]
- Cuervo, A.; Valdés, L.; Salazar, N.; de los Reyes-Gavilán, C.G.; Ruas-Madiedo, P.; Gueimonde, M.; González, S. Pilot study of diet and microbiota: Interactive associations of fibers and polyphenols with human intestinal bacteria. J. Agric. Food Chem. 2014, 62, 5330–5336. [Google Scholar] [CrossRef]
- Cuervo, A.; de los Reyes-Gavilán, C.G.; Ruas-Madiedo, P.; Lopez, P.; Suarez, A.; Gueimonde, M.; González, S. Red wine consumption is associated with fecal microbiota and malondialdehyde in a human population. J. Am. Coll. Nutr. 2015, 34, 135–141. [Google Scholar] [CrossRef]
- Centro de Ensenanza Superior de Nutricion Humana y Dietetica (CESNID). Tablas de Composicion de Alimentos por Medidas Caseras de Consumo Habitual en España; McGrawHill, Ed.; Publicaciones y Ediciones de la Universidad de Barcelona: Barcelona, Spain, 2008. [Google Scholar]
- U.S. Department of Agriculture Agricultural Research Service. FoodData Central. Available online: fdc.nal.usda.gov (accessed on 12 November 2019).
- Neveu, V.; Perez-Jimenez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef] [PubMed]
- Marlett, J.A.; Cheung, T.-F. Database and quick methods of assessing typical dietary fiber intakes using data for 228 commonly consumed foods. J. Am. Diet. Assoc. 1997, 97, 1139–1151. [Google Scholar] [CrossRef]
- Fernández-Navarro, T.; Díaz, I.; Gutiérrez-Díaz, I.; Rodríguez-Carrio, J.; Suárez, A.; de los Reyes-Gavilán, C.G.; Gueimonde, M.; Salazar, N.; González, S. Exploring the interactions between serum free fatty acids and fecal microbiota in obesity through a machine learning algorithm. Food Res. Int. 2019, 121, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Gérard-Monnier, D.; Erdelmeier, I.; Régnard, K.; Moze-Henry, N.; Yadan, J.-C.; Chaudière, J. Reactions of 1-Methyl-2-phenylindole with Malondialdehyde and 4-Hydroxyalkenals. Analytical applications to a colorimetric assay of lipid peroxidation. Chem. Res. Toxicol. 1998, 11, 1176–1183. [Google Scholar] [CrossRef]
- Nogacka, A.M.; Salazar, N.; Arboleya, S.; Ruas-Madiedo, P.; Mancabelli, L.; Suarez, A.; Martinez-Faedo, C.; Ventura, M.; Tochio, T.; Hirano, K.; et al. In vitro evaluation of different prebiotics on the modulation of gut microbiota composition and function in morbid obese and normal-weight subjects. Int. J. Mol. Sci. 2020, 21, 906. [Google Scholar] [CrossRef]
- Valdes, L.; Salazar, N.; Gonzalez, S.; Arboleya, S.; Rios-Covian, D.; Genoves, S.; Ramon, D.; los Reyes-Gavilán, C.G.; Ruas-Madiedo, P.; Gueimonde, M. Selections of potential probiotic bifidobacteria and prebiotics for elderly by using in vitro fecal batch cultures. Eur. Food. Res. Technol. 2017, 243, 157–185. [Google Scholar] [CrossRef]
- Salazar, N.; Gueimonde, M.; Hernandez-Barranco, A.M.; Ruas-Madiedo, P.; de los Reyes-Gavilan, C.G. Exopolysaccharides produced by intestinal bifidobacterium strains act as fermentable substrates for human intestinal bacteria. Appl. Environ. Microbiol. 2008, 74, 4737–4745. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Keski-Rahkonen, P.; Robinot, N.; Assi, N.; Casagrande, C.; Jenab, M.; Ferrari, P.; Boutron-Ruault, M.; Mahamat-Saleh, Y.; Mancini, F.R.; et al. A metabolomic study of biomarkers of habitual coffee intake in four european countries. Mol. Nutr. Food Res. 2019, 63, 1900659. [Google Scholar] [CrossRef]
- Chrysant, S.G. The impact of coffee consumption on blood pressure, cardiovascular disease and diabetes mellitus. Expert Rev. Cardiovasc. Ther. 2017, 15, 151–156. [Google Scholar] [CrossRef]
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Bes-Rastrollo, M.; Galvano, F.; Martinez-Gonzalez, M. Long-term coffee consumption is associated with decreased incidence of new-onset hypertension: A dose–response meta-analysis. Nutrients 2017, 9, 890. [Google Scholar] [CrossRef]
- Santos, R.M.M.; Lima, D.R.A. Coffee consumption, obesity and type 2 diabetes: A mini-review. Eur. J. Nutr. 2016, 55, 1345–1358. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Backhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Bhandarkar, N.S.; Mouatt, P.; Goncalves, P.; Thomas, T.; Brown, L.; Panchal, S.K. Modulation of gut microbiota by spent coffee grounds attenuates diet-induced metabolic syndrome in rats. FASEB J. 2020, 34, 4783–4797. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Clifford, M.N. Colonic metabolites of berry polyphenols: The missing link to biological activity? Br. J. Nutr. 2010, 104, S48–S66. [Google Scholar] [CrossRef]
- Aura, A.-M.; Martin-Lopez, P.; O’Leary, K.A.; Williamson, G.; Oksman-Caldentey, K.-M.; Poutanen, K.; Santos-Buelga, C. In vitro metabolism of anthocyanins by human gut microflora. Eur. J. Nutr. 2005, 44, 133–142. [Google Scholar] [CrossRef]
- Aura, A.-M. Microbial metabolism of dietary phenolic compounds in the colon. Phytochem. Rev. 2008, 7, 407–429. [Google Scholar] [CrossRef]
- Couteau, D.; McCartney, A.L.; Gibson, G.R.; Williamson, G.; Faulds, C.B. Isolation and characterization of human colonic bacteria able to hydrolyse chlorogenic acid. J. Appl. Microbiol. 2001, 90, 873–881. [Google Scholar] [CrossRef]
- Godos, J.; Pluchinotta, F.R.; Marventano, S.; Buscemi, S.; Li Volti, G.; Galvano, F.; Grosso, G. Coffee components and cardiovascular risk: Beneficial and detrimental effects. Int. J. Food Sci. Nutr. 2014, 65, 925–936. [Google Scholar] [CrossRef]
- Martini, D.; Del Bo’, C.; Tassotti, M.; Riso, P.; Del Rio, D.; Brighenti, F.; Porrini, M. Coffee consumption and oxidative stress: A review of human intervention studies. Molecules 2016, 21, 979. [Google Scholar] [CrossRef]
- Moreira, A.S.P.; Nunes, F.M.; Domingues, M.R.; Coimbra, M.A. Coffee melanoidins: Structures, mechanisms of formation and potential health impacts. Food Funct. 2012, 3, 903. [Google Scholar] [CrossRef] [PubMed]
- Mulak, A. Brain-gut-microbiota axis in Parkinson’s disease. World J. Gastroenterol. 2015, 21, 10609. [Google Scholar] [CrossRef] [PubMed]
- Cowan, T.E.; Palmnäs, M.S.A.; Yang, J.; Bomhof, M.R.; Ardell, K.L.; Reimer, R.A.; Vogel, H.J.; Shearer, J. Chronic coffee consumption in the diet-induced obese rat: Impact on gut microbiota and serum metabolomics. J. Nutr. Biochem. 2014, 25, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.-H.; Kim, N.-J.; Song, J.-K.; Chun, K.-H. Kahweol inhibits lipid accumulation and induces Glucose-uptake through activation of AMP-activated protein kinase (AMPK). BMB Rep. 2017, 50, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Farias-Pereira, R.; Oshiro, J.; Kim, K.-H.; Park, Y. Green coffee bean extract and 5-O-caffeoylquinic acid regulate fat metabolism in caenorhabditis elegans. J. Funct. Foods 2018, 48, 586–593. [Google Scholar] [CrossRef]
- Lally, J.S.V.; Jain, S.S.; Han, X.X.; Snook, L.A.; Glatz, J.F.C.; Luiken, J.J.F.P.; McFarlan, J.; Holloway, G.P.; Bonen, A. Caffeine-stimulated fatty acid oxidation is blunted in CD36 null mice. Acta Physiol. 2012, 205, 71–81. [Google Scholar] [CrossRef]
- Su, S.-H.; Shyu, H.-W.; Yeh, Y.-T.; Chen, K.-M.; Yeh, H.; Su, S.-J. Caffeine inhibits adipogenic differentiation of primary adipose-derived stem cells and bone marrow stromal cells. Toxicol. In Vitro 2013, 27, 1830–1837. [Google Scholar] [CrossRef]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef]

| Microbial Group | Primer Sequence (5′-3′) | Tm. (°C) |
|---|---|---|
| Akkermansia | F: CAGCACGTGAAGGTGGGGAC R: CCTTGCGGTTGGCTTCAGAT | 60 |
| Bacteroides-Prevotella-Porphyromonas | F: GAGAGGAAGGTCCCCCAC R: CGCKACTTGGCTGGTTCAG | 60 |
| Bifidobacterium | F: GATTCTGGCTCAGGATGAACGC R: CTGATAGGACGCGACCCCAT | 60 |
| Clostridia cluster XIVa group | F: CGGTACCTGACTAAGAAGC R: AGTTTYATTCTTGCGAACG | 55 |
| Faecalibacterium prausnitzii | F: GGAGGAAGAAGGTCTTCGG R: AATTCCGCCTACCTCTGCACT | 60 |
| Lactobacillus group | F: AGCAGTAGGGAATCTTCCA R: CATGGAGTTCCACTGTCCTC | 60 |
| Characteristic | Coffee (mL/day) | ||
|---|---|---|---|
| T1 (0–3) (n = 49) | T2 (>3–45) (n = 49) | T3 (>45–500) (n = 49) | |
| Age (years) | 58.8 ± 18.62 a | 67.57 ± 14.77 b | 47.10 ± 10.86 c |
| Gender (% female) | 69% | 71% | 71% |
| BMI (kg/m2) | 28.08 ± 4.52 a | 27.32 ± 3.55 a | 26.73 ± 5.16 a |
| Sleep duration (h/day) | 6.78 ± 1.06 a | 6.73 ± 1.07 a | 7.00 ± 1.31 a |
| Energy intake (Kcal/day) | 1906.93 ± 494.27 a | 1776.89 ± 531.64 a | 2040.23 ± 622.47 a |
| Coffee consumption (mL/day) | 0.15 ± 0.59 a | 27.53 ± 11.14 b | 151.84 ± 92.10 c |
| Tobacco user (%) | 25% | 28% | 25% |
| Depositions (nº/week) | 8.89 ± 6.26 a | 6.49 ± 2.68 b | 7.74 ± 3.84 a,b |
| Coffee (mL/day) | |||
|---|---|---|---|
| T1 (0–3) | T2 (>3–45) | T3(>45–500) | |
| Model 1. Microbial group (Log n cells/gram feces) (n, 138) * | |||
| Akkermansia | 5.70 ± 0.40 a | 5.76 ± 0.33 a | 6.30 ± 0.36 a |
| Bacteroides-Prevotella-Porphyromonas | 8.03 ± 0.27 a | 8.74 ± 0.27 a,b | 9.14 ± 0.30 b |
| Bifidobacterium | 7.61 ± 0.26 a | 7.73 ± 0.26 a | 8.19 ± 0.28 a |
| Clostridia cluster XIVa group | 7.48 ± 0.29 a | 7.49 ± 0.29 a | 7.50 ± 0.31 a |
| Lactobacillus group | 6.28 ± 0.27 a | 5.97 ± 0.27 a | 5.97 ± 0.29 a |
| Faecalibacterium prausnitzii | 7.10 ± 0.17 a | 7.29 ± 0.17 a | 7.51 ± 0.19 a |
| Model 2. Fecal SCFA concentration (mM) (n, 132) * | |||
| Acetic acid | 36.77 ± 2.51 a | 36.80 ± 2.48 a | 33.99 ± 2.58 a |
| Propionic acid | 12.55 ± 1.09 a | 13.97 ± 1.08 a | 12.56± 1.12 a |
| Butyric acid | 10.17 ± 1.18 a | 10.90 ± 1.17 a | 10.10 ± 1.21 a |
| Model 3. Blood parameters * | |||
| Serum MDA (µM) (n,102) | 2.51 ± 0.11 a | 2.28 ± 0.07 a,b | 1.89 ± 0.20 b |
| C reactive protein (mg/L) (n,108) | 1.37 ± 0.24 a | 1.27 ± 0.17 a | 0.69 ± 0.46 a |
| Leptin (ng/mL) (n,102) | 11.05 ± 1.21 a | 11.15 ± 0.85 a | 8.34 ± 2.34 a |
| LDL–HDL ratio (n,125) | 2.51 ± 0.18 a | 2.86 ± 0.13 a | 2.85 ± 0.35 a |
| Triglycerides (mg/dL) (n,125) | 121.70 ± 11.12 a | 122.08 ± 7.80 a | 103.14 ± 21.44 a |
| Glucose (mg/dL) (n,125) | 100.12 ± 5.62 a | 103.05 ± 3.94 a | 103.73 ± 10.84 a |
| Antioxidant capacity (mM) (n,72) | 0.36 ± 0.02 a | 0.34 ± 0.01 a | 0.34 ± 0.04 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, S.; Salazar, N.; Ruiz-Saavedra, S.; Gómez-Martín, M.; de los Reyes-Gavilán, C.G.; Gueimonde, M. Long-Term Coffee Consumption is Associated with Fecal Microbial Composition in Humans. Nutrients 2020, 12, 1287. https://doi.org/10.3390/nu12051287
González S, Salazar N, Ruiz-Saavedra S, Gómez-Martín M, de los Reyes-Gavilán CG, Gueimonde M. Long-Term Coffee Consumption is Associated with Fecal Microbial Composition in Humans. Nutrients. 2020; 12(5):1287. https://doi.org/10.3390/nu12051287
Chicago/Turabian StyleGonzález, Sonia, Nuria Salazar, Sergio Ruiz-Saavedra, María Gómez-Martín, Clara G. de los Reyes-Gavilán, and Miguel Gueimonde. 2020. "Long-Term Coffee Consumption is Associated with Fecal Microbial Composition in Humans" Nutrients 12, no. 5: 1287. https://doi.org/10.3390/nu12051287
APA StyleGonzález, S., Salazar, N., Ruiz-Saavedra, S., Gómez-Martín, M., de los Reyes-Gavilán, C. G., & Gueimonde, M. (2020). Long-Term Coffee Consumption is Associated with Fecal Microbial Composition in Humans. Nutrients, 12(5), 1287. https://doi.org/10.3390/nu12051287





