The Effect of Lactobacillus plantarum 299v on Iron Status and Physical Performance in Female Iron-Deficient Athletes: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Study Product
2.4. Recruitment and Screening
2.5. Demographics and Anthropometrics
2.6. Blood Sampling and Analyses
2.7. Ergometer Cycling Test
2.8. Profile of Mood States (POMS)
2.9. Safety and Gastrointestinal (GI) Well-Being
2.10. Statistical Methods
3. Results
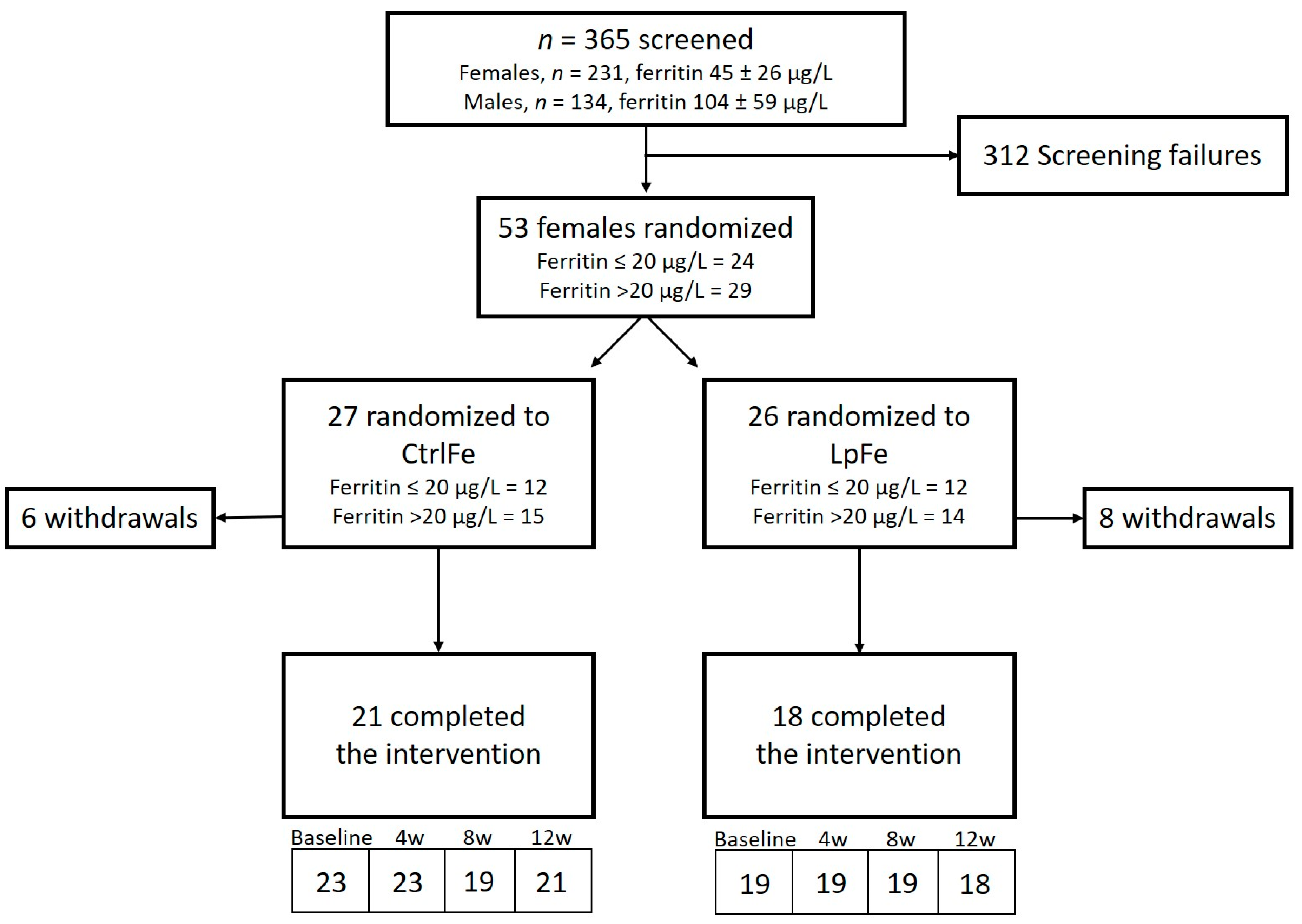
3.1. Subject Disposition and Baseline Characteristics
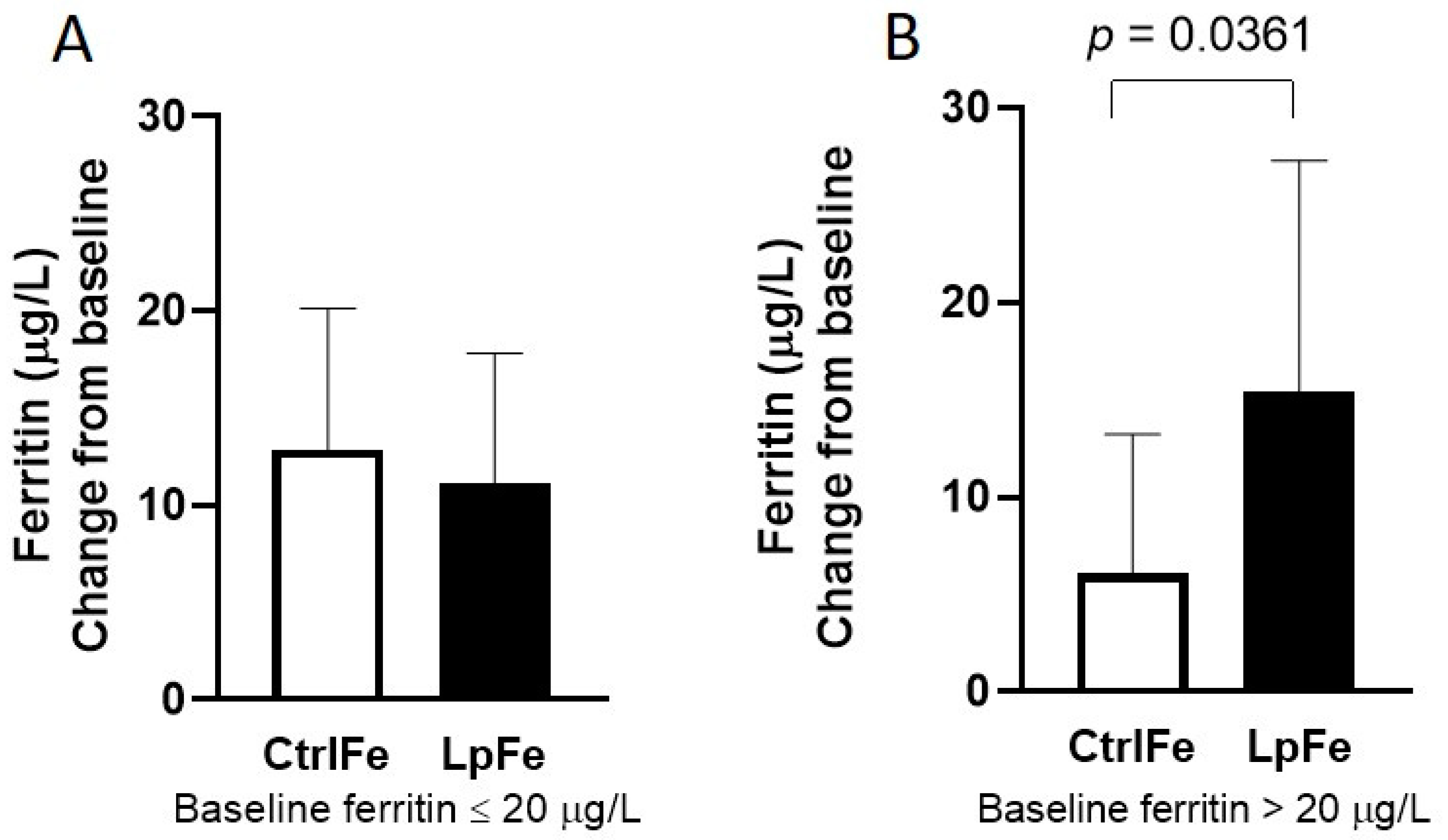
3.2. Iron Status
3.3. Inflammatory Parameters
3.4. Physical Performance
3.5. Profile of Mood Score
3.6. Safety and GI Well-Being
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kassebaum, N.J. The Global Burden of Anemia. Hematol. Clin. North Am. 2016, 30, 247–308. [Google Scholar] [CrossRef] [PubMed]
- Stoltzfus, R.J. Iron deficiency: Global prevalence and consequences. Food Nutr. Bull. 2003, 24, S99–S103. [Google Scholar] [CrossRef] [PubMed]
- Evstatiev, R.; Gasche, C. Iron sensing and signalling. Gut 2011, 61, 933–952. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.D.; Brownlie, T. Iron deficiency and reduced work capacity: A critical review of the research to determine a causal relationship. J. Nutr. 2001, 131, 676S–690S. [Google Scholar] [CrossRef]
- Rubeor, A.; Goojha, C.; Manning, J.; White, J. Does Iron Supplementation Improve Performance in Iron-Deficient Nonanemic Athletes? Sports Health 2018, 10, 400–405. [Google Scholar] [CrossRef]
- Dellavalle, D.M.; Haas, J.D. Impact of iron depletion without anemia on performance in trained endurance athletes at the beginning of a training season: A study of female collegiate rowers. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 501–506. [Google Scholar] [CrossRef]
- Dubnov, G.; Constantini, N.W. Prevalence of iron depletion and anemia in top-level basketball players. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 30–37. [Google Scholar] [CrossRef]
- Landahl, G.; Adolfsson, P.; Börjesson, M.; Mannheimer, C.; Rödjer, S. Iron deficiency and anemia: A common problem in female elite soccer players. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 689–694. [Google Scholar] [CrossRef]
- Dubnov-Raz, G.; Foldes, A.J.; Mann, G.; Magazanik, A.; Siderer, M.; Constantini, N. High Prevalence of Iron Deficiency and Anemia in Female Military Recruits. Mil. Med. 2006, 171, 866–869. [Google Scholar] [CrossRef]
- Di Santolo, M.; Stel, G.; Banfi, G.; Gonano, F.; Cauci, S. Anemia and iron status in young fertile non-professional female athletes. Eur. J. Appl. Physiol. 2008, 102, 703–709. [Google Scholar] [CrossRef]
- Sandström, G.; Börjesson, M.; Rödjer, S. Iron Deficiency in Adolescent Female Athletes—Is Iron Status Affected by Regular Sporting Activity? Clin. J. Sport Med. 2012, 22, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Dellavalle, D.M. Iron Supplementation for Female Athletes. Curr. Sports Med. Rep. 2013, 12, 234–239. [Google Scholar] [CrossRef]
- Parks, R.B.; Hetzel, S.J.; Brooks, M.A. Iron Deficiency and Anemia among Collegiate Athletes. Med. Sci. Sports Exerc. 2017, 49, 1711–1715. [Google Scholar] [CrossRef] [PubMed]
- Alaunyte, I.; Stojceska, V.; Plunkett, A. Iron and the female athlete: A review of dietary treatment methods for improving iron status and exercise performance. J. Int. Soc. Sports Nutr. 2015, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Auersperger, I.; Skof, B.; Leskošek, B.; Knap, B.; Jerin, A.; Lainscak, M. Exercise-Induced Changes in Iron Status and Hepcidin Response in Female Runners. PLoS ONE 2013, 8, e58090. [Google Scholar] [CrossRef] [PubMed]
- Alaunyte, I.; Stojceska, V.; Plunkett, A.; Derbyshire, E. Dietary iron intervention using a staple food product for improvement of iron status in female runners. J. Int. Soc. Sports Nutr. 2014, 11, 50. [Google Scholar] [CrossRef]
- Malczewska-Lenczowska, J.; Orysiak, J.; Szczepańska, B.; Turowski, D.; Burkhard-Jagodzinska, K.; Gajewski, J. Reticulocyte and erythrocyte hypochromia markers in detection of iron deficiency in adolescent female athletes. Boil. Sport 2017, 34, 111–118. [Google Scholar] [CrossRef]
- Malczewska, J.; Raczynski, G.; Stupnicki, R. Iron status in female endurance athletes and in non-athletes. Int. J. Sport Nutr. Exerc. Metab. 2000, 10, 260–276. [Google Scholar] [CrossRef]
- DeRuisseau, K.C.; Cheuvront, S.N.; Haymes, E.M.; Sharp, R.G. Sweat iron and zinc losses during prolonged exercise. Int. J. Sport Nutr. Exerc. Metab. 2002, 12, 428–437. [Google Scholar] [CrossRef]
- Akiboye, R.D.; Sharma, D.M. Haematuria in Sport: A Review. Eur. Urol. Focus 2019, 5, 912–916. [Google Scholar] [CrossRef]
- Dellavalle, D.M.; Haas, J.D. Iron Supplementation Improves Energetic Efficiency in Iron-Depleted Female Rowers. Med. Sci. Sports Exerc. 2014, 46, 1204–1215. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, E.P.; Burini, R.C. The impact of physical exercise on the gastrointestinal tract. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 533–538. [Google Scholar] [CrossRef]
- Beard, J.; Han, O. Systemic iron status. Biochim. et Biophys. Acta (BBA) -Gen. Subj. 2009, 1790, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Pasricha, S.R.; Low, M.S.Y.; Thompson, J.F.; Farrell, A.; De-Regil, L.M. Iron Supplementation Benefits Physical Performance in Women of Reproductive Age: A Systematic Review and Meta-Analysis. J. Nutr. 2014, 144, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, S.; Horner, K.; De Vito, G.; Conway, G.E. The Role of Mineral and Trace Element Supplementation in Exercise and Athletic Performance: A Systematic Review. Nutrients 2019, 11, 696. [Google Scholar] [CrossRef] [PubMed]
- Tolkien, Z.; Stecher, L.; Mander, A.P.; Pereira, D.; Powell, J.J. Ferrous Sulfate Supplementation Causes Significant Gastrointestinal Side-Effects in Adults: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0117383. [Google Scholar] [CrossRef]
- Eid, R.; Arab, N.T.; Greenwood, M.T. Iron mediated toxicity and programmed cell death: A review and a re-examination of existing paradigms. Biochim. et Biophys. Acta (BBA)-Bioenerg. 2017, 1864, 399–430. [Google Scholar] [CrossRef]
- Waterman, J.J.; Kapur, R. Upper Gastrointestinal Issues in Athletes. Curr. Sports Med. Rep. 2012, 11, 99–104. [Google Scholar] [CrossRef]
- De Oliveira, E.P.; Burini, R.C.; Jeukendrup, A. Gastrointestinal complaints during exercise: Prevalence, etiology, and nutritional recommendations. Sports Med. 2014, 44, 79–85. [Google Scholar] [CrossRef]
- Kortman, G.A.M.; Boleij, A.; Swinkels, R.W.; Tjalsma, H. Iron Availability Increases the Pathogenic Potential of Salmonella Typhimurium and Other Enteric Pathogens at the Intestinal Epithelial Interface. PLoS ONE 2012, 7, e29968. [Google Scholar] [CrossRef]
- Bering, S.; Suchdev, S.; Sjøltov, L.; Berggren, A.; Tetens, I.; Bukhave, K. A lactic acid-fermented oat gruel increases non-haem iron absorption from a phytate-rich meal in healthy women of childbearing age. Br. J. Nutr. 2006, 96, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, M.; Önning, G.; Hulthen, L. Freeze-dried Lactobacillus plantarum 299v increases iron absorption in young females—Double isotope sequential single-blind studies in menstruating women. PLoS ONE 2017, 12, e0189141. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, M.; Önning, G.; Berggren, A.; Hulthén, L. Probiotic strain Lactobacillus plantarum 299v increases iron absorption from an iron-supplemented fruit drink: A double-isotope cross-over single-blind study in women of reproductive age. Br. J. Nutr. 2015, 114, 1195–1202. [Google Scholar] [CrossRef]
- Jäger, R.; Mohr, A.E.; Carpenter, K.C.; Kerksick, C.; Purpura, M.; Moussa, A.; Townsend, J.R.; Lamprecht, M.; West, N.P.; Black, K.; et al. International Society of Sports Nutrition Position Stand: Probiotics. J. Int. Soc. Sports Nutr. 2019, 16, 62. [Google Scholar] [CrossRef] [PubMed]
- Borg, E.; Kaijser, L. A comparison between three rating scales for perceived exertion and two different work tests. Scand. J. Med. Sci. Sports 2006, 16, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E.; Wallis, G.A. Measurement of Substrate Oxidation During Exercise by Means of Gas Exchange Measurements. Int. J. Sports Med. 2005, 26, 28–37. [Google Scholar] [CrossRef] [PubMed]
- McNair, D.M.; Lorr, M.; Droppleman, L.F. Manual for the Profile of Mood States; Educational and Industrial Testing Services: San Diego, CA, USA, 1971. [Google Scholar]
- Guyonnet, D.; Naliboff, B.; Rondeau, P.; Mayer, E.; Chassany, O. Gastrointestinal well-being in subjects reporting mild gastrointestinal discomfort: Characteristics and properties of a global assessment measure. Br. J. Nutr. 2013, 110, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Gelaw, Y.; Woldu, B.; Melku, M. The Role of Reticulocyte Hemoglobin Content for Diagnosis of Iron Deficiency and Iron Deficiency Anemia, and Monitoring of Iron Therapy: A Literature Review. Clin. Lab. 2019, 65, 65. [Google Scholar] [CrossRef]
- Toki, Y.; Ikuta, K.; Kawahara, Y.; Niizeki, N.; Kon, M.; Enomoto, M.; Tada, Y.; Hatayama, M.; Yamamoto, M.; Ito, S.; et al. Reticulocyte hemoglobin equivalent as a potential marker for diagnosis of iron deficiency. Int. J. Hematol. 2017, 106, 116–125. [Google Scholar] [CrossRef]
- Adiki, S.K.; Perla, C.K.; Saha, G.; Katakam, P.; Theendra, V. Enhancement in Iron Absorption on Intake of Chemometrically Optimized Ratio of Probiotic Strain Lactobacillus plantarum 299v with Iron Supplement Pearl Millet. Boil. Trace Element Res. 2018, 190, 150–156. [Google Scholar] [CrossRef]
- Vonderheid, S.C.; Tussing-Humphreys, L.; Park, C.; Pauls, H.; Hemphill, N.O.; LaBomascus, B.; McLeod, A.; Koenig, M.D. A Systematic Review and Meta-Analysis on the Effects of Probiotic Species on Iron Absorption and Iron Status. Nutrients 2019, 11, 2938. [Google Scholar] [CrossRef]
- Sandberg, A.S.; Önning, G.; Engström, N.; Scheers, N. Iron Supplements Containing Lactobacillus plantarum 299v Increase Ferric Iron and Up-regulate the Ferric Reductase DCYTB in Human Caco-2/HT29 MTX Co-Cultures. Nutrients 2018, 10, 1949. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Cherayil, B.J. Iron and inflammation—The gut reaction. Metallomics 2017, 9, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Guida, C.; Altamura, S.; Klein, F.A.; Galy, B.; Boutros, M.; Ulmer, A.J.; Hentze, M.W.; Muckenthaler, M.U. A novel inflammatory pathway mediating rapid hepcidin-independent hypoferremia. Blood 2015, 125, 2265–2275. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Sánchez-Oliver, A.J.; Mata-Ordoñez, F.; Feria-Madueño, A.; Grimaldi-Puyana, M.; López-Samanes, Á.; Pérez-López, A. Effects of an Acute Exercise Bout on Serum Hepcidin Levels. Nutrients 2018, 10, 209. [Google Scholar] [CrossRef]
- Malik, M.; Suboc, T.M.; Tyagi, S.; Salzman, N.; Wang, J.; Ying, R.; Tanner, M.J.; Kakarla, M.; Baker, J.E.; Widlansky, M.E. Lactobacillus plantarum 299v Supplementation Improves Vascular Endothelial Function and Reduces Inflammatory Biomarkers in Men With Stable Coronary Artery Disease. Circ. Res. 2018, 123, 1091–1102. [Google Scholar] [CrossRef]
- McNaught, C.; Woodcock, N.; Anderson, A.; MacFie, J. A prospective randomised trial of probiotics in critically ill patients. Clin. Nutr. 2005, 24, 211–219. [Google Scholar] [CrossRef]
- Naruszewicz, M.; Johansson, M.L.; Zapolska-Downar, D.; Bukowska, H. Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. Am. J. Clin. Nutr. 2002, 76, 1249–1255. [Google Scholar] [CrossRef]
- McClung, J.P.; Karl, J.P.; Cable, S.J.; Williams, K.W.; Nindl, B.C.; Young, A.J.; Lieberman, H.R. Randomized, double-blind, placebo-controlled trial of iron supplementation in female soldiers during military training: Effects on iron status, physical performance, and mood. Am. J. Clin. Nutr. 2009, 90, 124–131. [Google Scholar] [CrossRef]
- Johansson, M.L.; Nobaek, S.; Berggren, A.; Nyman, M.E.; Björck, I.; Ahrné, S.; Jeppsson, B.; Molin, G. Survival of Lactobacillus plantarum DSM 9843 (299v), and effect on the short-chain fatty acid content of faeces after ingestion of a rose-hip drink with fermented oats. Int. J. Food Microbiol. 1998, 42, 29–38. [Google Scholar] [CrossRef]
- Nobaek, S.; Johansson, M.L.; Molin, G.; Ahrné, S.; Jeppsson, B. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am. J. Gastroenterol. 2000, 95, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Niedzielin, K.; Kordecki, H.; Birkenfeld, B. A controlled, double-blind, randomized study on the efficacy of Lactobacillus plantarum 299V in patients with irritable bowel syndrome. Eur. J. Gastroenterol. Hepatol. 2001, 13, 1143–1147. [Google Scholar] [CrossRef] [PubMed]
- Ducrotté, P.; Sawant, P.; Jayanthi, V. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J. Gastroenterol. 2012, 18, 4012–4018. [Google Scholar]
- Rask, C.; Adlerberth, I.; Berggren, A.; Ahren, I.L.; Wold, A. Differential effect on cell-mediated immunity in human volunteers after intake of different lactobacilli. Clin. Exp. Immunol. 2013, 172, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Spence, L.; Brown, W.J.; Pyne, D.; Nissen, M.; Sloots, T.P.; McCormack, J.G.; Locke, A.S.; Fricker, P.A. Incidence, Etiology, and Symptomatology of Upper Respiratory Illness in Elite Athletes. Med. Sci. Sports Exerc. 2007, 39, 577–586. [Google Scholar] [CrossRef]
- Colbey, C.; Cox, A.J.; Pyne, D.; Zhang, P.; Cripps, A.W.; West, N.P. Upper Respiratory Symptoms, Gut Health and Mucosal Immunity in Athletes. Sports Med. 2018, 48, 65–77. [Google Scholar] [CrossRef] [PubMed]
| Variables | CtrlFe | LpFe |
|---|---|---|
| n | 23 | 19 |
| Age (years; SD) | 21.6 (6.0) | 22.3 (3.5) |
| Height (cm; SD) | 171 (6) | 169 (5) |
| Body weight (kg; SD) | 67 (7) | 66 (6) |
| BMI (kg/m2; SD) | 22.9 (1.8) | 23.3 (2.5) |
| Average training hours per week (SD) * | 7.6 (2.0) | 8.2 (2.0) |
| VO2max mL × kg−1 × min−1 | 2.8 (0.3) | 2.9 (0.3) |
| Variables | CtrlFe | LpFe |
|---|---|---|
| n | 22–23 | 17–18 |
| B-Hb (g/L) | 129.4 (9.3) | 130.4 (8.7) |
| P-Ferritin (µg/L) | 19.8 (6.8) | 19.5 (9.3) |
| P-Iron (µg/L) | 14.7 (6.4) | 18.1 (7.5) |
| P-Transferrin (g/L) | 3.2 (0.5) | 3.3 (0.5) |
| P-Transferrin sat. (%) | 18.8 (7.9) | 22.3 (10.5) |
| B-EVF (%) | 39 (3.0) | 39 (2.0) |
| B-Reticulocytes (pg/L) | 46.6 (12.3) | 48.5 (11.9) |
| B-Ret-Hb (pg) | 31.3 (2.0) | 31.5 (2.4) |
| sTfR (mg/L) | 1.3 (0.3) | 1.5 (0.4) |
| Hepcidin (nmol/L) | 1.6 (2.1) | 1.9 (2.0) |
| hCRP (mg/L) | 0.9 (0.6) | 0.8 (0.3) |
| Variable | Week | CtrlFe | LpFe | p |
|---|---|---|---|---|
| B-Hb (g/L) | Week 4 | 0.55 (8.9) | −1.2 (9.3) | 0.7201 |
| Week 8 | 2.8 (10.7) | −1.06 (7.2) | 0.1876 | |
| Week 12 | 3.9 (8.7) | 0.67 (7.4) | 0.5072 | |
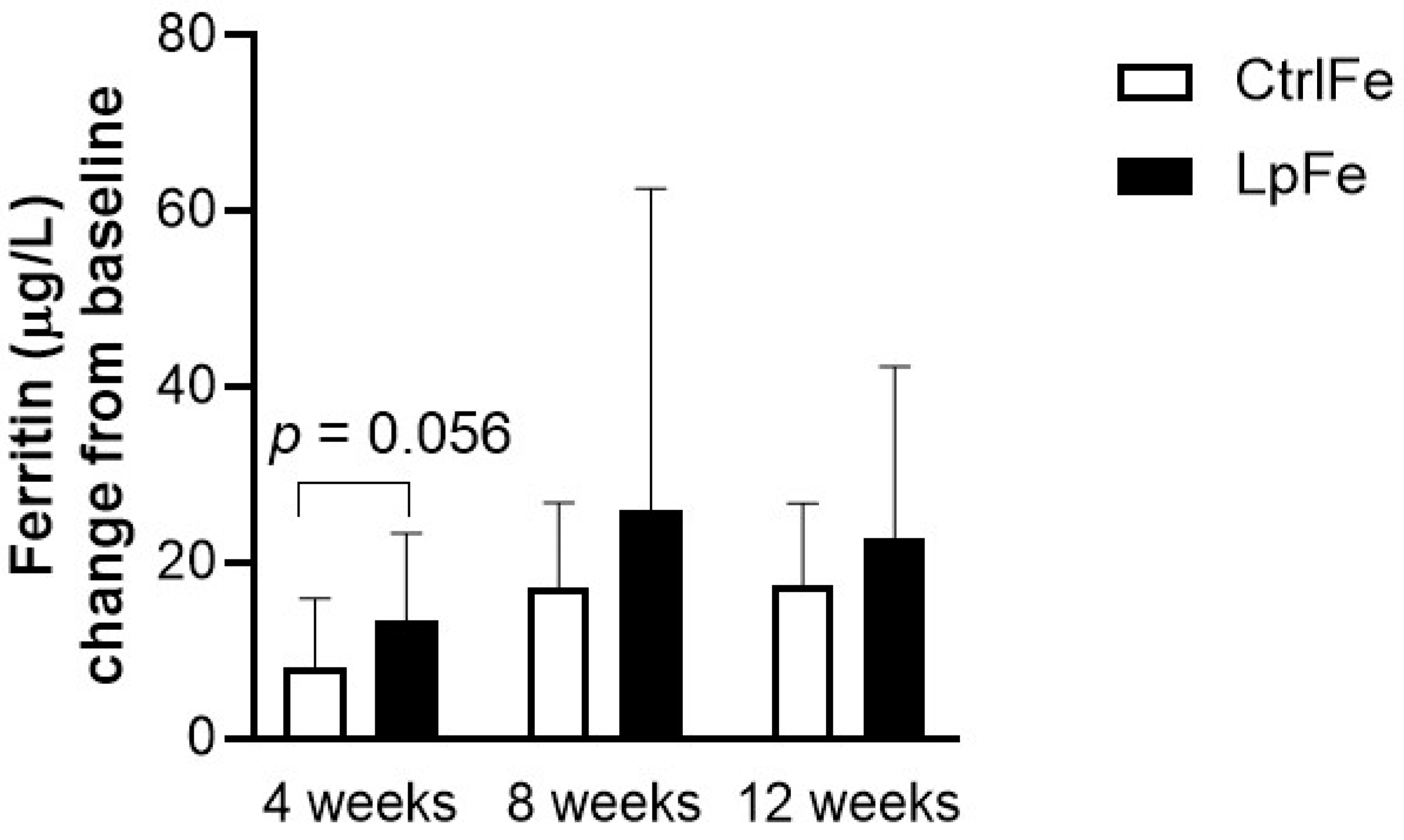
| P-Ferritin (µg/L) | Week 4 | 8.2 (7.7) | 13.6 (9.9) | 0.0565 |
| Week 8 | 17.3 (9.6) | 25.9 (36.7) | 0.9504 | |
| Week 12 | 17.4 (9.4) | 19.5 (19.5) | 0.4491 | |
| P-Iron (µmol/L) | Week 4 | 5.6 (10.1) | −1.6 (12.5) | 0.0411 |
| Week 8 | 2.1 (8.7) | 0.81 (11.8) | 0.8305 | |
| Week 12 | 2.6 (6.7) | −0.27 (10.5) | 0.4373 | |
| P-Transferrin (g/L) | Week 4 | −0.16 (0.31) | −0.29 (0.33) | 0.3313 |
| Week 8 | −0.31 (0.44) | −0.39 (0.33) | 0.8443 | |
| Week 12 | −0.18 (0.34) | −0.36 (0.32) | 0.1644 | |
| P-Transferrin sat. (%) | Week 4 | 8.2 (12.5) | −1.6 (15.23) | 0.0655 |
| Week 8 | 4.6 (10.9) | 3.56 (15.45) | 0.9778 | |
| Week 12 | 5.1 (10.3) | 2.53 (14.67) | 0.4056 | |
| B-EVF (%) | Week 4 | 0 (0.03) | 0 (0.03) | 0.8531 |
| Week 8 | −0.01 (0.02) | −0.01 (0.02) | 0.3274 | |
| Week 12 | −0.01 (0.02) | −0.01 (0.02) | 0.4128 | |
| B-Reticulocytes (pg/L) | Week 4 | 1.4 (9.2) | 0.4 (14.0) | 0.5148 |
| Week 8 | 1.1 (10.9) | −1.3 (11.2) | 0.6245 | |
| Week 12 | 3.5 (9.7) | 3.9 (9.3) | 0.8014 | |
| B-Ret-Hb (pg) | Week 4 | 1.1 (1.8) | 1.0 (1.6) | 0.9354 |
| Week 8 | 0.8 (1.3) | 1.3 (0.5) | 0.2749 | |
| Week 12 | 0.8 (0.9) | 1.3 (0.8) | 0.0834 | |
| sTfR (mg/L) | Week 4 | −0.14 (0.20) | −0.12 (0.18) | 0.7314 |
| Week 8 | −0.24 (0.27) | −0.15 (0.21) | 0.3327 | |
| Week 12 | −0.19 (0.15) | −0.18 (0.36) | 0.3112 | |
| Hepcidin (nmol/L) | Week 4 | 2.7 (7.3) | 0.8 (3.1) | 0.3615 |
| Week 8 | 1.1 (3.6) | 5.9 (8.7) | 0.1597 | |
| Week 12 | 2.5 (4.1) | 3.9 (6.8) | 0.8076 | |
| hCRP (mg/L) | Week 4 | −0.05 (0.5) | 0.07 (0.4) | 0.7876 |
| Week 8 | −0.15 (0.5) | 0.01 (0.3) | 0.8789 | |
| Week 12 | −0.02 (0.6) | 0.05 (0.5) | 0.9915 |
| Parameter | Change | CtrlFe | LpFe | p |
|---|---|---|---|---|
| Endurance (time to exhaustion, min) | ΔV3–V2 | 0.29 (0.54) | 0.35 (0.35) | 0.8888 |
| ΔV4–V2 | −0.17 (1.28) | 0.52 (0.78) | 0.2428 | |
| ΔV5–V2 | −0.30 (1.74) | 0.20 (0.69) | 0.8467 | |
| Heart rate (beats/min) | ΔV3–V2 | 0.45 (4.56) | 0.40 (4.10) | 0.9935 |
| ΔV4–V2 | −3.22 (6.50) | −2.00 (2.87) | 0.7634 | |
| ΔV5–V2 | −2.33 (5.12) | 1.29 (4.51) | 0.1243 | |
| VO2max (L/min) | ΔV3–V2 | 0.07 (0.14) | 0.08 (0.13) | 0.7357 |
| ΔV4–V2 | −0.08 (0.32) | 0.09 (0.18) | 0.2863 | |
| ΔV5–V2 | −0.03 (0.26) | 0.02 (0.14) | 0.7717 | |
| Lactate (mmol/L) | ΔV3–V2 | −0.72 (2.05) | 0.47 (1.84) | 0.0562 |
| ΔV4–V2 | −1.22 (2.50) | −0.01 (1.42) | 0.2743 | |
| ΔV5–V2 | −1.05 (2.48) | 0.02 (2.06) | 0.3346 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Axling, U.; Önning, G.; Combs, M.A.; Bogale, A.; Högström, M.; Svensson, M. The Effect of Lactobacillus plantarum 299v on Iron Status and Physical Performance in Female Iron-Deficient Athletes: A Randomized Controlled Trial. Nutrients 2020, 12, 1279. https://doi.org/10.3390/nu12051279
Axling U, Önning G, Combs MA, Bogale A, Högström M, Svensson M. The Effect of Lactobacillus plantarum 299v on Iron Status and Physical Performance in Female Iron-Deficient Athletes: A Randomized Controlled Trial. Nutrients. 2020; 12(5):1279. https://doi.org/10.3390/nu12051279
Chicago/Turabian StyleAxling, Ulrika, Gunilla Önning, Maile A. Combs, Alemtsehay Bogale, Magnus Högström, and Michael Svensson. 2020. "The Effect of Lactobacillus plantarum 299v on Iron Status and Physical Performance in Female Iron-Deficient Athletes: A Randomized Controlled Trial" Nutrients 12, no. 5: 1279. https://doi.org/10.3390/nu12051279
APA StyleAxling, U., Önning, G., Combs, M. A., Bogale, A., Högström, M., & Svensson, M. (2020). The Effect of Lactobacillus plantarum 299v on Iron Status and Physical Performance in Female Iron-Deficient Athletes: A Randomized Controlled Trial. Nutrients, 12(5), 1279. https://doi.org/10.3390/nu12051279





