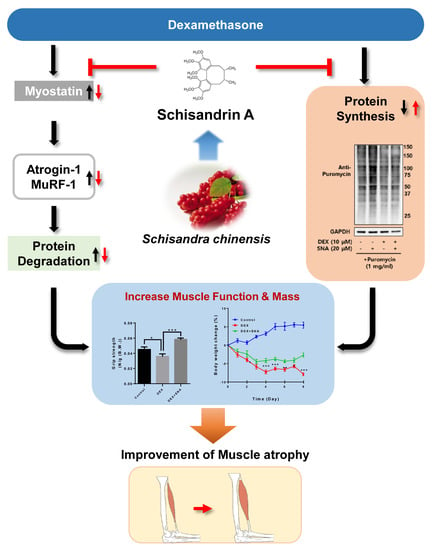
Preventive Effects of Schisandrin A, A Bioactive Component of Schisandra chinensis, on Dexamethasone-Induced Muscle Atrophy
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Induction of Muscle Atrophy and Treatment with SNA
2.3. Cell Culture
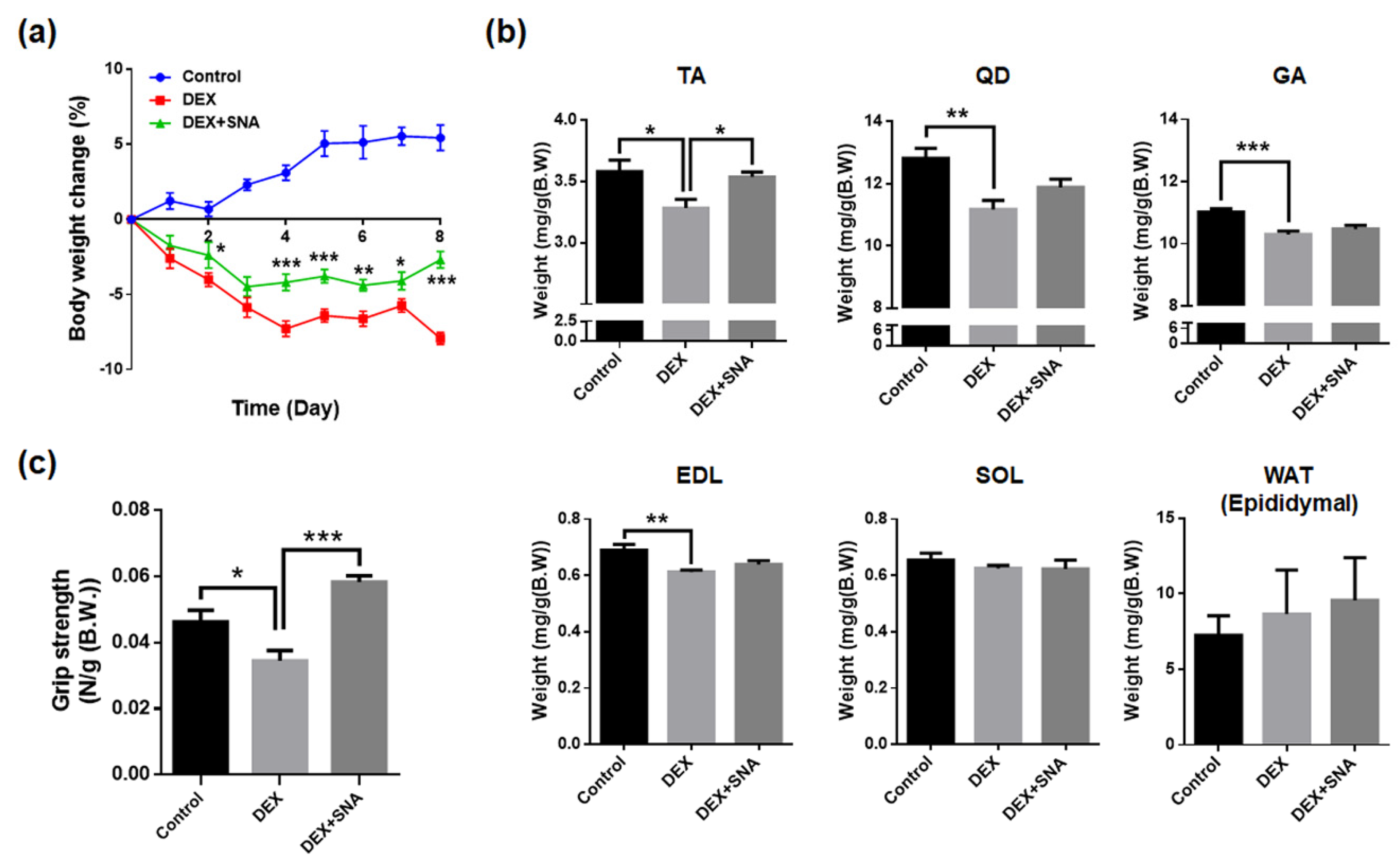
2.4. Measurement of Grip Strength
2.5. Tissue Collection
2.6. Histology
2.7. Real-Time Polymerase Chain Reaction (PCR) Analysis
2.8. Western Blot Analysis
2.9. Surface Sensing of Translation (SUnSET) Assay
2.10. Measurement of Myotube Diameter in C2C12 Myotubes
2.11. Statistical Analysis
3. Results
3.1. SNA Increased Muscle Weight and Enhanced Grip Strength in DEX-Administered Mice
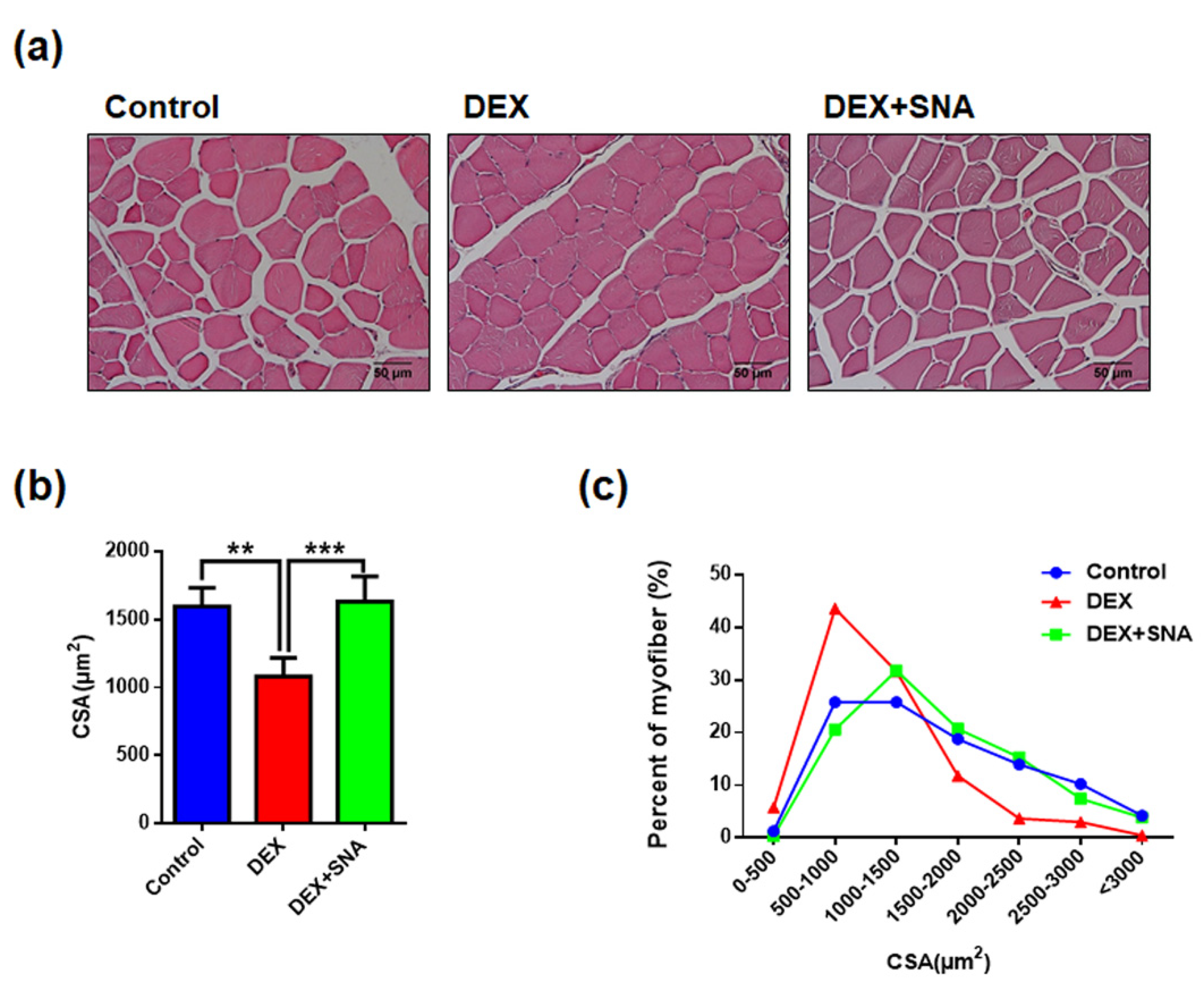
3.2. SNA Increased the Muscle Fiber Size in the DEX-Administered Mice
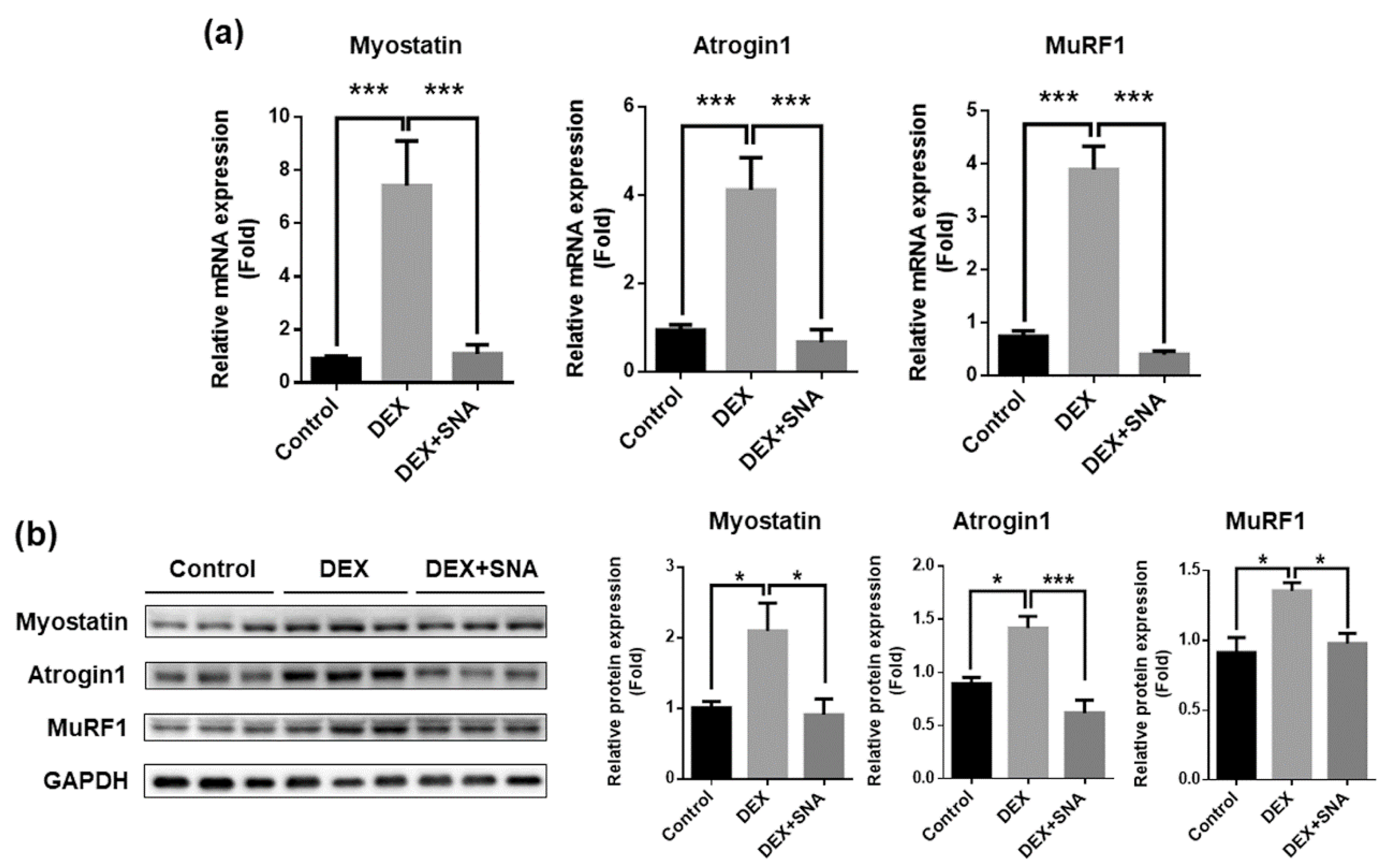
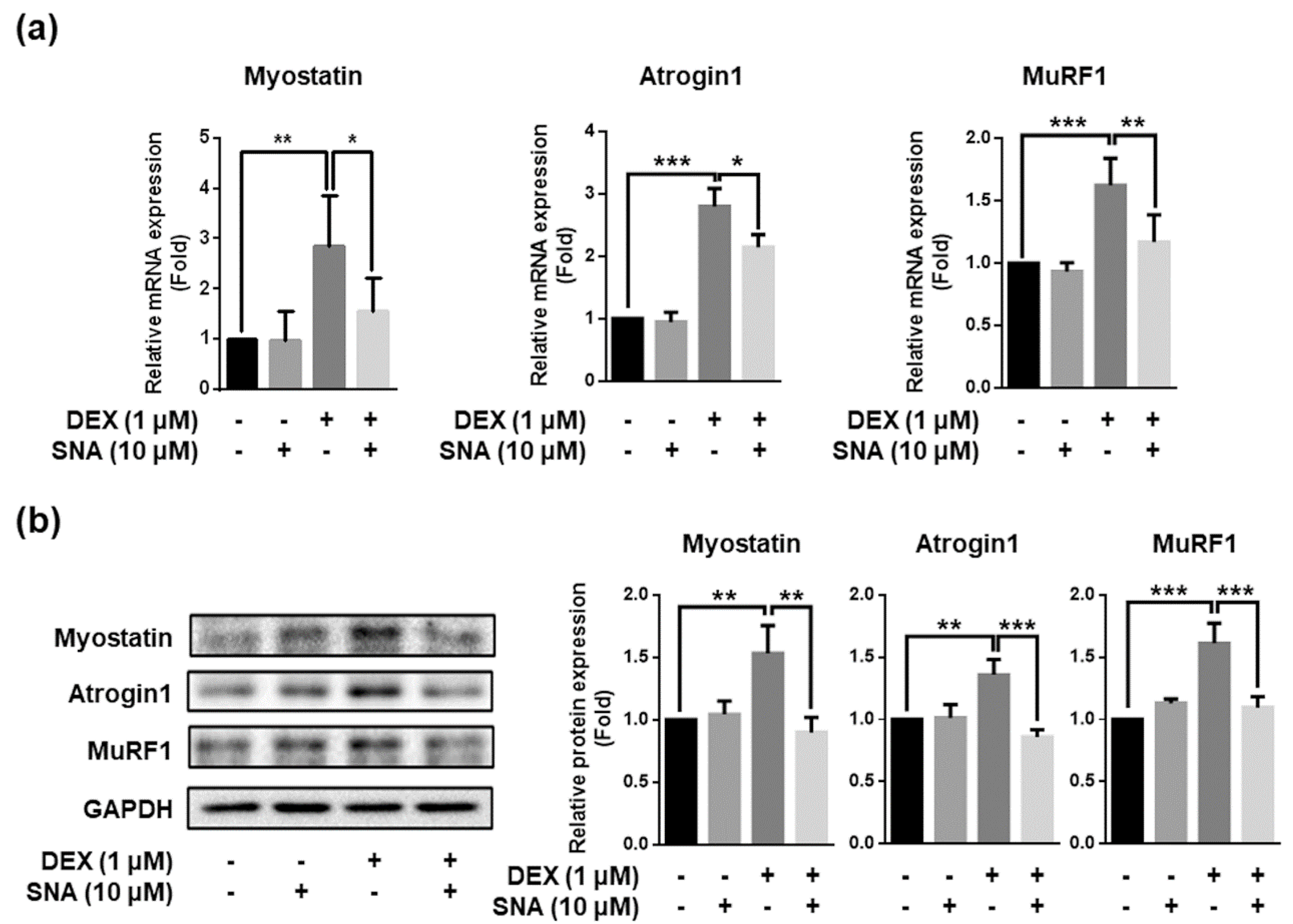
3.3. SNA Inhibited the Expression of Muscle Degradation Factors in the TA Muscles of DEX-Administered Mice
3.4. SNA Decreased the Expression of Muscle Degradation Factors in C2C12 Myotubes
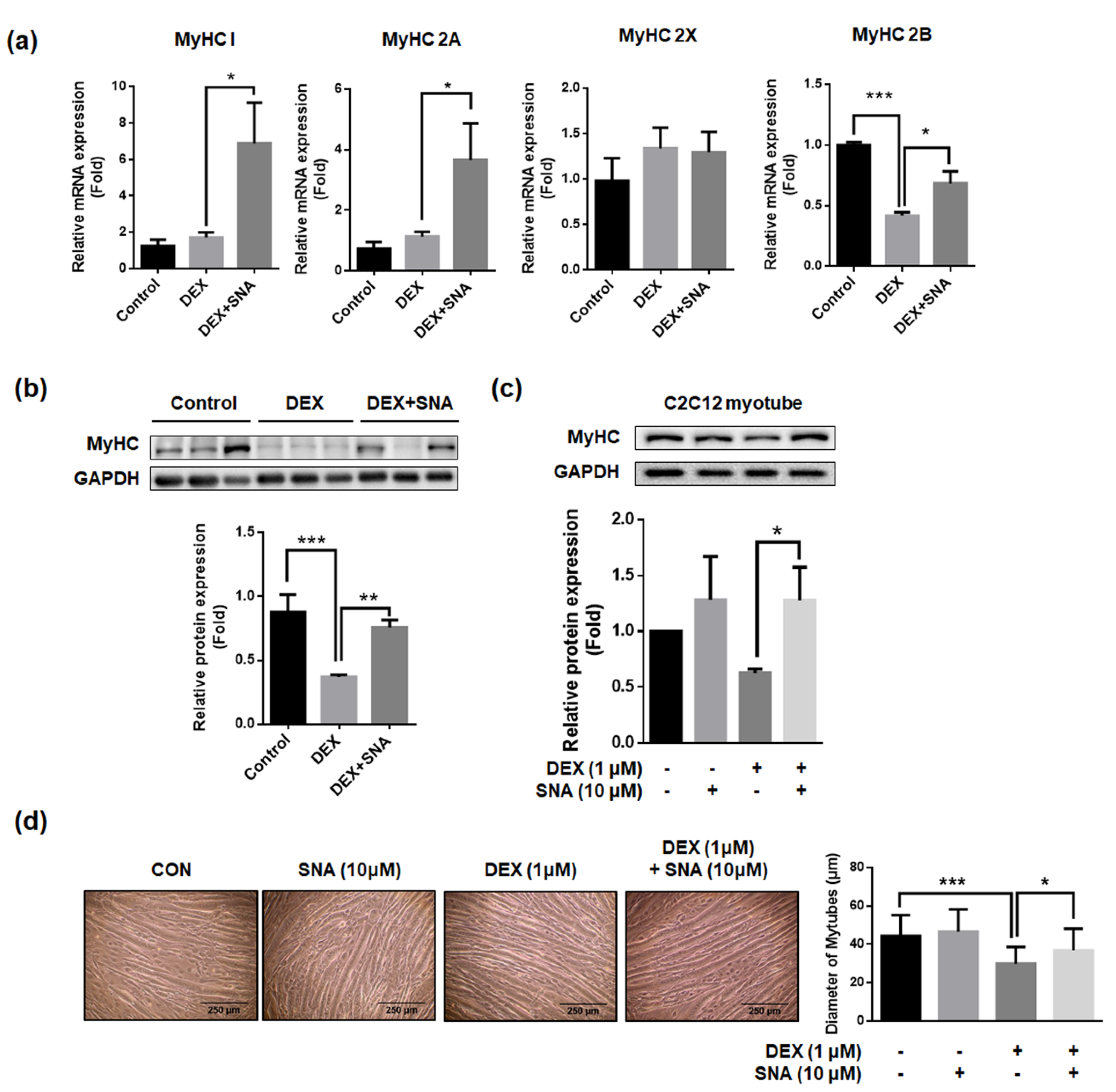
3.5. SNA Increased mRNA and Protein Expression of the Myosin Heavy Chain (MyHC) in DEX-Administered Mice and in C2C12 Myotubes
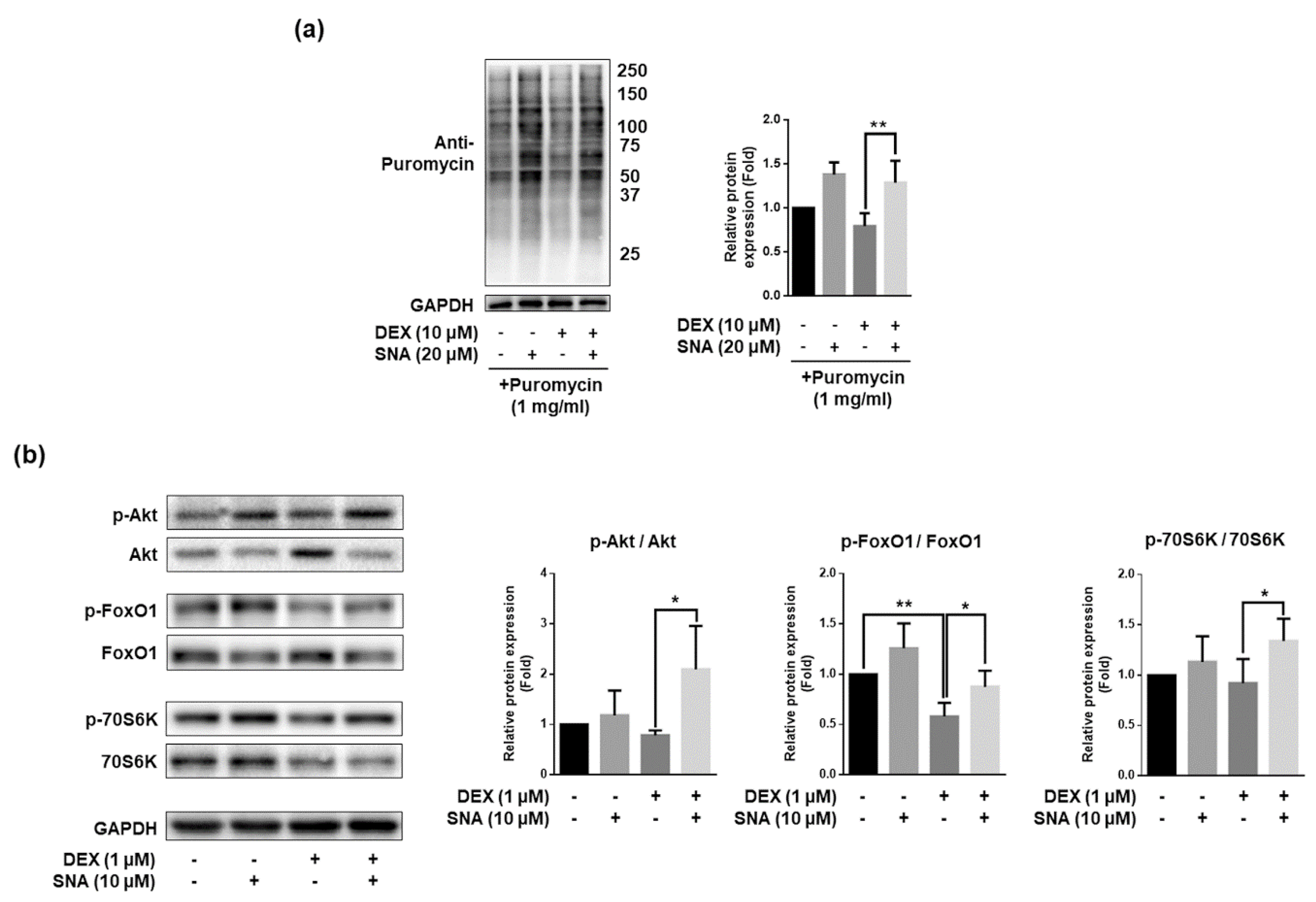
3.6. SNA Increased the Protein Synthesis and Expression of pAkt, pFoxO, and p70S6K in C2C12 Myotubes
4. Discussion
5. Conclusion
Author Contributions
Funding
Conflicts of Interest
Data Availability Statement
References
- Cohen, S.; Nathan, J.A.; Goldberg, A.L. Muscle wasting in disease: Molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 2015, 14, 58–74. [Google Scholar] [CrossRef]
- Fanzani, A.; Conraads, V.M.; Penna, F.; Martinet, W. Molecular and cellular mechanisms of skeletal muscle atrophy: An update. J. Cachexia Sarcopenia Muscle 2012, 3, 163–179. [Google Scholar] [CrossRef]
- Ali, S.; Garcia, J.M. Sarcopenia, cachexia and aging: Diagnosis, mechanisms and therapeutic options—A mini-review. Gerontology 2014, 60, 294–305. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Q.-B.; Zhou, Y.; Chen, S.; Huang, P.-P.; Liu, Y.; Xu, Y.-H. The mechanisms and treatments of muscular pathological changes in immobilization-induced joint contracture: A literature review. Chin. J. Traumatol. 2019, 22, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Malavaki, C.J.; Sakkas, G.K.; Mitrou, G.I.; Kalyva, A.; Stefanidis, I.; Myburgh, K.H.; Karatzaferi, C. Skeletal muscle atrophy: Disease-induced mechanisms may mask disuse atrophy. J. Muscle Res. Cell Motil. 2015, 36, 405–421. [Google Scholar] [CrossRef] [PubMed]
- Brioche, T.; Pagano, A.F.; Py, G.; Chopard, A. Muscle wasting and aging: Experimental models, fatty infiltrations, and prevention. Mol. Asp. Med. 2016, 50, 56–87. [Google Scholar] [CrossRef]
- Hirata, Y.; Nomura, K.; Senga, Y.; Okada, Y.; Kobayashi, K.; Okamoto, S.; Minokoshi, Y.; Imamura, M.; Takeda, S.; Hosooka, T.; et al. Hyperglycemia induces skeletal muscle atrophy via a wwp1/klf15 axis. JCI Insight 2019, 4, e124952. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Ku, S.K.; Han, M.H.; Kim, K.Y.; Kim, S.G.; Kim, G.Y.; Hwang, H.J.; Kim, B.W.; Kim, C.M.; Choi, Y.H. The administration of fructus schisandrae attenuates dexamethasone-induced muscle atrophy in mice. Int. J. Mol. Med. 2015, 36, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.S.; Lo, M.J.; Kau, M.M. Glucocorticoids and aging. J. Med. Assoc. 1997, 96, 792–801. [Google Scholar]
- Yi, C.X.; Foppen, E.; Abplanalp, W.; Gao, Y.; Alkemade, A.; la Fleur, S.E.; Serlie, M.J.; Fliers, E.; Buijs, R.M.; Tschop, M.H.; et al. Glucocorticoid signaling in the arcuate nucleus modulates hepatic insulin sensitivity. Diabetes 2012, 61, 339–345. [Google Scholar] [CrossRef]
- Kuo, T.; Harris, C.A.; Wang, J.C. Metabolic functions of glucocorticoid receptor in skeletal muscle. Mol. Cell. Endocrinol. 2013, 380, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Z.; Ren, S.; Yan, X.T.; Li, H.P.; Li, W.; Zheng, B.; Wang, Z.; Liu, Y.Y. Improvement of cisplatin-induced renal dysfunction by schisandra chinensis stems via anti-inflammation and anti-apoptosis effects. J. Ethnopharmacol. 2018, 217, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Qu, X.N.; Han, Y.; Zheng, S.W.; Wang, J.; Wang, Y.P. Ameliorative effects of 5-hydroxymethyl-2-furfural (5-hmf) from schisandra chinensis on alcoholic liver oxidative injury in mice. Int. J. Mol. Sci. 2015, 16, 2446–2457. [Google Scholar] [CrossRef] [PubMed]
- Bunel, V.; Antoine, M.H.; Nortier, J.; Duez, P.; Stevigny, C. Protective effects of schizandrin and schizandrin b towards cisplatin nephrotoxicity in vitro. J. Appl. Toxicol. 2014, 34, 1311–1319. [Google Scholar] [CrossRef]
- Kim, J.W.; Ku, S.K.; Kim, K.Y.; Kim, S.G.; Han, M.H.; Kim, G.Y.; Hwang, H.J.; Kim, B.W.; Kim, C.M.; Choi, Y.H. Schisandrae fructus supplementation ameliorates sciatic neurectomy-induced muscle atrophy in mice. Oxid. Med. Cell. Longev. 2015, 2015, 872428. [Google Scholar] [CrossRef]
- Cho, S.; Hong, R.; Yim, P.; Yeom, M.; Lee, B.; Yang, W.M.; Hong, J.; Lee, H.S.; Hahm, D.H. An herbal formula consisting of schisandra chinensis (turcz.) baill, lycium chinense mill and eucommia ulmoides oliv alleviates disuse muscle atrophy in rats. J. Ethnopharmacol. 2018, 213, 328–339. [Google Scholar] [CrossRef]
- Szopa, A.; Ekiert, R.; Ekiert, H. Current knowledge of schisandra chinensis (turcz.) baill. (chinese magnolia vine) as a medicinal plant species: A review on the bioactive components, pharmacological properties, analytical and biotechnological studies. Phytochem. Rev. 2017, 16, 195–218. [Google Scholar] [CrossRef]
- Guo, L.Y.; Hung, T.M.; Bae, K.H.; Shin, E.M.; Zhou, H.Y.; Hong, Y.N.; Kang, S.S.; Kim, H.P.; Kim, Y.S. Anti-inflammatory effects of schisandrin isolated from the fruit of schisandra chinensis baill. Eur. J. Pharm. 2008, 591, 293–299. [Google Scholar] [CrossRef]
- Sun, N.; Pan, S.Y.; Zhang, Y.; Wang, X.Y.; Zhu, P.L.; Chu, Z.S.; Yu, Z.L.; Zhou, S.F.; Ko, K.M. Dietary pulp from fructus schisandra chinensis supplementation reduces serum/hepatic lipid and hepatic glucose levels in mice fed a normal or high cholesterol/bile salt diet. Lipids Health Dis. 2014, 13, 46. [Google Scholar] [CrossRef]
- Schmidt, E.K.; Clavarino, G.; Ceppi, M.; Pierre, P. Sunset, a nonradioactive method to monitor protein synthesis. Nat. Methods 2009, 6, 275–277. [Google Scholar] [CrossRef]
- Sun, H.; Gong, Y.; Qiu, J.; Chen, Y.; Ding, F.; Zhao, Q. Traf6 inhibition rescues dexamethasone-induced muscle atrophy. Int. J. Mol. Sci. 2014, 15, 11126–11141. [Google Scholar] [CrossRef]
- Shibaguchi, T.; Hoshi, M.; Yoshihara, T.; Naito, H.; Goto, K.; Yoshioka, T.; Sugiura, T. Impact of different temperature stimuli on the expression of myosin heavy chain isoforms during recovery from bupivacaine-induced muscle injury in rats. J. Appl. Physiol. 2019, 127, 178–189. [Google Scholar] [CrossRef]
- Barany, M. Atpase activity of myosin correlated with speed of muscle shortening. J. Gen. Physiol. 1967, 50 (Suppl. 6), 197–218. [Google Scholar] [CrossRef]
- Han, X.; Pires, L.; Browne, J.D.; Sullivan, C.A.; Zhao, W.; Feng, X. Increased expression of murf1 is associated with radiation-induced laryngeal muscle atrophy. Anticancer Res. 2015, 35, 6049–6056. [Google Scholar] [PubMed]
- Egerman, M.A.; Glass, D.J. Signaling pathways controlling skeletal muscle mass. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 59–68. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G. Pharmacology of schisandra chinensis bail.: An overview of russian research and uses in medicine. J. Ethnopharmacol. 2008, 118, 183–212. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Seo, E.; Yeon, M.; Kim, M.S.; Hur, H.J.; Oh, B.C.; Jun, H.S. Anti-aging effects of schisandrae chinensis fructus extract: Improvement of insulin sensitivity and muscle function in aged mice. Evid. Based Complement. Altern. Med. 2019, 2019, 5642149. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.H.; Minh, N.V.; Lee, S.H.; Lim, S.W.; Lee, Y.M.; Lee, K.S.; Kim, D.K. Deoxyschisandrin inhibits h2o2-induced apoptotic cell death in intestinal epithelial cells through nuclear factor-kappab. Int. J. Mol. Med. 2010, 26, 401–406. [Google Scholar]
- Choi, Y.H. Schisandrin a prevents oxidative stress-induced DNA damage and apoptosis by attenuating ros generation in c2c12 cells. BioMed Pharm. 2018, 106, 902–909. [Google Scholar] [CrossRef]
- Jeong, M.J.; Kim, S.R.; Jung, U.J. Schizandrin a supplementation improves nonalcoholic fatty liver disease in mice fed a high-fat and high-cholesterol diet. Nutr. Res. 2019, 64, 64–71. [Google Scholar] [CrossRef]
- Tu, C.; Huang, X.; Xiao, Y.; Song, M.; Ma, Y.; Yan, J.; You, H.; Wu, H. Schisandrin a inhibits the il-1beta-induced inflammation and cartilage degradation via suppression of mapk and nf-kappab signal pathways in rat chondrocytes. Front. Pharm. 2019, 10, 41. [Google Scholar] [CrossRef]
- Kwon, D.H.; Cha, H.J.; Choi, E.O.; Leem, S.H.; Kim, G.Y.; Moon, S.K.; Chang, Y.C.; Yun, S.J.; Hwang, H.J.; Kim, B.W.; et al. Schisandrin a suppresses lipopolysaccharide-induced inflammation and oxidative stress in raw 264.7 macrophages by suppressing the nf-kappab, mapks and pi3k/akt pathways and activating nrf2/ho-1 signaling. Int. J. Mol. Med. 2018, 41, 264–274. [Google Scholar]
- Zhou, F.; Wang, M.; Ju, J.; Wang, Y.; Liu, Z.; Zhao, X.; Yan, Y.; Yan, S.; Luo, X.; Fang, Y. Schizandrin a protects against cerebral ischemia-reperfusion injury by suppressing inflammation and oxidative stress and regulating the ampk/nrf2 pathway regulation. Am. J. Transl. Res. 2019, 11, 199–209. [Google Scholar] [PubMed]
- Huang, N.; Kny, M.; Riediger, F.; Busch, K.; Schmidt, S.; Luft, F.C.; Slevogt, H.; Fielitz, J. Deletion of nlrp3 protects from inflammation-induced skeletal muscle atrophy. Intensive Care Med. Exp. 2017, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Abrigo, J.; Elorza, A.A.; Riedel, C.A.; Vilos, C.; Simon, F.; Cabrera, D.; Estrada, L.; Cabello-Verrugio, C. Role of oxidative stress as key regulator of muscle wasting during cachexia. Oxid. Med. Cell. Longev. 2018, 2018, 2063179. [Google Scholar] [CrossRef] [PubMed]
- Rhen, T.; Cidlowski, J.A. Antiinflammatory action of glucocorticoids—New mechanisms for old drugs. N. Engl. J. Med. 2005, 353, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Wing, S.S.; Goldberg, A.L. Glucocorticoids activate the atp-ubiquitin-dependent proteolytic system in skeletal muscle during fasting. Am. J. Physiol. 1993, 264, E668–E676. [Google Scholar] [CrossRef] [PubMed]
- Tiao, G.; Fagan, J.; Roegner, V.; Lieberman, M.; Wang, J.J.; Fischer, J.E.; Hasselgren, P.O. Energy-ubiquitin-dependent muscle proteolysis during sepsis in rats is regulated by glucocorticoids. J. Clin. Investig. 1996, 97, 339–348. [Google Scholar] [CrossRef]
- Hall-Angeras, M.; Angeras, U.; Zamir, O.; Hasselgren, P.O.; Fischer, J.E. Effect of the glucocorticoid receptor antagonist ru 38486 on muscle protein breakdown in sepsis. Surgery 1991, 109, 468–473. [Google Scholar]
- Prezant, D.J.; Karwa, M.L.; Richner, B.; Maggiore, D.; Gentry, E.I.; Cahill, J. Gender-specific effects of dexamethasone treatment on rat diaphragm structure and function. J. Appl. Physiol. 1997, 82, 125–133. [Google Scholar] [CrossRef]
- Hong, Y.; Lee, J.H.; Jeong, K.W.; Choi, C.S.; Jun, H.S. Amelioration of muscle wasting by glucagon-like peptide-1 receptor agonist in muscle atrophy. J. Cachexia Sarcopenia Muscle 2019, 10, 903–918. [Google Scholar] [CrossRef] [PubMed]
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Model. Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, L.; Wan, L.; Huo, Y.; Huang, J.; Li, J.; Lu, J.; Xin, B.; Yang, Q.; Guo, C. Matrine improves skeletal muscle atrophy by inhibiting e3 ubiquitin ligases and activating the akt/mtor/foxo3alpha signaling pathway in c2c12 myotubes and mice. Oncol. Rep. 2019, 42, 479–494. [Google Scholar] [PubMed]
- Umeki, D.; Ohnuki, Y.; Mototani, Y.; Shiozawa, K.; Suita, K.; Fujita, T.; Nakamura, Y.; Saeki, Y.; Okumura, S. Protective effects of clenbuterol against dexamethasone-induced masseter muscle atrophy and myosin heavy chain transition. PLoS ONE 2015, 10, e0128263. [Google Scholar] [CrossRef] [PubMed]
- Crossland, H.; Smith, K.; Atherton, P.J.; Wilkinson, D.J. A novel puromycin decorporation method to quantify skeletal muscle protein breakdown: A proof-of-concept study. Biochem. Biophys. Res. Commun. 2017, 494, 608–614. [Google Scholar] [CrossRef]
| No. | Primer | Sense | Anti-Sense |
|---|---|---|---|
| 1 | MuRF1 | AGGACTCCTGCAGAGTGACCAA | TTCTCGTCCAGGATGGCGTA |
| 2 | Atrogin1 | GCAAACACTGCCACATTCTCTC | CTTGAGGGGAAAGTGAGACG |
| 3 | Myostatin | GGCCATGATCTTGCTGTAA | TTGGGTGCGATAATCCAGTC |
| 4 | MyHC * 1 | CCAAGGGCCTGAATGAGGAG | GCAAAGGCTCCAGGTCTGAG |
| 5 | MyHC * 2A | AAGCGAAGAGTAAGGCTGTC | GTGATTGCTTGCAAAGGAAC |
| 6 | MyHC * 2X | CACCGTCTGGATGAGGCTGA | TGTTTGCGCAGACCCTTGATAG |
| 7 | MyHC * 2B | ACAAGCTGCGGGTGAAGAGC | CAGGACAGTGACAAAGAACG |
| 8 | Cyclophilin B | TGGAGAGCACCAAGACAGACA | TGCCGGAGTCGACAATGAT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeon, M.; Choi, H.; Jun, H.-S. Preventive Effects of Schisandrin A, A Bioactive Component of Schisandra chinensis, on Dexamethasone-Induced Muscle Atrophy. Nutrients 2020, 12, 1255. https://doi.org/10.3390/nu12051255
Yeon M, Choi H, Jun H-S. Preventive Effects of Schisandrin A, A Bioactive Component of Schisandra chinensis, on Dexamethasone-Induced Muscle Atrophy. Nutrients. 2020; 12(5):1255. https://doi.org/10.3390/nu12051255
Chicago/Turabian StyleYeon, MyeongHoon, Hojung Choi, and Hee-Sook Jun. 2020. "Preventive Effects of Schisandrin A, A Bioactive Component of Schisandra chinensis, on Dexamethasone-Induced Muscle Atrophy" Nutrients 12, no. 5: 1255. https://doi.org/10.3390/nu12051255
APA StyleYeon, M., Choi, H., & Jun, H.-S. (2020). Preventive Effects of Schisandrin A, A Bioactive Component of Schisandra chinensis, on Dexamethasone-Induced Muscle Atrophy. Nutrients, 12(5), 1255. https://doi.org/10.3390/nu12051255