Anserine Reverses Exercise-Induced Oxidative Stress and Preserves Cellular Homeostasis in Healthy Men
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
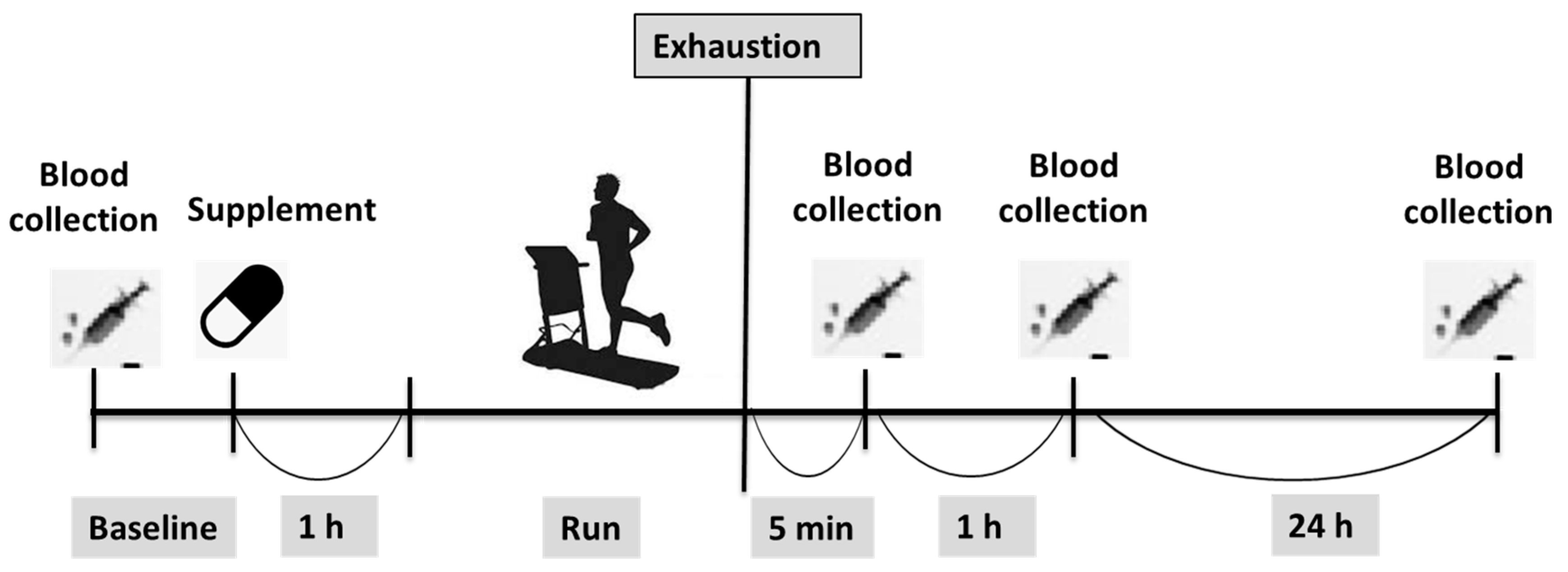
2.2. Procedures and Protocol
2.3. Baseline Assessment
2.4. TTE
2.5. Supplementation Protocol
2.6. Blood Sampling and Biochemistry
2.7. Data Analysis and Statistics
3. Results
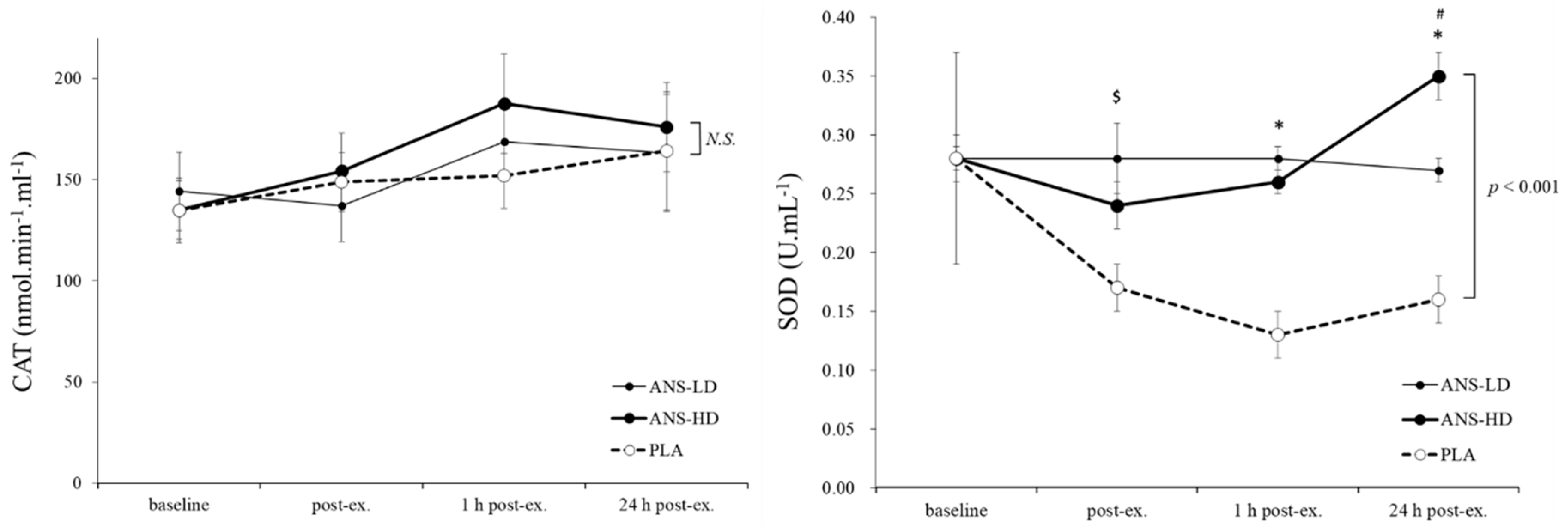
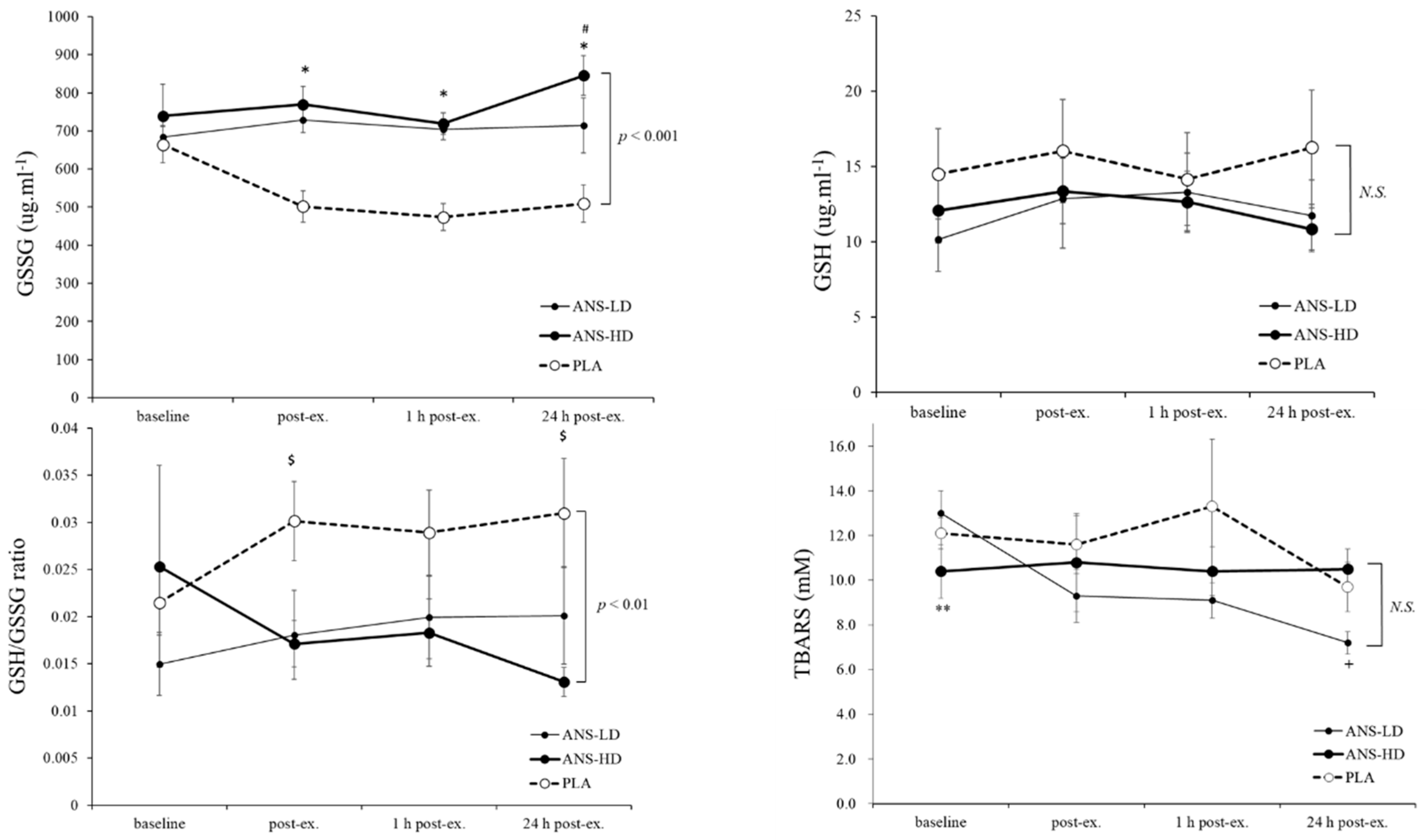
3.1. Antioxidatants Response to Anserine Supplementation
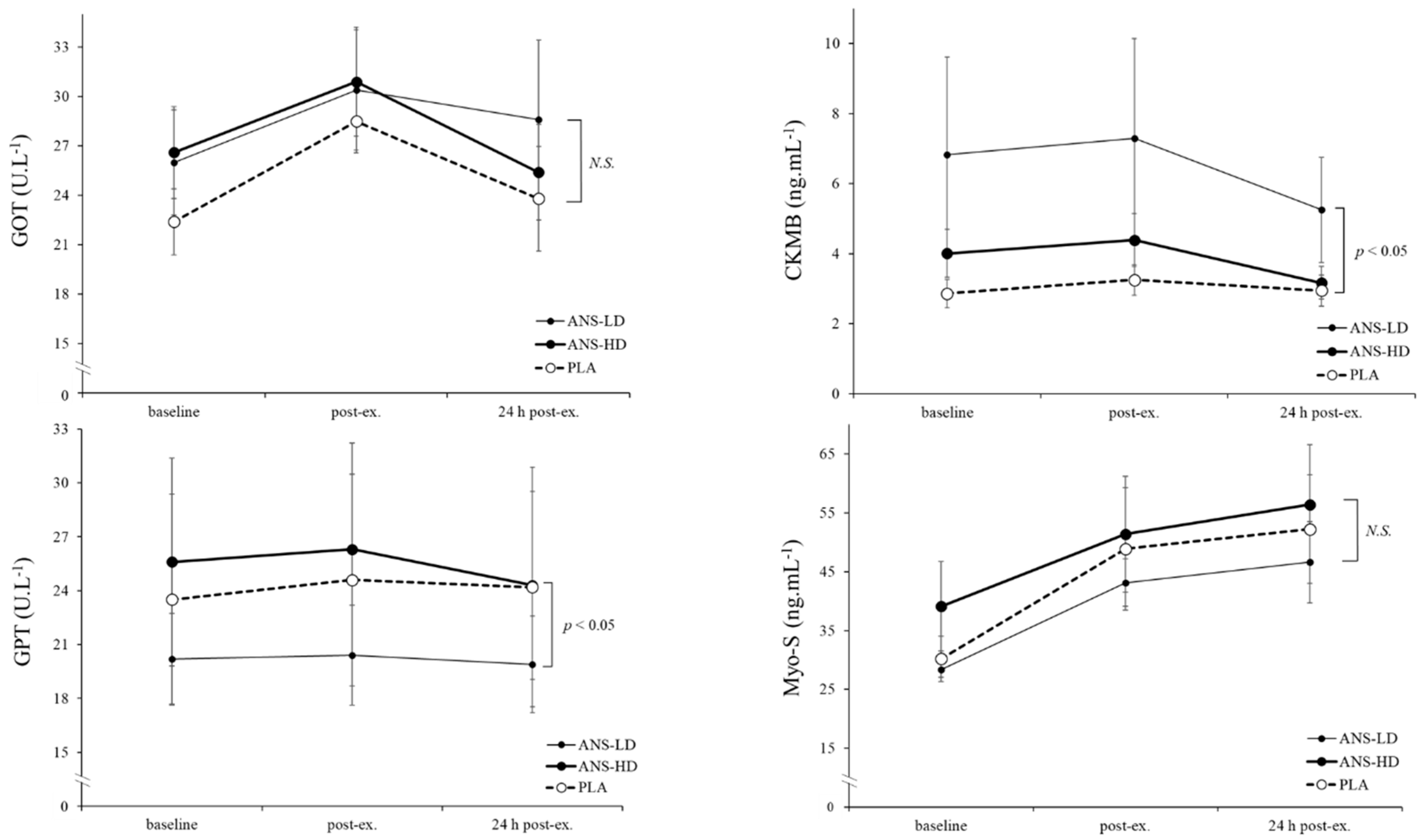
3.2. Cell Damage Biomarkers
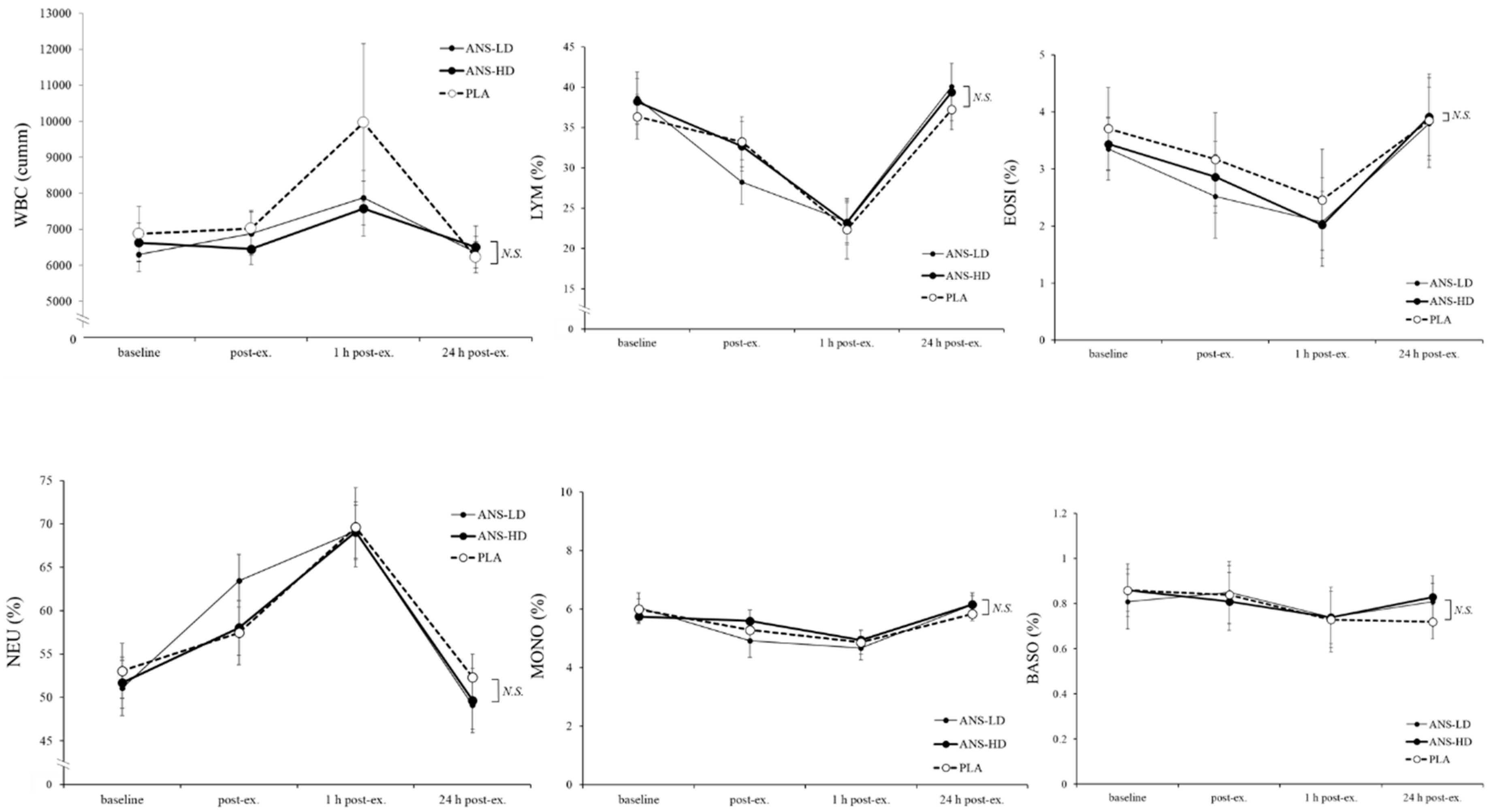
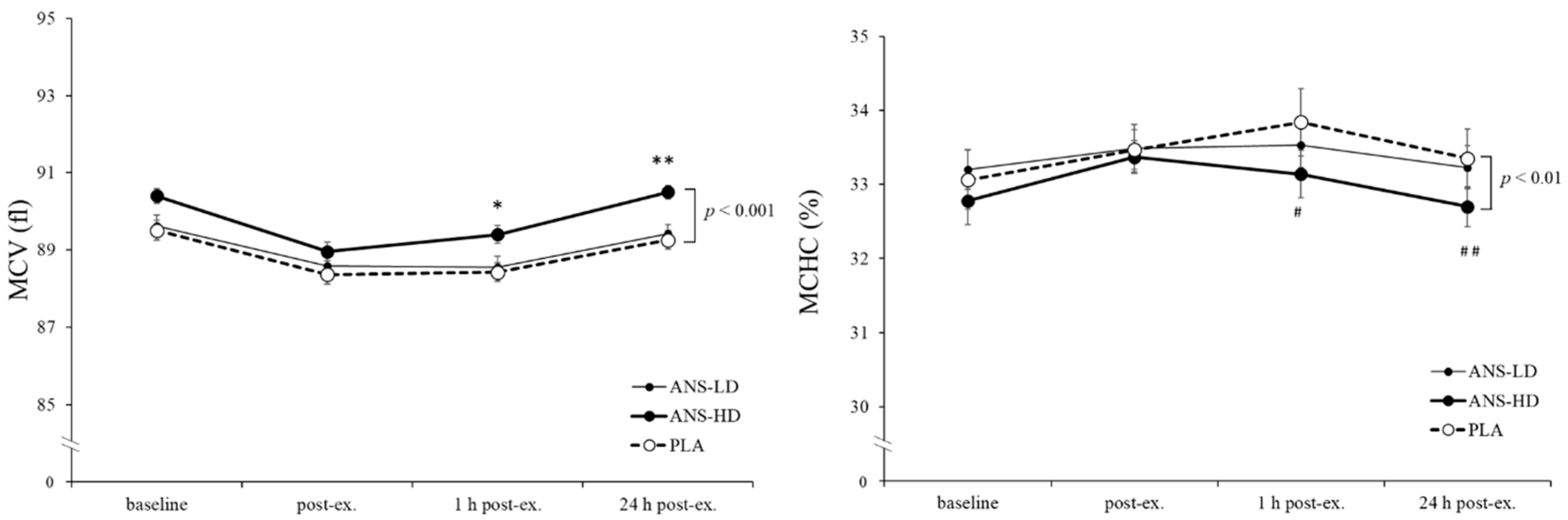
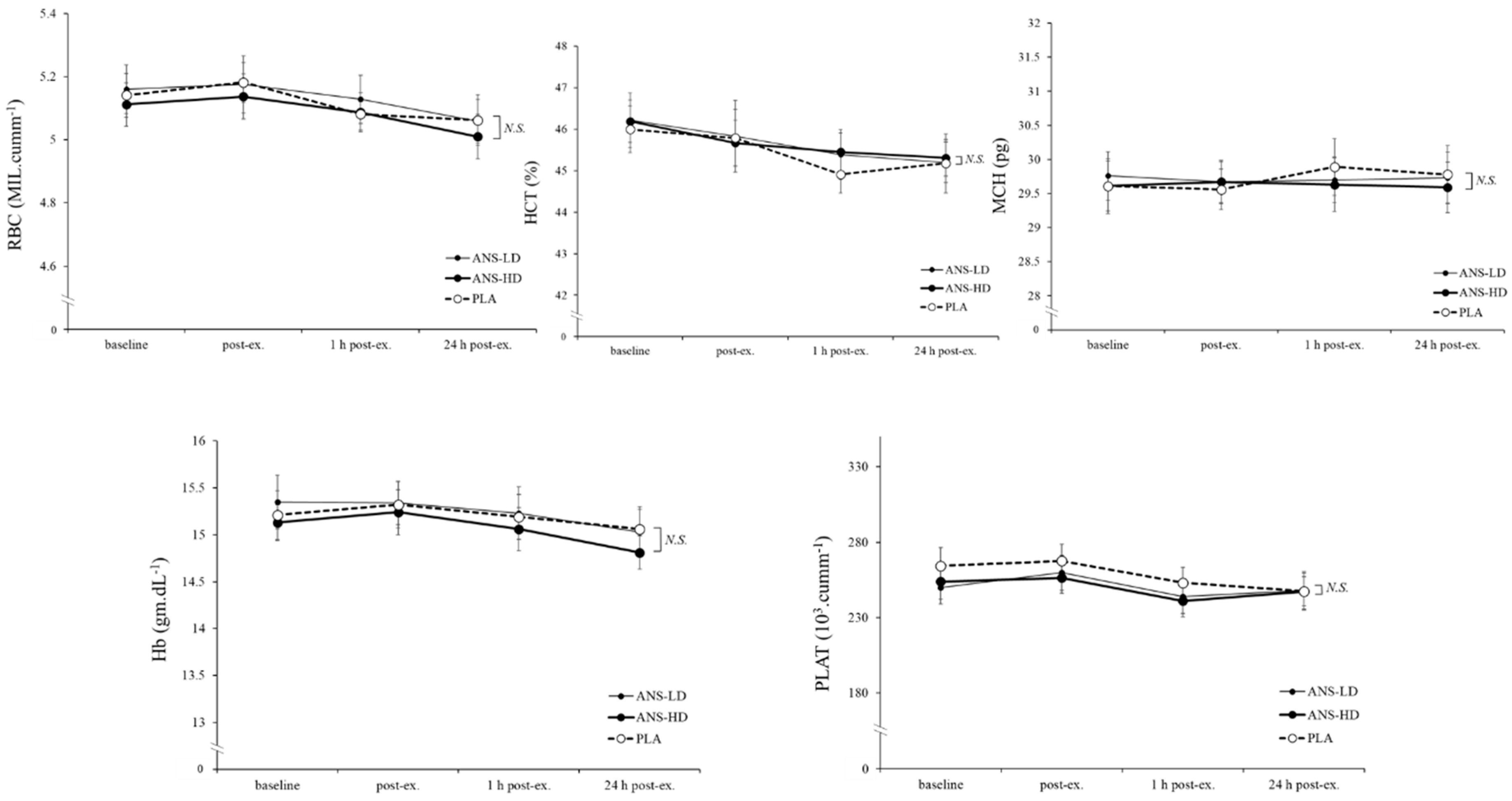
3.3. Blood Haematology Biomarkers
3.4. Anserine Effects on Exercise Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Y.F.; He, R.R.; Tsoi, B.; Kurihara, H. Bioactivities of chicken essence. J. Food Sci. 2012, 77, R105–R110. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, A.A.; Sergei, S.E. The histidine-containing dipeptides, carnosine and anserine: Distribution, properties and biological significance. Adv. Enzym. Regul. 1990, 30, 175–194. [Google Scholar] [CrossRef]
- Wu, H.C.; Shiau, C.Y.; Chen, H.M.; Chiou, T.K. Antioxidant activities of carnosine, anserine, some free amino acids and their combination. J. Food Drug Anal. 2003, 11, 148–153. [Google Scholar]
- Kim, S.K.; Kim, Y.; Baek, I.K.; Auh, J.H. Carnosine and anserine in chicken: Distribution, age-dependency and their anti-glycation activity. Korean J. Food Sci. Anim. Resour. 2012, 32, 45–48. [Google Scholar] [CrossRef]
- Katakura, Y.; Totsuka, M.; Imabayashi, E.; Matsuda, H.; Hisatsune, T. Anserine/Carnosine Supplementation Suppresses the Expression of the Inflammatory Chemokine CCL24 in Peripheral Blood Mononuclear Cells from Elderly People. Nutrients 2017, 9, 1199. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Lin, C.I.; Chiu, C.C.; Lin, Y.T.; Huang, W.K.; Huang, H.Y.; Huang, C.C. Chicken essence improves exercise performance and ameliorates physical fatigue. Nutrients 2014, 6, 2681–2696. [Google Scholar] [CrossRef]
- Huang, S.W.; Hsu, Y.J.; Lee, M.C.; Li, H.S.; Yeo, P.C.W.; Lim, A.L.; Huang, C.C. In Vitro and In Vivo Functional Characterization of Essence of Chicken as an Ergogenic Aid. Nutrients 2018, 10, 1943. [Google Scholar] [CrossRef]
- Aydin, A.F.; Bingul, I.; Kucukgergin, C.; Dogan-Ekici, I.; Dogru Abbasoglu, S.; Uysal, M. Carnosine decreased oxidation and glycation products in serum and liver of high-fat diet and low-dose streptozotocin-induced diabetic rats. Int. J. Exp. Pathol. 2017, 98, 278–288. [Google Scholar] [CrossRef]
- Everaert, I.; Baron, G.; Barbaresi, S.; Gilardoni, E.; Coppa, C.; Carini, M.; Vistoli, G.; Bex, T.; Stautemas, J.; Blancquaert, L.; et al. Development and validation of a sensitive LC–MS/MS assay for the quantification of anserine in human plasma and urine and its application to pharmacokinetic study. Amino Acids 2019, 51, 103–114. [Google Scholar] [CrossRef]
- Chou, M.Y.; Chen, Y.J.; Lin, L.H.; Nakao, Y.; Lim, A.L.; Wang, M.F.; Yong, S.M. Protective Effects of Hydrolyzed Chicken Extract (Probeptigen®/Cmi-168) on Memory Retention and Brain Oxidative Stress in Senescence-Accelerated Mice. Nutrients 2019, 11, 1870. [Google Scholar] [CrossRef]
- Wu, J.F.; Saovieng, S.; Cheng, I.S.; Jensen, J.; Jean, W.H.; Alkhatib, A.; Kao, C.L.; Huang, C.Y.; Kuo, C.H. Satellite cells depletion in exercising human skeletal muscle is restored by ginseng component Rg1 supplementation. J. Funct. Foods 2019, 58, 27–33. [Google Scholar] [CrossRef]
- Byrne, C.; Twist, C.; Eston, R. Neuromuscular function after exercise-induced muscle damage: Theoretical and applied implications. Sports Med. 2004, 34, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chou, W.Y.; Ko, J.Y.; Lee, M.S.; Wu, R.W. Early Recovery of Exercise-Related Muscular Injury by HBOT. Biomed. Res. Int. 2019, 6289380. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Kwak, Y.S. Changes of cardiac biomarkers after ultradistance and standard-distance triathlon. J. Exerc. Rehab. 2019, 15, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Varamenti, E.; Nikolovski, Z.; Elgingo, M.I.; Jamurtas, A.Z.; Cardinale, M. Training-Induced Variations in Haematological and Biochemical Variables in Adolescent Athletes of Arab Origin throughout an Entire Athletic Season. J. Hum. Kinet. 2018, 64, 123–135. [Google Scholar] [CrossRef]
- Goto, K.; Maemura, H.; Takamatsu, K.; Ishii, N. Hormonal responses to resistance exercise after ingestion of carnosine and anserine. J. Strength Cond. Res. 2011, 25, 398–405. [Google Scholar] [CrossRef]
- Bruce, R.A.; Kusumi, F.; Hosmer, D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am. Heart J. 1973, 85, 546–562. [Google Scholar] [CrossRef]
- Peters, V.; Calabrese, V.; Forsberg, E.; Volk, N.; Fleming, T.; Baelde, H.; Weigand, T.; Thiel, C.; Trovato, A.; Scuto, M.; et al. Protective Actions of Anserine Under Diabetic Conditions. Int. J. Mol. Sci. 2018, 19, 2751. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Centner, C.; Zdzieblik, D.; Dressler, P.; Fink, B.; Gollhofer, A.; König, D. Acute effects of blood flow restriction on exercise-induced free radical production in young and healthy subjects. Free Radic. Res. 2018, 52, 446–454. [Google Scholar] [CrossRef]
- Ebbeling, C.B.; Clarkson, P.M. Exercise-induced muscle damage and adaptation. Sports Med. 1989, 7, 207–234. [Google Scholar] [CrossRef] [PubMed]
- Yimcharoen, M.; Kittikunnathum, S.; Suknikorn, C.; Nak-On, W.; Yeethong, P.; Anthony, T.G.; Bunpo, P. Effects of ascorbic acid supplementation on oxidative stress markers in healthy women following a single bout of exercise. J. Int. Soc. Sports Nutr. 2019, 16, 2. [Google Scholar] [CrossRef]
- Mashiko, T.; Umeda, T.; Nakaji, S.; Sugawara, K. Effects of exercise on the physical condition of college rugby players during summer training camp. Br. J. Sports Med. 2004, 38, 186–190. [Google Scholar] [CrossRef]
- Chen, K.; Xie, K.; Liu, Z.; Nakasone, Y.; Sakao, K.; Hossain, A.; Hou, D.X. Preventive Effects and Mechanisms of Garlic on Dyslipidemia and Gut Microbiome Dysbiosis. Nutrients 2019, 11, 1225. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.; Sobrino, T.; Ramos-Cabrer, P.; Castellanos, M.; Blanco, M.; Rodríguez-Yáñez, M.; Serena, J.; Leira, R.; Castillo, J. High blood glutamate oxaloacetate transaminase levels are associated with good functional outcome in acute ischemic stroke. J. Cereb. Blood Flow Metab. 2011, 31, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Jastrzebska, M.; Kaczmarczyk, M.; Suárez, A.D.; Sánchez, G.F.L.; Jastrzebska, J.; Radziminski, L.; Jastrzebski, Z. Iron, Hematological Parameters and Blood Plasma Lipid Profile in Vitamin D Supplemented and Non-Supplemented Young Soccer Players Subjected to High-Intensity Interval Training. J. Nutr. Sci. Vitaminol. 2017, 63, 357–364. [Google Scholar] [CrossRef]
- Mairbäurl, H. Red blood cells in sports: Effects of exercise and training on oxygen supply by red blood cells. Front. Physiol. 2013, 4, 332. [Google Scholar] [CrossRef] [PubMed]
- Finkler, M.; Hochman, A.; Pinchuk, I.; Lichtenberg, D. In Healthy Young Men, a Short Exhaustive Exercise Alters the Oxidative Stress Only Slightly, Independent of the Actual Fitness. Oxid. Med. Cell. Longev. 2016, 9107210. [Google Scholar] [CrossRef]
- Apicella, J.M.; Lee, E.C.; Bailey, B.L.; Saenz, C.; Anderson, J.M.; Craig, S.A.; Kraemer, W.J.; Volek, J.S.; Maresh, C.M. Betaine supplementation enhances anabolic endocrine and Akt signaling in response to acute bouts of exercise. Eur. J. Appl. Physiol. 2013, 113, 793–802. [Google Scholar] [CrossRef]
- Hyldahl, R.D.; Chen, T.C.; Nosaka, K. Mechanisms and Mediators of the Skeletal Muscle Repeated Bout Effect. Exerc. Sport Sci. Rev. 2017, 45, 24–33. [Google Scholar] [CrossRef]
| Blood Biomarkers | Area Under the Curve (AUC) (mean ± SEM) | p-Values (Main ANOVA Effect) | p-Values (Post Hoc) | ||||
|---|---|---|---|---|---|---|---|
| PLA | ANS-LD | ANS-HD | PLA vs. ANS-LD | PLA vs. ANS-HD | ANS-LD vs. ANS-HD | ||
| CAT (nmol·min−1·mL−1) | 900.8 ± 80.3 | 919.5 ± 67.4 | 994.7 ± 87.6 | 0.21 | 0.43 | 0.22 | 0.14 |
| SOD (U/mL) | 1.0 ± 0.1 | 1.7 ± 0.1 | 1.6 ± 0.1 | 0.12 | <0.01 | <0.01 | 0.31 |
| GSH (ug/mL) | 91.2 ± 19.2 | 74.2 ± 11.4 | 74.9 ± 8.8 | <0.05 | 0.17 | 0.14 | 0.46 |
| GSSG (ug/mL) | 3123.8 ± 213.6 | 4262.1 ± 122.1 | 4561.2 ± 175.7 | 0.61 | <0.01 | <0.01 | 0.08 |
| GSH/GSSG Ratio | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.86 | <0.01 | <0.01 | 0.45 |
| TBARS (μM) | 71.6 ± 7.9 | 57.2 ± 3.7 | 63.3 ± 5.6 | 0.08 | 0.055 | 0.14 | 0.07 |
| GOT (U/L) | 103.2 ± 8.7 | 115.4 ± 14.5 | 113.8 ± 12.1 | 0.05 | 0.20 | 0.06 | 0.45 |
| GPT (U/L) | 96.9 ± 24.3 | 80.9 ± 10.7 | 102.5 ± 22.8 | <0.05 | 0.15 | 0.15 | 0.06 |
| CKMB (ng/mL) | 12.3 ± 1.6 | 12.7 ± 2.4 | 16.0 ± 2.5 | 0.08 | 0.38 | 0.057 | 0.12 |
| Myo-S (ng/mL) | 232.5 ± 38.1 | 227.6 ± 23.1 | 254.8 ± 46.3 | 0.39 | 0.42 | 0.26 | 0.25 |
| WBC (cumm) | 47075 ± 5060.2 | 42177 ± 2863.6 | 41210 ± 2478.7 | 0.08 | 0.09 | 0.11 | 0.33 |
| LYM (%) | 184.7 ± 16.1 | 181.9 ± 14.2 | 189.4 ± 15.3 | 0.07 | 0.32 | 0.35 | 0.22 |
| EOSI (%) | 18.8 ± 4.9 | 16.3 ± 4.1 | 17.1 ± 3.4 | 0.93 | 0.14 | 0.21 | 0.35 |
| NEU (%) | 359.6 ± 19.8 | 365.6 ± 14.7 | 355.6 ± 16.6 | 0.21 | 0.23 | 0.38 | 0.18 |
| MONO (%) | 32.2 ± 2.0 | 31.4 ± 2.5 | 33.0 ± 1.7 | <0.01 | 0.36 | 0.14 | 0.19 |
| BASO (%) | 4.7 ± 0.7 | 4.8 ± 0.7 | 4.8 ± 0.6 | 0.85 | 0.36 | 0.37 | 0.48 |
| MCV (fl) | 532.3 ± 4.2 | 533.3 ± 4.0 | 537.6 ± 4.3 | 0.17 | 0.31 | <0.05 | <0.01 |
| MCHC (%) | 201.0 ± 2.1 | 200.4 ± 1.5 | 198.5 ± 1.4 | 0.12 | 0.29 | <0.01 | <0.01 |
| RBC (MIL/cumm) | 30.7 ± 0.3 | 30.8 ± 0.5 | 30.6 ± 0.4 | 0.65 | 0.35 | 0.21 | 0.16 |
| HCT (%) | 272.6 ± 3.1 | 273.9 ± 3.9 | 273.7 ± 2.7 | 0.60 | 0.30 | 0.26 | 0.47 |
| MCH (pg) | 178.3 ± 2.2 | 178.2 ± 1.9 | 177.8 ± 2.0 | 0.71 | 0.45 | 0.14 | 0.15 |
| Hb (g/dL) | 91.3 ± 1.4 | 91.5 ± 1.5 | 90.5 ± 1.3 | 0.39 | 0.38 | 0.08 | 0.09 |
| PLAT (103/cumm) | 1553.3 ± 62.0 | 1505.4 ± 67.3 | 1496.3 ± 62.2 | 0.35 | 0.24 | 0.12 | 0.42 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhatib, A.; Feng, W.-H.; Huang, Y.-J.; Kuo, C.-H.; Hou, C.-W. Anserine Reverses Exercise-Induced Oxidative Stress and Preserves Cellular Homeostasis in Healthy Men. Nutrients 2020, 12, 1146. https://doi.org/10.3390/nu12041146
Alkhatib A, Feng W-H, Huang Y-J, Kuo C-H, Hou C-W. Anserine Reverses Exercise-Induced Oxidative Stress and Preserves Cellular Homeostasis in Healthy Men. Nutrients. 2020; 12(4):1146. https://doi.org/10.3390/nu12041146
Chicago/Turabian StyleAlkhatib, Ahmad, Wen-Hsin Feng, Yi-Jen Huang, Chia-Hua Kuo, and Chien-Wen Hou. 2020. "Anserine Reverses Exercise-Induced Oxidative Stress and Preserves Cellular Homeostasis in Healthy Men" Nutrients 12, no. 4: 1146. https://doi.org/10.3390/nu12041146
APA StyleAlkhatib, A., Feng, W.-H., Huang, Y.-J., Kuo, C.-H., & Hou, C.-W. (2020). Anserine Reverses Exercise-Induced Oxidative Stress and Preserves Cellular Homeostasis in Healthy Men. Nutrients, 12(4), 1146. https://doi.org/10.3390/nu12041146







