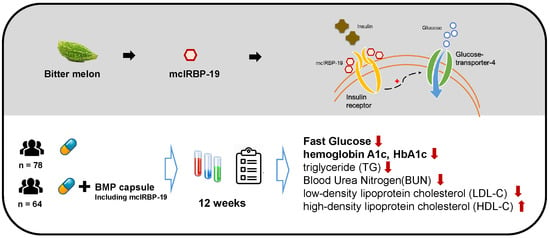
mcIRBP-19 of Bitter Melon Peptide Effectively Regulates Diabetes Mellitus (DM) Patients’ Blood Sugar Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Subject
2.2. Intervention Material
2.3. Measurement
3. Results
3.1. Demographic Characteristics and Concomitant Medication
3.2. BMP Intervention
4. Discussion
5. Conclusions
6. Limitations of the Study
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Human Rights Statements and Informed Consent
Appendix A
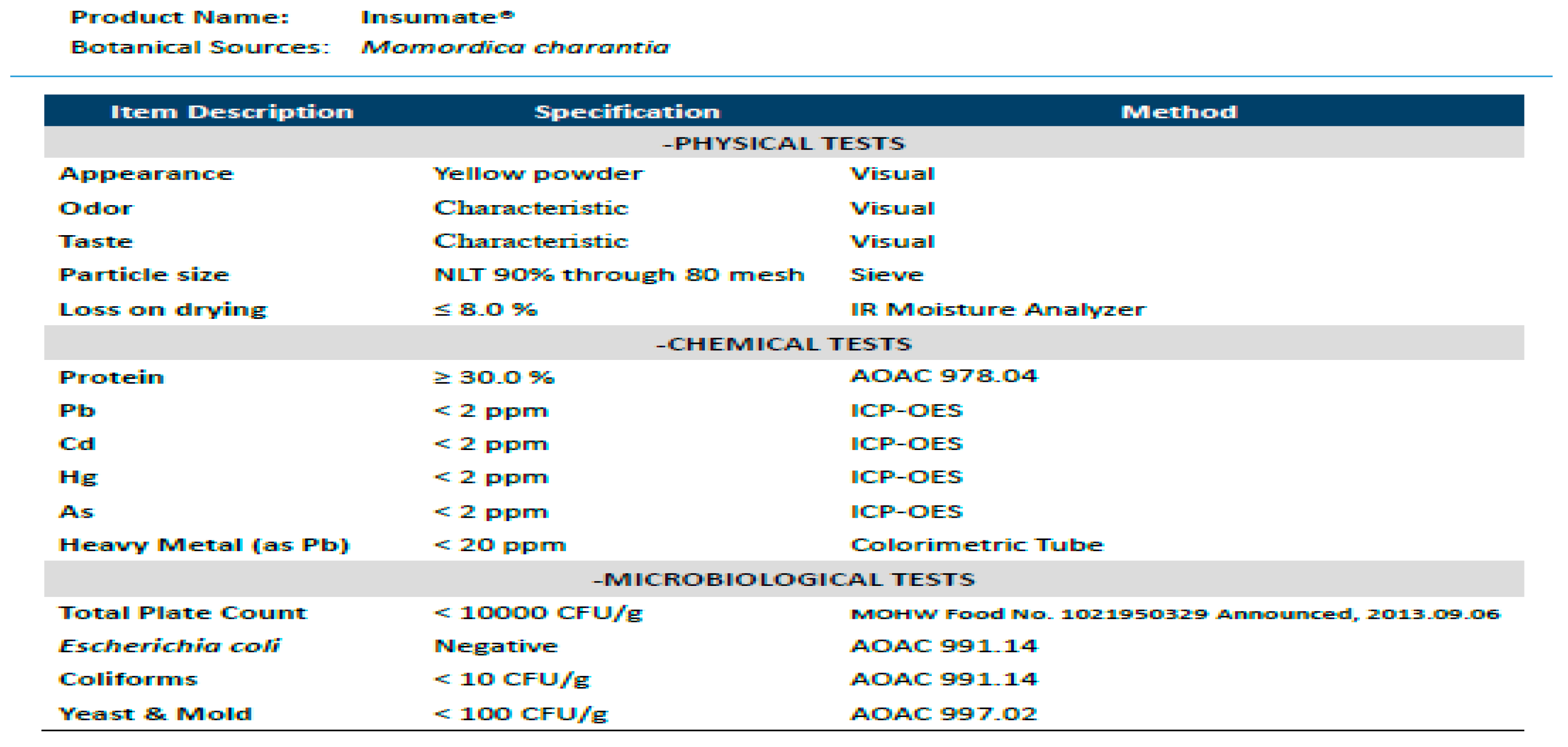
| Nutritional Facts per 100 g | ||
|---|---|---|
| Calories | Kcal/100 g | 359.2 |
| Protein | g/100 g | 31.1 |
| Total Fat | g/100 g | N. D. |
| Saturated Fat | g/100 g | N. D. |
| Trans Fat | g/100 g | N. D. |
| Total Carbohydrate | g/100 g | 58.7 |
| Sugar | g/100 g | N. D. |
| Water | g/100 g | 6.7 |
| Sodium | mg/100 g | 4.8 |
References
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Lauritzen, T.; Bojesen, A.; Christiansen, J.S. Multiple mealtime administration of biphasic insulin as part 30 versus traditional basal-bolus human insulin treatment in patients with type 1 diabetes. Diabetes Obes. Metab. 2006, 8, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Avilés-Santa, L.; Sinding, J.; Raskin, P. Effects of metformin in patients with poorly controlled, insulin-treated type 2 diabetes mellitus. A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 1999, 131, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Nakar, S.; Yitzhaki, G.; Rosenberg, R.; Vinker, S. Transition to insulin in Type 2 diabetes: Family physicians’ misconception of patients’ fears contributes to existing barriers. J. Diabetes Complicat. 2007, 21, 220–226. [Google Scholar] [CrossRef]
- Polonsky, W.H.; Fisher, L.; Guzman, S.; Villa-Caballero, L.; Edelman, S.V. Psychological insulin resistance in patients with type 2 diabetes: The scope of the problem. Diabetes Care 2005, 28, 2543–2545. [Google Scholar] [CrossRef]
- Khanna, P.; Jain, S.C.; Panagariya, A.; Dixit, V.P. Hypoglycemic activity of polypeptide-p from a plant source. J. Nat. Prod. 1981, 44, 648–655. [Google Scholar] [CrossRef]
- Grover, J.K.; Vats, V.; Rathi, S.S.; Dawar, R. Traditional Indian anti-diabetic attenuate progression of renal damage in streptozotocin induced diabetic mice. J. Ethnopharmacol. 2001, 76, 233–238. [Google Scholar] [CrossRef]
- Grover, J.K.; Yadav, S.; Vats, V. Medicinal plants of India with anti-diabetic potential. J. Ethnopharmacol. 2002, 81, 81–100. [Google Scholar] [CrossRef]
- Rathi, S.S.; Grover, J.K.; Vikrant, V.; Biswas, N.R. Prevention of experimental diabetic cataract by Indian Ayurvedic plant extracts. Phytother. Res. 2002, 16, 774–777. [Google Scholar] [CrossRef]
- Lo, H.Y.; Ho, T.Y.; Lin, C.; Li, C.C.; Hsiang, C.Y. Momordica charantia and its novel polypeptide regulate glucose homeostasis in mice via binding to insulin receptor. J. Agric. Food Chem. 2013, 61, 2461–2468. [Google Scholar] [CrossRef]
- Lo, H.Y.; Ho, T.Y.; Li, C.C.; Chen, J.C.; Liu, J.J.; Hsiang, C.Y. A novel insulin receptor-binding protein from Momordica charantia enhances glucose uptake and glucose clearance in vitro and in vivo through triggering insulin receptor signaling pathway. J. Agric. Food Chem. 2014, 62, 8952–8961. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.Y.; Li, C.C.; Ho, T.Y.; Hsiang, C.Y. Identification of the bioactive and consensus peptide motif from Momordica charantia insulin receptor-binding protein. Food Chem. 2016, 204, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, J. The Universal Declaration of Ethical Principles for Psychologists: Third Draft; International Union of Psychological Science, International Association of Applied Psychology, International Association for Cross-Cultural Psychology: Stockholm, Sweden, 2009. [Google Scholar]
- Tan, S.P.; Kha, T.C.; Parks, S.E.; Roach, P.D. Bitter melon (Momordica charantia L.) bioactive composition and health benefits: A review. Food Rev. Int. 2016, 32, 181–202. [Google Scholar] [CrossRef]
- Bhushan, M.S.; Rao, C.H.V.; Ojha, S.K.; Vijayakumar, M.; Verma, A. An analytical review of plants for anti-diabetic activity with their phytoconstituent and mechanism of action. Int. J. Pharm. Sci. Res. 2010, 1, 29–46. [Google Scholar]
- Chaturvedi, P. Antidiabetic potentials of Momordica charantia: Multiple mechanisms behind the effects. J. Med. Food 2012, 15, 101–107. [Google Scholar] [CrossRef]
- Joseph, B.; Jini, D. Antidiabetic effects of Momordica charantia (bitter melon) and its medicinal potency. Asian Pac. J. Trop. Dis. 2013, 3, 93–102. [Google Scholar] [CrossRef]
- Cummings, E.; Hundal, H.S.; Wackerhage, H.; Hope, M.; Belle, M.; Adeghate, E. Momordica charantia fruit juice stimulates glucose and amino acid uptakes in L6 myotubes. Mol. Cell. Biochem. 2004, 261, 99–104. [Google Scholar] [CrossRef]
- Després, J.P.; Moorjani, S.; Tremblay, A.; Ferland, M.; Lupien, P.J.; Nadeau, A.; Bouchard, C. Relation of high plasma triglyceride levels associated with obesity and regional adipose tissue distribution to plasma lipoprotein-lipid composition in premenopausal women. Clin. Investig. Med. 1989, 12, 374–380. [Google Scholar]
- Bao, B.; Chen, Y.G.; Zhang, L.; Xu, Y.L.N.; Wang, X.; Liu, J.; Qu, W. Momordica charantia (bitter melon) reduces obesity-associated macrophage and mast cell infiltration as well as inflammatory cytokine expression in adipose tissues. PLoS ONE 2013, 8, e84075. [Google Scholar] [CrossRef]
- Yu, Y.C.; Zhang, X.H.; Ebersole, B.; Ribnicky, D.; Wang, Z. Bitter melon extract attenuating hepatic steatosis may be mediated by FGF21 and AMPK/Sirt1 signaling in mice. Sci. Rep. 2013, 3, 3142. [Google Scholar] [CrossRef]
- Goldberg, I.J. Diabetic dyslipidemia: Causes and consequences. JCEM 2001, 86, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Vergès, B. Pathophysiology of diabetic dyslipidaemia: Where are we? Diabetologia 2015, 58, 886–899. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Bowe, B.; Li, T.; Xian, H.; Yan, Y.; Al-Aly, Z. Higher blood urea nitrogen is associated with increased risk of incident diabetes mellitus. Kidney Int. 2018, 93, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Stanton, R.C. Oxidative stress and diabetic kidney disease. Curr. Diabetes Rep. 2011, 11, 330–336. [Google Scholar] [CrossRef] [PubMed]
| Indices | Experiment n = 64 | Control n = 78 | pa | ||
|---|---|---|---|---|---|
| Mean | s.d. b | Mean | s.d. | ||
| Age (years) | 62.9 | 12.1 | 59.3 | 11.9 | 0.083 |
| Height (cm) | 163.5 | 6.4 | 159.7 | 20.5 | 0.153 |
| Weight (kg) | 69.7 | 14.1 | 69.8 | 16.6 | 0.803 |
| FG (mg/dL) | 136.8 | 64.0 | 123.4 | 42.6 | 0.138 |
| Insulin (mU/mL) | 14.8 | 17.7 | 12.1 | 12.1 | 0.956 |
| HbA1c (%) | 7.8 | 1.4 | 7.5 | 1.2 | 0.187 |
| Creatinine (mg/dL) | 1.3 | 1.2 | 2.0 | 5.7 | 0.323 |
| AST (U/L) | 29.0 | 13.7 | 27.0 | 17.6 | 0.462 |
| ALT (U/L) | 29.7 | 19.8 | 26.8 | 12.8 | 0.288 |
| Items | Base (n = 78) | 3-Month (n = 78) | pa | ||
|---|---|---|---|---|---|
| Mean | s.d. b | Mean | s.d. | ||
| FG (mg/dL) | 123.4 | 42.6 | 131.5 | 51.9 | 0.155 |
| HbA1c (%) | 7.5 | 1.2 | 7.5 | 1.1 | 0.852 |
| Insulin (mU/mL) | 12.1 | 12.1 | 14.7 | 19.4 | 0.321 |
| TG (mg/dL) | 163.0 | 149.0 | 165.2 | 140.3 | 0.902 |
| TC (mg/dL) | 182.2 | 43.1 | 181.8 | 33.6 | 0.866 |
| HDL-C (mg/dL) | 49.2 | 14.2 | 48.8 | 12.3 | 0.885 |
| LDL-C (mg/dL) | 109.4 | 37.9 | 108.1 | 30.0 | 0.890 |
| AST (U/L) | 27.0 | 17.6 | 26.3 | 13.5 | 0.387 |
| ALT (U/L) | 26.8 | 12.8 | 28.9 | 18.7 | 0.293 |
| Creatinine (mg/dL) | 2.0 | 5.7 | 4.8 | 18.8 | 0.093 |
| BUN (mg/dL) | 20.6 | 8.1 | 19.7 | 6.0 | 0.166 |
| Items | Base (n = 64) | 3-Month (n = 64) | pa | ||
|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | ||
| FG (mg/dL) | 136.8 | 63.5 | 118.0 | 35.5 | 0.007 |
| HbA1c (%) | 7.8 | 1.4 | 7.4 | 1.1 | 0.000 |
| Insulin (mU/mL) | 14.8 | 17.6 | 13.3 | 11.5 | 0.345 |
| TG (mg/dL) | 147.9 | 85.9 | 114.7 | 64.8 | 0.000 |
| TC (mg/dL) | 165.5 | 31.6 | 163.7 | 27.4 | 0.554 |
| HDL-C (mg/dL) | 46.5 | 10.5 | 49.1 | 12.0 | 0.050 |
| LDL-C (mg/dL) | 96.4 | 25.9 | 95.5 | 25.8 | 0.676 |
| AST (U/L) | 29.0 | 13.7 | 29.0 | 17.2 | 0.991 |
| ALT (U/L) | 29.7 | 19.8 | 29.5 | 18.8 | 0.881 |
| Creatinine (mg/dL) | 1.3 | 1.2 | 1.2 | 0.7 | 0.100 |
| BUN (mg/dL) | 20.5 | 8.0 | 19.5 | 7.0 | 0.041 |
| Time | FG | HbA1c | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | s.d. | △ Mean | t | pb | △% | Mean | s.d. | △ Mean | t | pb | △% | |
| E a T0 | 136.8 | 64.0 | 7.8 | 1.4 | ||||||||
| E T1 | 129.7 | 47.3 | −7.1 | 1.40 | 0.165 | −5.19 | 7.7 | 1.4 | −0.1 | 1.44 | 0.155 | −0.98 |
| E T2 | 120.3 | 32.3 | −9.4 | 2.22 | 0.030 | −7.23 | 7.5 | 1.4 | −0.2 | 2.32 | 0.024 | −3.16 |
| E T3 | 118.0 | 35.8 | −2.3 | 0.62 | 0.539 | −1.92 | 7.3 | 1.5 | −0.2 | 1.12 | 0.269 | −2.32 |
| C a T0 | 123.6 | 42.6 | 7.5 | 1.2 | ||||||||
| C T3 | 131.5 | 51.9 | 8.1 | 1.44 | 0.155 | 6.89 | 7.5 | 1.1 | 0.0 | −0.19 | 0.852 | 0.36 |
| Indices | Experiment | s.d. | Control | s.d. | t-test | Sig. |
|---|---|---|---|---|---|---|
| Mean | Mean | t | pa | |||
| Weight (kg) | 66.7 | 20.3 | 63.6 | 27.2 | 0.76 | 0.447 |
| BMI | 24.7 | 6.9 | 24.4 | 9.6 | 0.22 | 0.826 |
| Pulse (bpm) | 74.6 | 19.6 | 69.4 | 28.4 | 1.24 | 0.217 |
| Body fat (%) | 27.7 | 8.7 | 28.3 | 12.6 | −0.29 | 0.769 |
| HbAlc | 7.3 | 1.5 | 7.5 | 1.1 | −1.04 | 0.300 |
| FG | 118.0 | 35.8 | 131.5 | 51.9 | −1.76 | 0.080 |
| Insulin (mU/mL) | 13.0 | 11.6 | 14.7 | 19.4 | −0.58 | 0.560 |
| TC | 161.1 | 34.2 | 179.5 | 39.4 | −2.97 | 0.004 |
| TG | 112.9 | 66.4 | 163.1 | 141.6 | −2.61 | 0.010 |
| LDL-C | 94.0 | 28.4 | 106.7 | 32.4 | −2.49 | 0.014 |
| HDL-C | 48.3 | 13.5 | 47.5 | 14.5 | 0.32 | 0.746 |
| Creatinine | 1.2 | 0.7 | 4.8 | 18.9 | −1.54 | 0.125 |
| AST | 29.0 | 17.2 | 26.3 | 13.5 | 1.01 | 0.313 |
| ALT | 29.5 | 18.8 | 28.9 | 18.7 | 0.20 | 0.845 |
| BUN | 19.4 | 7.0 | 19.0 | 7.1 | 0.40 | 0.690 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, P.-K.; Pan, F.F.C.; Hsieh, C.-S. mcIRBP-19 of Bitter Melon Peptide Effectively Regulates Diabetes Mellitus (DM) Patients’ Blood Sugar Levels. Nutrients 2020, 12, 1252. https://doi.org/10.3390/nu12051252
Hsu P-K, Pan FFC, Hsieh C-S. mcIRBP-19 of Bitter Melon Peptide Effectively Regulates Diabetes Mellitus (DM) Patients’ Blood Sugar Levels. Nutrients. 2020; 12(5):1252. https://doi.org/10.3390/nu12051252
Chicago/Turabian StyleHsu, Pang-Kuei, Frank F. C. Pan, and Ching-Sen Hsieh. 2020. "mcIRBP-19 of Bitter Melon Peptide Effectively Regulates Diabetes Mellitus (DM) Patients’ Blood Sugar Levels" Nutrients 12, no. 5: 1252. https://doi.org/10.3390/nu12051252
APA StyleHsu, P.-K., Pan, F. F. C., & Hsieh, C.-S. (2020). mcIRBP-19 of Bitter Melon Peptide Effectively Regulates Diabetes Mellitus (DM) Patients’ Blood Sugar Levels. Nutrients, 12(5), 1252. https://doi.org/10.3390/nu12051252