Factors Associated with Frequency of Peanut Consumption in Korea: A National Population-Based Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Populations
2.2. Data Variables
2.3. Statistical Analysis
3. Results
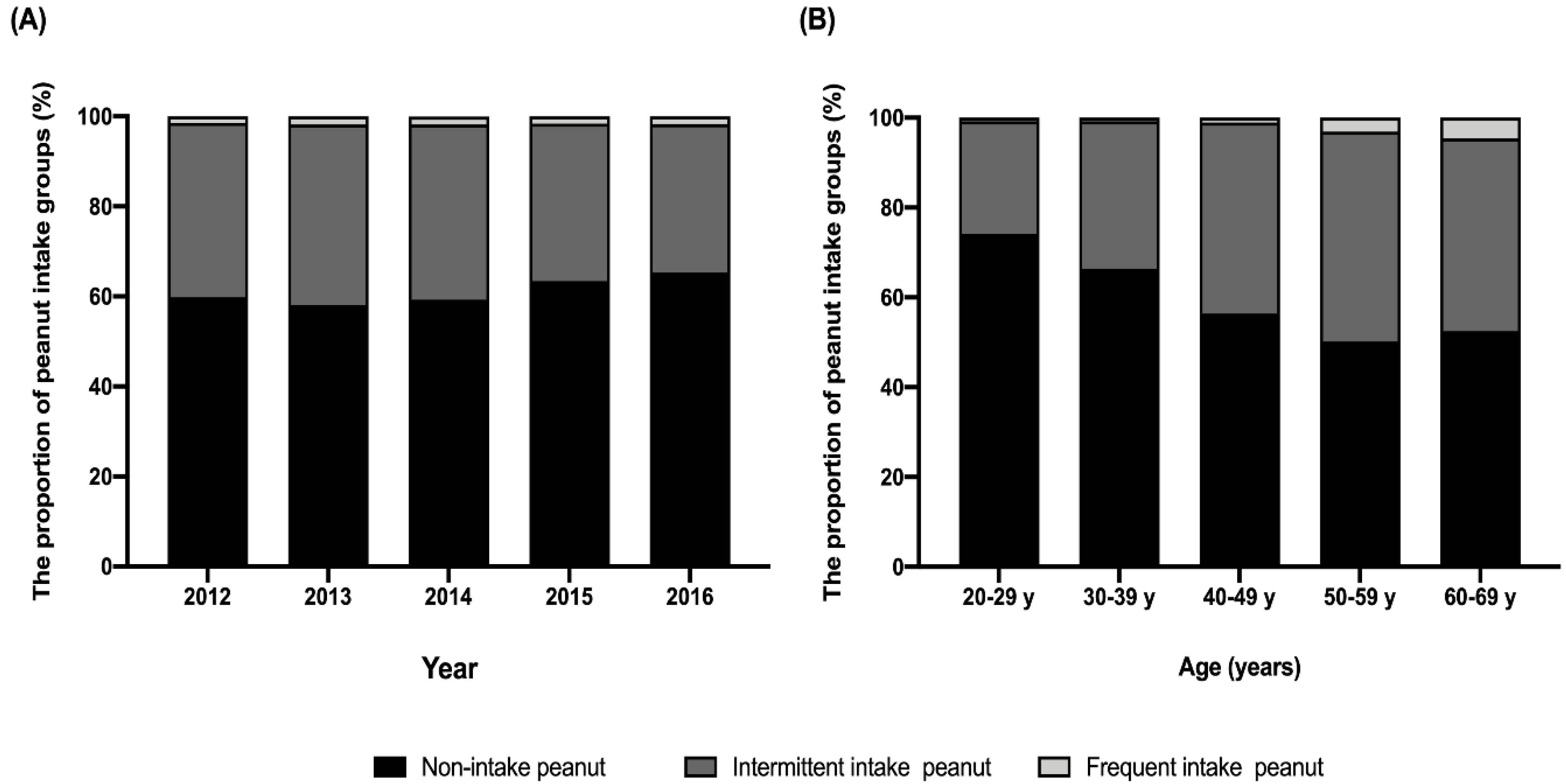
3.1. Sociodemographic Characteristics of Study Participants
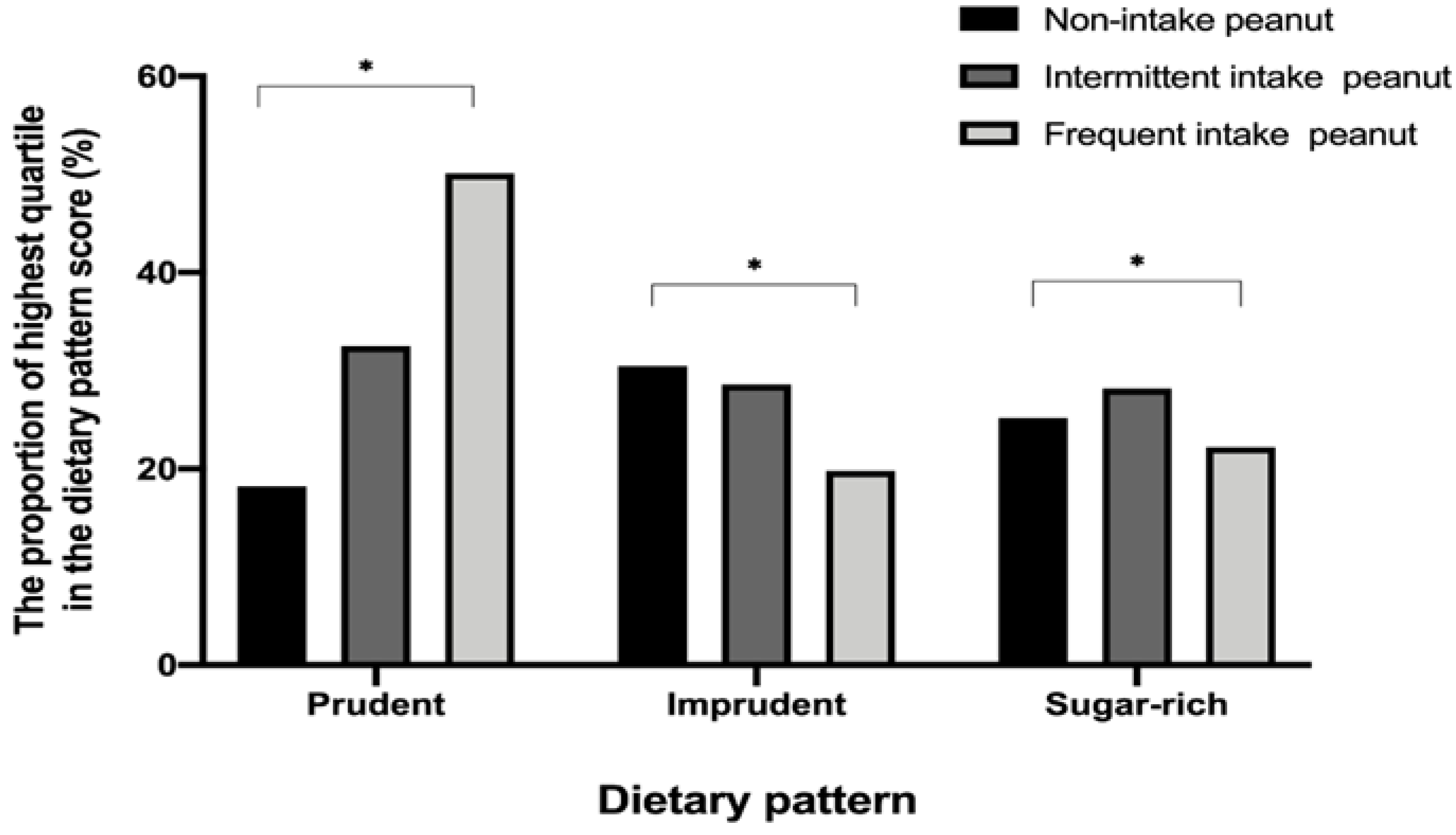
3.2. Dietary Pattern of Study Participants
3.3. Sociodemographic and Dietary Factors Associated with Frequent Peanut Consumption in Korean
4. Discussion
4.1. Peanut Consumption in Korea
4.2. Perspectives for Peanut Allergy
4.3. Study Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Toomer, O.T. Nutritional chemistry of the peanut (arachis hypogaea). Crit. Rev. Food Sci. Nutr. 2018, 58, 3042–3053. [Google Scholar] [CrossRef]
- Akhtar, S.; Khalid, N.; Ahmed, I.; Shahzad, A.; Suleria, H.A.R. Physicochemical characteristics, functional properties, and nutritional benefits of peanut oil: A review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1562–1575. [Google Scholar] [CrossRef]
- Arya, S.S.; Salve, A.R.; Chauhan, S. Peanuts as functional food: A review. J. Food Sci. Technol. 2016, 53, 31–41. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Hu, F.B.; Ros, E.; Sabate, J. The role of tree nuts and peanuts in the prevention of coronary heart disease: Multiple potential mechanisms. J. Nutr. 2008, 138, 1746s–1751s. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, C.; Bolling, B.W. Review of nut phytochemicals, fat-soluble bioactives, antioxidant components and health effects. Br. J. Nutr. 2015, 113 (Suppl. S2), S68–S78. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.E.; Mocan, A.; Ferreira, I.; Popa, D.S. Health benefits of nut consumption in middle-aged and elderly population. Antioxidants 2019, 8, 302. [Google Scholar] [CrossRef] [PubMed]
- Coates, A.M.; Hill, A.M.; Tan, S.Y. Nuts and cardiovascular disease prevention. Curr. Atheroscler. Rep. 2018, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Tomas, N.; Paz-Graniel, I.; WC Kendall, C.; Kahleova, H.; Rahelic, D.; Sievenpiper, J.L.; Salas-Salvado, J. Nut consumption and incidence of cardiovascular diseases and cardiovascular disease mortality: A meta-analysis of prospective cohort studies. Nutr. Rev. 2019, 77, 691–709. [Google Scholar] [CrossRef] [PubMed]
- Jafari Azad, B.; Daneshzad, E.; Azadbakht, L. Peanut and cardiovascular disease risk factors: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2020, 60, 1123–1140. [Google Scholar] [CrossRef]
- Liu, G.; Guasch-Ferre, M.; Hu, Y.; Li, Y.; Hu, F.B.; Rimm, E.B.; Manson, J.E.; Rexrode, K.M.; Sun, Q. Nut consumption in relation to cardiovascular disease incidence and mortality among patients with diabetes mellitus. Circ. Res. 2019, 124, 920–929. [Google Scholar] [CrossRef]
- Amba, V.; Murphy, G.; Etemadi, A.; Wang, S.; Abnet, C.C.; Hashemian, M. Nut and peanut butter consumption and mortality in the national institutes of health-aarp diet and health study. Nutrients 2019, 11, 1508. [Google Scholar] [CrossRef] [PubMed]
- Hashemian, M.; Murphy, G.; Etemadi, A.; Dawsey, S.M.; Liao, L.M.; Abnet, C.C. Nut and peanut butter consumption and the risk of esophageal and gastric cancer subtypes. Am. J. Clin. Nutr. 2017, 106, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Barbour, J.A.; Howe, P.R.C.; Buckley, J.D.; Bryan, J.; Coates, A.M. Cerebrovascular and cognitive benefits of high-oleic peanut consumption in healthy overweight middle-aged adults. Nutr. Neurosci. 2017, 20, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, N.B.; Guffey, D.; Anagnostou, K.; Coleman, N.E.; Davis, C.M. Epidemiology of anaphylaxis in critically ill children in the united states and canada. J. Allergy Clin. Immunol. Pract. 2019, 7, 2241–2249. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.J.; Jerschow, E.; Umasunthar, T.; Lin, R.; Campbell, D.E.; Boyle, R.J. Fatal anaphylaxis: Mortality rate and risk factors. J. Allergy Clin. Immunol. Pract. 2017, 5, 1169–1178. [Google Scholar] [CrossRef]
- Umasunthar, T.; Leonardi-Bee, J.; Hodes, M.; Turner, P.J.; Gore, C.; Habibi, P.; Warner, J.O.; Boyle, R.J. Incidence of fatal food anaphylaxis in people with food allergy: A systematic review and meta-analysis. Clin. Exp. Allergy 2013, 43, 1333–1341. [Google Scholar] [CrossRef]
- Lange, L. Quality of life in the setting of anaphylaxis and food allergy. Allergo J. Int. 2014, 23, 252–260. [Google Scholar] [CrossRef]
- Prescott, S.L.; Pawankar, R.; Allen, K.J.; Campbell, D.E.; Sinn, J.; Fiocchi, A.; Ebisawa, M.; Sampson, H.A.; Beyer, K.; Lee, B.W. A global survey of changing patterns of food allergy burden in children. World Allergy Organ. J. 2013, 6, 21. [Google Scholar] [CrossRef]
- Flokstra-de Blok, B.M.; van der Velde, J.L.; Vlieg-Boerstra, B.J.; Oude Elberink, J.N.; DunnGalvin, A.; Hourihane, J.O.; Duiverman, E.J.; Dubois, A.E. Health-related quality of life of food allergic patients measured with generic and disease-specific questionnaires. Allergy 2010, 65, 1031–1038. [Google Scholar] [CrossRef]
- Greenhawt, M. Food allergy quality of life and living with food allergy. Curr. Opin. Allergy Clin. Immunol. 2016, 16, 284–290. [Google Scholar] [CrossRef]
- Avery, N.J.; King, R.M.; Knight, S.; Hourihane, J.O. Assessment of quality of life in children with peanut allergy. Pediatr. Allergy Immunol. 2003, 14, 378–382. [Google Scholar] [CrossRef]
- Shemesh, E.; Annunziato, R.A.; Ambrose, M.A.; Ravid, N.L.; Mullarkey, C.; Rubes, M.; Chuang, K.; Sicherer, M.; Sicherer, S.H. Child and parental reports of bullying in a consecutive sample of children with food allergy. Pediatrics 2013, 131, e10–e17. [Google Scholar] [CrossRef] [PubMed]
- Savage, J.; Sicherer, S.; Wood, R. The natural history of food allergy. J. Allergy Clin. Immunol. Pract. 2016, 4, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, G.; Roberts, G.; Sayre, P.H.; Bahnson, H.T.; Radulovic, S.; Santos, A.F.; Brough, H.A.; Phippard, D.; Basting, M.; Feeney, M.; et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N. Engl. J. Med. 2015, 372, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Togias, A.; Cooper, S.F.; Acebal, M.L.; Assa’ad, A.; Baker, J.R., Jr.; Beck, L.A.; Block, J.; Byrd-Bredbenner, C.; Chan, E.S.; Eichenfield, L.F.; et al. Addendum guidelines for the prevention of peanut allergy in the united states: Report of the national institute of allergy and infectious diseases-sponsored expert panel. J. Allergy Clin. Immunol. 2017, 139, 29–44. [Google Scholar] [CrossRef]
- Netting, M.J.; Campbell, D.E.; Koplin, J.J.; Beck, K.M.; McWilliam, V.; Dharmage, S.C.; Tang, M.L.K.; Ponsonby, A.L.; Prescott, S.L.; Vale, S.; et al. An australian consensus on infant feeding guidelines to prevent food allergy: Outcomes from the australian infant feeding summit. J. Allergy Clin. Immunol. Pract. 2017, 5, 1617–1624. [Google Scholar] [CrossRef]
- Greer, F.R.; Sicherer, S.H.; Burks, A.W. The effects of early nutritional interventions on the development of atopic disease in infants and children: The role of maternal dietary restriction, breastfeeding, hydrolyzed formulas, and timing of introduction of allergenic complementary foods. Pediatrics 2019, 143, e20190281. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.Y.; Jeon, H.Y.; Yang, H.K.; Lee, K.J.; Han, Y.; Kim, Y.H.; Kim, J.; Ahn, K. Prevalence of immediate-type food allergy in korean schoolchildren in 2015: A nationwide, population-based study. Allergy Asthma Immunol. Res. 2017, 9, 410–416. [Google Scholar] [CrossRef]
- Shek, L.P.; Cabrera-Morales, E.A.; Soh, S.E.; Gerez, I.; Ng, P.Z.; Yi, F.C.; Ma, S.; Lee, B.W. A population-based questionnaire survey on the prevalence of peanut, tree nut, and shellfish allergy in 2 asian populations. J. Allergy Clin. Immunol. 2010, 126, 324–331. [Google Scholar] [CrossRef]
- Venter, C.; Maslin, K.; Patil, V.; Kurukulaaratchy, R.; Grundy, J.; Glasbey, G.; Twiselton, R.; Dean, T.; Arshad, S.H. The prevalence, natural history and time trends of peanut allergy over the first 10 years of life in two cohorts born in the same geographical location 12 years apart. Pediatr. Allergy Immunol. 2016, 27, 804–811. [Google Scholar] [CrossRef]
- Peters, R.L.; Allen, K.J.; Dharmage, S.C.; Koplin, J.J.; Dang, T.; Tilbrook, K.P.; Lowe, A.; Tang, M.L.; Gurrin, L.C. Natural history of peanut allergy and predictors of resolution in the first 4 years of life: A population-based assessment. J. Allergy Clin. Immunol. 2015, 135, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Tham, E.H.; Shek, L.P.; Van Bever, H.P.; Vichyanond, P.; Ebisawa, M.; Wong, G.W.; Lee, B.W. Early introduction of allergenic foods for the prevention of food allergy from an asian perspective-an asia pacific association of pediatric allergy, respirology & immunology (apapari) consensus statement. Pediatr. Allergy Immunol. 2018, 29, 18–27. [Google Scholar] [PubMed]
- Irvine, A.D.; McLean, W.H.; Leung, D.Y. Filaggrin mutations associated with skin and allergic diseases. N. Engl. J. Med. 2011, 365, 1315–1327. [Google Scholar] [CrossRef] [PubMed]
- Brough, H.A.; Liu, A.H.; Sicherer, S.; Makinson, K.; Douiri, A.; Brown, S.J.; Stephens, A.C.; Irwin McLean, W.H.; Turcanu, V.; Wood, R.A.; et al. Atopic dermatitis increases the effect of exposure to peanut antigen in dust on peanut sensitization and likely peanut allergy. J. Allergy Clin. Immunol. 2015, 135, 164–170. [Google Scholar] [CrossRef]
- Brough, H.A.; Kull, I.; Richards, K.; Hallner, E.; Soderhall, C.; Douiri, A.; Penagos, M.; Melen, E.; Bergstrom, A.; Turcanu, V.; et al. Environmental peanut exposure increases the risk of peanut sensitization in high-risk children. Clin. Exp. Allergy 2018, 48, 586–593. [Google Scholar] [CrossRef]
- Fox, A.T.; Sasieni, P.; du Toit, G.; Syed, H.; Lack, G. Household peanut consumption as a risk factor for the development of peanut allergy. J. Allergy Clin. Immunol. 2009, 123, 417–423. [Google Scholar] [CrossRef]
- World Health Organization. Classification of Overweight and Obesity by Bmi, Waist Circumference, and Associated Disease Risks. Available online: https://www.nhlbi.nih.gov/health/educational/lose_wt/BMI/bmi_dis.htm (accessed on 20 March 2020).
- Hou, Y.Y.; Ojo, O.; Wang, L.L.; Wang, Q.; Jiang, Q.; Shao, X.Y.; Wang, X.H. A randomized controlled trial to compare the effect of peanuts and almonds on the cardio-metabolic and inflammatory parameters in patients with type 2 diabetes mellitus. Nutrients 2018, 10, 1565. [Google Scholar] [CrossRef]
- Wien, M.; Oda, K.; Sabate, J. A randomized controlled trial to evaluate the effect of incorporating peanuts into an american diabetes association meal plan on the nutrient profile of the total diet and cardiometabolic parameters of adults with type 2 diabetes. Nutr. J. 2014, 13, 10. [Google Scholar] [CrossRef]
- Castro-Quezada, I.; Roman-Vinas, B.; Serra-Majem, L. The mediterranean diet and nutritional adequacy: A review. Nutrients 2014, 6, 231–248. [Google Scholar] [CrossRef]
- Eslamparast, T.; Sharafkhah, M.; Poustchi, H.; Hashemian, M.; Dawsey, S.M.; Freedman, N.D.; Boffetta, P.; Abnet, C.C.; Etemadi, A.; Pourshams, A.; et al. Nut consumption and total and cause-specific mortality: Results from the golestan cohort study. Int. J. Epidemiol. 2017, 46, 75–85. [Google Scholar] [CrossRef]
- Bao, Y.; Han, J.; Hu, F.B.; Giovannucci, E.L.; Stampfer, M.J.; Willett, W.C.; Fuchs, C.S. Association of nut consumption with total and cause-specific mortality. N. Engl. J. Med. 2013, 369, 2001–2011. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, A.J.; de Souza, R.J.; Meyre, D.; Anand, S.S.; Mente, A. A systematic review and meta-analysis of nut consumption and incident risk of cvd and all-cause mortality. Br. J. Nutr. 2016, 115, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, M.S.; Lee, M.S.; Park, Y.S.; Lee, H.J.; Kang, S.-A.; Lee, H.S.; Lee, K.-E.; Yang, H.J.; Kim, M.J.; et al. Korean diet: Characteristics and historical background. J. Ethn. Foods 2016, 3, 26–31. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, Y. Jongka, the traditional korean family: Exploring jongka food in the context of Korean food categories. J. Ethn. Foods 2018, 5, 40–53. [Google Scholar] [CrossRef]
- Finger, J.D.; Tylleskar, T.; Lampert, T.; Mensink, G.B. Dietary behaviour and socioeconomic position: The role of physical activity patterns. PLoS ONE 2013, 8, e78390. [Google Scholar] [CrossRef] [PubMed]
- Wadolowska, L.; Hamulka, J.; Kowalkowska, J.; Ulewicz, N.; Hoffmann, M.; Gornicka, M.; Bronkowska, M.; Leszczynska, T.; Glibowski, P.; Korzeniowska-Ginter, R. Changes in sedentary and active lifestyle, diet quality and body composition nine months after an education program in polish students aged 11(–)12 years: Report from the abc of healthy eating study. Nutrients 2019, 11, 331. [Google Scholar] [CrossRef]
- Thorpe, M.G.; Milte, C.M.; Crawford, D.; McNaughton, S.A. Education and lifestyle predict change in dietary patterns and diet quality of adults 55 years and over. Nutr. J. 2019, 18, 67. [Google Scholar] [CrossRef]
- Aranceta, J.; Perez Rodrigo, C.; Naska, A.; Vadillo, V.R.; Trichopoulou, A. Nut consumption in Spain and other countries. Br. J. Nutr. 2006, 96 (Suppl. S2), S3–S11. [Google Scholar] [CrossRef]
- Griel, A.E.; Eissenstat, B.; Juturu, V.; Hsieh, G.; Kris-Etherton, P.M. Improved diet quality with peanut consumption. J. Am. Coll. Nutr. 2004, 23, 660–668. [Google Scholar] [CrossRef]
- Brown, R.C.; Tey, S.L.; Gray, A.R.; Chisholm, A.; Smith, C.; Fleming, E.; Parnell, W. Nut consumption is associated with better nutrient intakes: Results from the 2008/09 new zealand adult nutrition survey. Br. J. Nutr. 2016, 115, 105–112. [Google Scholar] [CrossRef]
- O’Neil, C.E.; Keast, D.R.; Nicklas, T.A.; Fulgoni, V.L., 3rd. Out-of-hand nut consumption is associated with improved nutrient intake and health risk markers in us children and adults: National health and nutrition examination survey 1999–2004. Nutr. Res. 2012, 32, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Freisling, H.; Noh, H.; Slimani, N.; Chajes, V.; May, A.M.; Peeters, P.H.; Weiderpass, E.; Cross, A.J.; Skeie, G.; Jenab, M.; et al. Nut intake and 5-year changes in body weight and obesity risk in adults: Results from the epic-panacea study. Eur. J. Nutr. 2018, 57, 2399–2408. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, S.; Neuenschwander, M.; Schwedhelm, C.; Hoffmann, G.; Bechthold, A.; Boeing, H.; Schwingshackl, L. Food groups and risk of overweight, obesity, and weight gain: A systematic review and dose-response meta-analysis of prospective studies. Adv. Nutr. 2019, 10, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Hao, T.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Changes in diet and lifestyle and long-term weight gain in women and men. N. Engl. J. Med. 2011, 364, 2392–2404. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.P.; Johnston, C.A.; El-Mubasher, A.A.; Papaioannou, M.A.; Tyler, C.; Gee, M.; Foreyt, J.P. Peanut consumption in adolescents is associated with improved weight status. Nutr. Res. 2013, 33, 552–556. [Google Scholar] [CrossRef]
- Nikodijevic, C.J.; Probst, Y.C.; Batterham, M.J.; Tapsell, L.C.; Neale, E.P. Nut consumption in a representative survey of australians: A secondary analysis of the 201–2012 national nutrition and physical activity survey. Public Health Nutr. 2020, 1–11. [Google Scholar] [CrossRef]
- Relja, A.; Miljkovic, A.; Gelemanovic, A.; Boskovic, M.; Hayward, C.; Polasek, O.; Kolcic, I. Nut consumption and cardiovascular risk factors: A cross-sectional study in a mediterranean population. Nutrients 2017, 9, 1296. [Google Scholar] [CrossRef]
- Lee, J.; Shin, A.; Oh, J.H.; Kim, J. The relationship between nut intake and risk of colorectal cancer: A case control study. Nutr. J. 2018, 17, 37. [Google Scholar] [CrossRef]
- Wang, Y.; Allen, K.J.; Suaini, N.H.A.; Peters, R.L.; Ponsonby, A.L.; Koplin, J.J. Asian children living in australia have a different profile of allergy and anaphylaxis than australian-born children: A state-wide survey. Clin. Exp. Allergy 2018, 48, 1317–1324. [Google Scholar] [CrossRef]
- Sasaki, M.; Peters, R.L.; Koplin, J.J.; Field, M.J.; McWilliam, V.; Sawyer, S.M.; Vuillermin, P.J.; Pezic, A.; Gurrin, L.C.; Douglass, J.A.; et al. Risk factors for food allergy in early adolescence: The schoolnuts study. J. Allergy Clin. Immunol. Pract. 2018, 6, 496–505. [Google Scholar] [CrossRef]
- Panjari, M.; Koplin, J.J.; Dharmage, S.C.; Peters, R.L.; Gurrin, L.C.; Sawyer, S.M.; McWilliam, V.; Eckert, J.K.; Vicendese, D.; Erbas, B.; et al. Nut allergy prevalence and differences between asian-born children and australian-born children of asian descent: A state-wide survey of children at primary school entry in victoria, australia. Clin. Exp. Allergy 2016, 46, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shoshan, M.; Soller, L.; Harrington, D.W.; Knoll, M.; La Vieille, S.; Fragapane, J.; Joseph, L.; St. Pierre, Y.; Wilson, K.; Elliott, S.J.; et al. Eczema in early childhood, sociodemographic factors and lifestyle habits are associated with food allergy: A nested case-control study. Int. Arch. Allergy Immunol. 2015, 166, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shoshan, M.; Harrington, D.W.; Soller, L.; Fragapane, J.; Joseph, L.; Pierre, Y.S.; Godefroy, S.B.; Elliott, S.J.; Clarke, A.E. Demographic predictors of peanut, tree nut, fish, shellfish, and sesame allergy in canada. J. Allergy 2012, 2012, 1–6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brough, H.A.; Santos, A.F.; Makinson, K.; Penagos, M.; Stephens, A.C.; Douiri, A.; Fox, A.T.; Du Toit, G.; Turcanu, V.; Lack, G. Peanut protein in household dust is related to household peanut consumption and is biologically active. J. Allergy Clin. Immunol. 2013, 132, 630–638. [Google Scholar] [CrossRef]
- Trendelenburg, V.; Ahrens, B.; Wehrmann, A.K.; Kalb, B.; Niggemann, B.; Beyer, K. Peanut allergen in house dust of eating area and bed—A risk factor for peanut sensitization? Allergy 2013, 68, 1460–1462. [Google Scholar] [CrossRef]
- Foong, R.X.; Brough, H. The role of environmental exposure to peanut in the development of clinical allergy to peanut. Clin. Exp. Allergy 2017, 47, 1232–1238. [Google Scholar] [CrossRef]
- Choi, J.H.; Woo, H.D.; Lee, J.H.; Kim, J. Dietary patterns and risk for metabolic syndrome in Korean women: A cross-sectional study. Medicine 2015, 94, e1424. [Google Scholar] [CrossRef]
- Lee, S. Associations between dietary patterns and handgrip strength: The Korea national health and nutrition examination survey 2014–2016. J. Am. Coll. Nutr. 2019, 1–7. [Google Scholar] [CrossRef]
- Song, S.; Kim, J.; Kim, J. Gender differences in the association between dietary pattern and the incidence of hypertension in middle-aged and older adults. Nutrients 2018, 10, 252. [Google Scholar] [CrossRef]
| Variable | Non-Intake (n = 10,552) | Intermittent Intake (n = 6726) | Frequent Intake (n = 347) | p-Value * |
|---|---|---|---|---|
| Sex | <0.001 | |||
| Male | 57.3 (0.7) | 41.3 (0.7) | 1.4 (0.1) | |
| Female | 65.1 (0.6) | 33.1 (0.6) | 1.8 (0.1) | |
| Age group | <0.001 | |||
| 20–29 years | 74.2 (1.0) | 24.9 (1.0) | 0.8 (0.2) | |
| 30–39 years | 66.4 (1.0) | 32.8 (0.9) | 0.8 (0.2) | |
| 40–49 years | 56.4 (1.0) | 42.5 (1.0) | 1.0 (0.2) | |
| 50–59 years | 50.2 (1.0) | 46.7 (0.9) | 3.1 (0.3) | |
| 60–69 years | 52.5 (1.3) | 42.9 (1.2) | 4.6 (0.5) | |
| Resident area | 0.532 | |||
| Rural | 61.2 (0.6) | 37.1 (0.5) | 1.6 (0.1) | |
| Urban | 60.5 (1.3) | 37.9 (1.2) | 1.7 (0.3) | |
| Household income | <0.001 | |||
| Lowest | 68.3 (1.6) | 30.1 (1.5) | 1.6 (0.3) | |
| Middle–low | 64.1 (0.9) | 34.5 (0.9) | 1.4 (0.2) | |
| Middle–high | 60.9 (0.9) | 37.7 (0.8) | 1.4 (0.2) | |
| Highest | 57.3 (0.8) | 40.7 (0.8) | 2.0 (0.2) | |
| Household composition | <0.001 | |||
| Living alone | 60.2 (1.0) | 37.9 (1.0) | 1.9 (0.2) | |
| Single generation household | 61.2 (0.6) | 37.3 (0.6) | 1.5 (0.1) | |
| Multigeneration household | 62.6 (1.6) | 35.5 (1.6) | 1.9 (0.4) | |
| Education level | 0.001 | |||
| <Elementary | 62.3 (1.5) | 35.0 (1.5) | 2.7 (0.4) | |
| Middle school | 60.1 (1.6) | 37.8 (1.6) | 2.1 (0.4) | |
| High school | 63.0 (0.8) | 35.4 (0.8) | 1.6 (0.2) | |
| College or higher | 59.4 (0.8) | 39.2 (0.8) | 1.4 (0.2) | |
| Occupation | <0.001 | |||
| Unemployed | 65.9 (0.8) | 39.5 (1.1) | 1.6 (0.2) | |
| Unskilled workers | 61.9 (1.6) | 40.0 (0.7) | 1.2 (0.3) | |
| Non-manual, skilled workers | 58.5 (0.7) | 36.9 (0.7) | 1.6 (0.2) | |
| Professionals and managers | 58.9 (1.2) | 32.4 (0.8) | 1.6 (0.2) | |
| Smoking status | <0.001 | |||
| None | 63.1 (0.6) | 35.1 (0.6) | 1.8 (0.1) | |
| Former | 53.3 (1.1) | 45.1 (1.1) | 1.5 (0.2) | |
| Current | 63.5 (1.0) | 35.2 (1.1) | 1.4 (0.2) | |
| Alcohol consumption | 0.001 | |||
| None | 63.0 (1.2) | 34.8 (1.2) | 2.1 (0.3) | |
| Moderate | 60.1 (0.7) | 38.4 (0.7) | 1.5 (0.2) | |
| Heavy | 60.3 (2.2) | 37.8 (2.2) | 1.8 (0.5) | |
| Body mass index, | <0.001 | |||
| Underweight (<18.5 kg/m2) | 71.7 (1.9) | 26.8 (1.8) | 1.5 (0.6) | |
| Normal (18.5–24.9 kg/m2) | 61.3 (0.6) | 37.1 (0.6) | 1.7 (0.1) | |
| Overweight (25.0–29.9 kg/ m2) | 58.2 (0.9) | 40.3 (0.9) | 1.6 (0.2) | |
| Obese (≥30.0 kg/m2) | 65.7 (2.0) | 33.0 (2.0) | 1.3 (0.4) | |
| History of cardiovascular disease † or diabetes mellitus | 16.2 (15.2) | 20.6 (0.7) | 27.5 (3.0) | <0.001 |
| Food Group | Prudent | Imprudent | Sugar-Rich |
|---|---|---|---|
| Beef, pork and poetry | 0.184 | 0.445 * | −0.007 |
| Dairy product | 0.228 | 0.168 | −0.133 |
| Fruits and vegetables | 0.462 * | 0.010 | −0.072 |
| Refined grain | −0.025 | 0.602 * | 0.043 |
| Whole grain | 0.217 | −0.553 * | −0.024 |
| Sweets | −0.003 | −0.030 | 0.680 * |
| Seafoods | 0.417 * | 0.005 | 0.042 |
| Legume | 0.423 * | −0.008 | 0.037 |
| Egg | 0.274 | 0.215 | −0.106 |
| Kimchi | 0.294 | −0.159 | 0.155 |
| Sugar beverages | 0.026 | 0.033 | 0.664 * |
| Alcohol | 0.004 | 0.178 | 0.182 |
| Seaweed | 0.371 | −0.005 | 0.016 |
| Variable | Non-Intake (n = 10,552) | Intermittent Intake (n = 6726) | Frequent intake (n = 347) | p-Value * |
|---|---|---|---|---|
| Total energy intake, kcal/d | 2041.6 (10.2) | 2287.3 (13.6) | 2340.0 (60.6) | <0.001 |
| Carbohydrate intake, g/day | 317.4 (1.4) | 352.6 (1.9) | 354.9 (8.4) | <0.001 |
| Protein intake, g/day | 66.65 (0.4) | 76.9 (0.6) | 80.5 (2.3) | <0.001 |
| Total fat intake, g/day | 41.7 (0.3) | 48.1 (0.4) | 55.8 (1.9) | <0.001 |
| Polyunsaturated fatty acid, g/day | 10.7 (0.1) | 12.8 (0.1) | 15.9 (0.5) | <0.001 |
| Monounsaturated fatty acid, g/day | 12.95 (0.1) | 15.0 (0.2) | 18.6 (0.7) | <0.001 |
| Saturated fatty acid, g/day | 12.63 (0.1) | 13.9 (0.1) | 14.81 (0.6) | 0.039 |
| Cholesterol, mg/day | 262.1 (2.3) | 292.8 (3.0) | 291.9 (14.7) | 0.002 |
| Fiber, g/day | 19.1 (0.1) | 23.8 (0.2) | 28.1 (0.7) | <0.001 |
| Iron intake, g/day | 13.3 (0.1) | 15.9 (0.1) | 17.0 (0.4) | <0.001 |
| Vitamin A RAE, µg/day | 594.4 (4.1) | 715.3 (5.3) | 824.4 (25.0) | <0.001 |
| Vitamin B1, mg/day | 1.8 (0.1) | 2.1 (0.1) | 2.2 (0.1) | <0.001 |
| Vitamin B2, mg/day | 1.3 (0.1) | 1.5 (0.1) | 1.6 (0.1) | 0.001 |
| Vitamin B3, mg/day | 13.2 (0.1) | 15.9 (0.1) | 19.3 (0.5) | <0.001 |
| Vitamin C, mg/day | 102.7 (1.1) | 131.4 (1.3) | 176.7 (7.1) | <0.001 |
| Variable | Model 1 aOR (95% CI) | Model 2 aOR (95% CI) | Model 3 aOR (95% CI) |
|---|---|---|---|
| Age | 1.03 (1.02–1.04) * | 1.03 (1.02–1.04) * | 1.03 (1.03–1.04) * |
| Male | 1.42 (1.33–1.52) * | 1.42 (1.32–1.53) * | 1.67 (1.43–1.95) * |
| BMI | |||
| Underweight (<18.5 kg/m2) | Reference | Reference | Reference |
| Normal (18.5–24.9 kg/m2) | 1.16 (0.96–1.42) | 1.18 (0.95–1.46) | 1.11 (0.80–1.54) |
| Overweight (25.0–29.9 kg/ m2) | 1.16 (0.95–1.42) | 1.21 (0.97–1.51) | 1.22 (0.87–1.70) |
| Obese (≥30.0 kg/m2) | 0.95 (0.74–1.23) | 0.98 (0.74–1.30) | 1.07 (0.72–1.61) |
| Education | |||
| ≤Elementary school | Reference | Reference | Reference |
| Middle school | 1.26 (1.05–1.50) * | 1.28 (1.06–1.55) * | 1.27 (0.97–1.66) |
| High school | 1.82 (1.56–2.12) * | 1.83 (1.56–2.15) * | 1.73 (1.38–2.16) * |
| ≥college | 2.14 (1.83–2.50) * | 3.11 (1.78–2.50) * | 2.10 (1.63–2.65) * |
| Household income | |||
| Lowest | Reference | Reference | Reference |
| Middle–low | 1.30 (1.09–1.55) * | 1.33 (1.10–1.61) * | 1.16 (0.90–1.48) |
| Middle–high | 1.50 (1.26–1.77) * | 1.54 (1.28–1.85) * | 1.16 (0.91–1.49) |
| Highest | 1.71 (1.45–2.02) * | 1.74 (1.46–2.08) * | 1.11 (0.87–1.41) |
| Household composition | |||
| Living alone | Reference | Reference | Reference |
| Single generation household | 1.12 (1.02–1.24) * | 1.10 (0.99–1.22) | 0.94 (0.82–1.07) |
| Multigeneration household | 1.04 (0.90–1.21) | 1.01 (0.86–1.19) | 0.93 (0.75–1.17) |
| Occupation | |||
| Unemployed | Reference | Reference | Reference |
| Unskilled workers | 0.89 (0.77–1.04) | 0.87 (0.74–1.02) | 1.06 (0.85–1.32) |
| Non-manual, skilled workers | 1.12 (1.03–1.23) * | 1.11 (1.01–1.23) * | 1.17 (1.01–1.35) * |
| Professionals and managers | 1.26 (1.12–1.42) * | 1.22 (1.07–1.39) * | 0.97 (0.80–1.17) |
| History of cardiovascular diseases † or diabetes mellitus | 1.0 (0.85–1.17) | 1.02 (0.02–1.03) | 1.01 (0.86–1.19) |
| Alcohol consumption | |||
| None | Reference | References | Reference |
| Moderate | 1.15 (1.02–1.31) * | 1.20 (1.04–1.38) * | 1.24 (1.07–1.43) * |
| Heavy | 0.93 (0.75–1.17) | 1.05 (0.82–1.35) | 1.10 (0.84–1.43) |
| Smoking status | |||
| None | References | References | Reference |
| Former | 1.02 (0.90–1.15) | 1.0 (0.87–1.15) | 0.98 (0.82–1.16) |
| Current | 0.75 (0.66–0.84) * | 0.72 (0.63–0.82) * | 0.73 (0.61–0.87) * |
| Prudent dietary pattern | |||
| 1st quartile | Reference | Reference | Reference |
| 2nd quartile | 1.73 (1.54–1.93) * | 1.75 (1.55–1.99) * | 1.71 (1.47–1.99) * |
| 3rd quartile | 2.66 (2.49–2.95) * | 2.67 (2.38–3.01) * | 2.53 (2.16–2.97) * |
| 4th quartile | 3.85 (3.44–4.31) * | 3.91 (3.45–4.43) * | 3.72 (3.16–4.40) * |
| Imprudent dietary pattern | |||
| 1st quartile | Reference | Reference | Reference |
| 2nd quartile | 1.19 (1.06–1.33) * | 1.16 (1.03–1.30) * | 0.99 (0.83–1.18) |
| 3rd quartile | 1.07 (0.95–1.20) | 1.06 (0.94–1.20) | 1.06 (0.88–1.28) |
| 4th quartile | 1.20 (1.06–1.36) * | 1.19 (1.04–1.36) * | 1.02 (0.84–1.24) |
| Sugar-rich dietary pattern | |||
| 1st quartile | Reference | Reference | Reference |
| 2nd quartile | 1.02 (0.92–1.14) | 1.04 (0.93–1.17) | 1.03 (0.87–1.23) |
| 3rd quartile | 1.14 (1.03–1.26) * | 1.12 (1.01–1.26) * | 1.12 (0.94–1.33) |
| 4th quartile | 0.95 (0.85–1.06) | 0.95 (0.84–1.07) | 0.88 (0.74–1.06) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, M.; Kim, J.; Ahn, S.M. Factors Associated with Frequency of Peanut Consumption in Korea: A National Population-Based Study. Nutrients 2020, 12, 1207. https://doi.org/10.3390/nu12051207
Jung M, Kim J, Ahn SM. Factors Associated with Frequency of Peanut Consumption in Korea: A National Population-Based Study. Nutrients. 2020; 12(5):1207. https://doi.org/10.3390/nu12051207
Chicago/Turabian StyleJung, Minyoung, Jayun Kim, and Su Mi Ahn. 2020. "Factors Associated with Frequency of Peanut Consumption in Korea: A National Population-Based Study" Nutrients 12, no. 5: 1207. https://doi.org/10.3390/nu12051207
APA StyleJung, M., Kim, J., & Ahn, S. M. (2020). Factors Associated with Frequency of Peanut Consumption in Korea: A National Population-Based Study. Nutrients, 12(5), 1207. https://doi.org/10.3390/nu12051207





