Anti-Fatigue Effect of Prunus Mume Vinegar in High-Intensity Exercised Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of PV
2.3. Physicochemical Properties of PV
2.3.1. Total Acidity, Alcohol Content and Sugar Content in PV
2.3.2. Organic Acids and Free Amino Acids Content in PV
2.4. Cytotoxicity and Glycogen Accumulation in Vitro
2.4.1. Cell Culture and Differentiation
2.4.2. Sulforhodamine B (SRB) Assay
2.4.3. In Vitro Glycogen Content
2.5. Animal Experimental Design
2.5.1. Animals and Diets
2.5.2. Gradual Loaded Exercise Program and Running Endurance Test
2.6. Biochemical Parameters
2.6.1. Biomarkers Related to Fatigue
2.6.2. Analysis of Glycogen Levels in Liver and Muscle
2.6.3. Activities of Muscle Lactate Dehydrogenase (LDH) and Serum Creatine Kinase (CK) Activities
2.6.4. Levels of Malondialdehyde (MDA) and Glutathione Peroxidase (GPx) Activity
2.7. Determination of the Total Phenolic Content (TPC)
2.8. HPLC Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Contents of Organic Acids and Free Amino Acids in PV
3.2. Identification and Quantification of Phenolic Compounds in PV
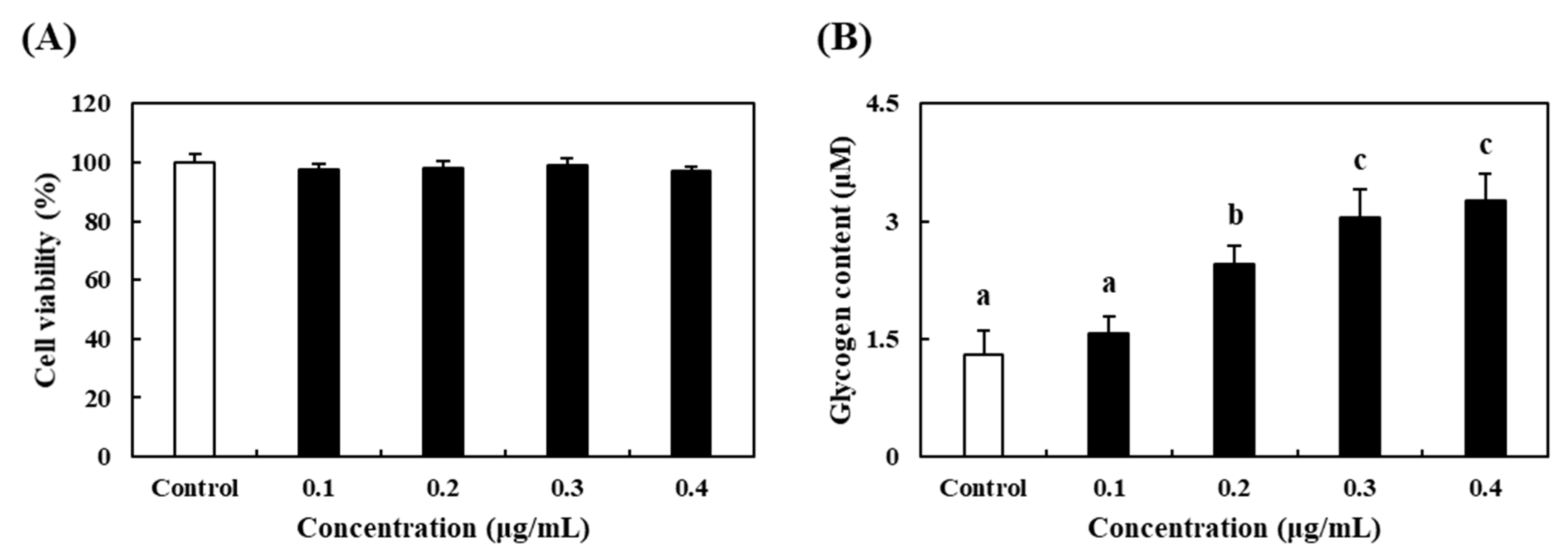
3.3. Effects of PV on Cell Proliferation and Glycogen Accumulation in C2C12 Myoblasts
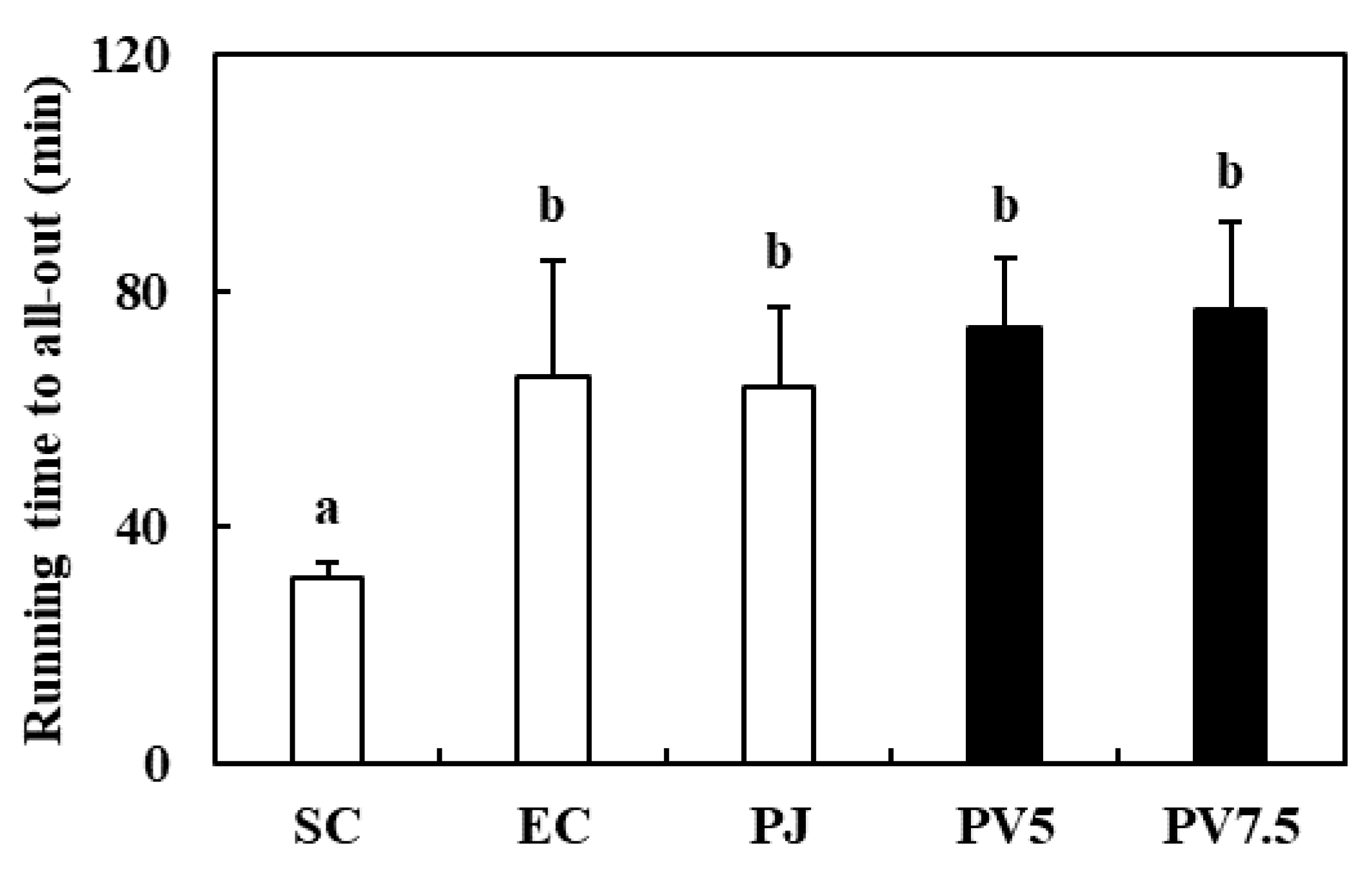
3.4. Effects of PV on Treadmill Running Time
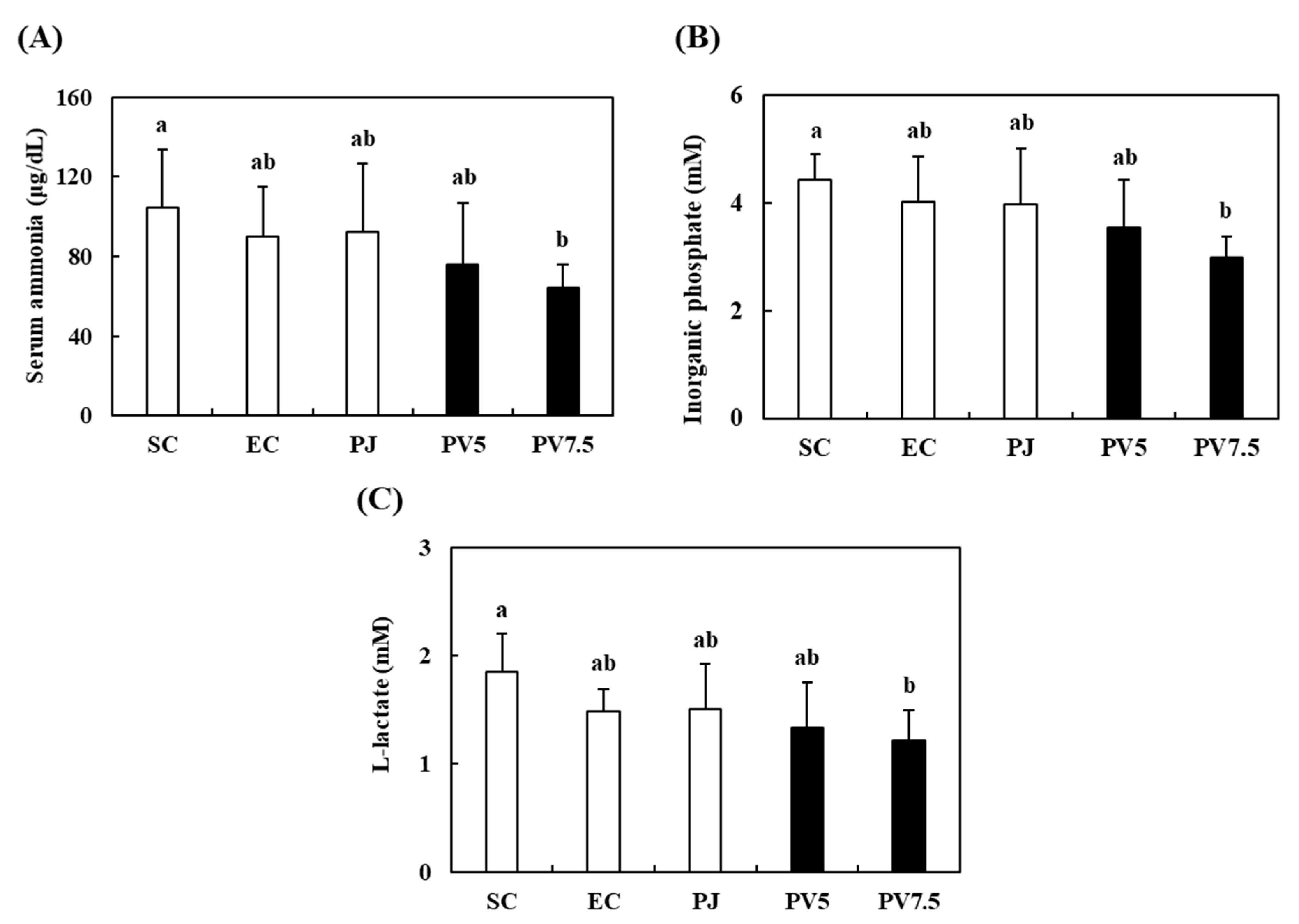
3.5. Effects of PV on Serum Biomarkers Related to Fatigue
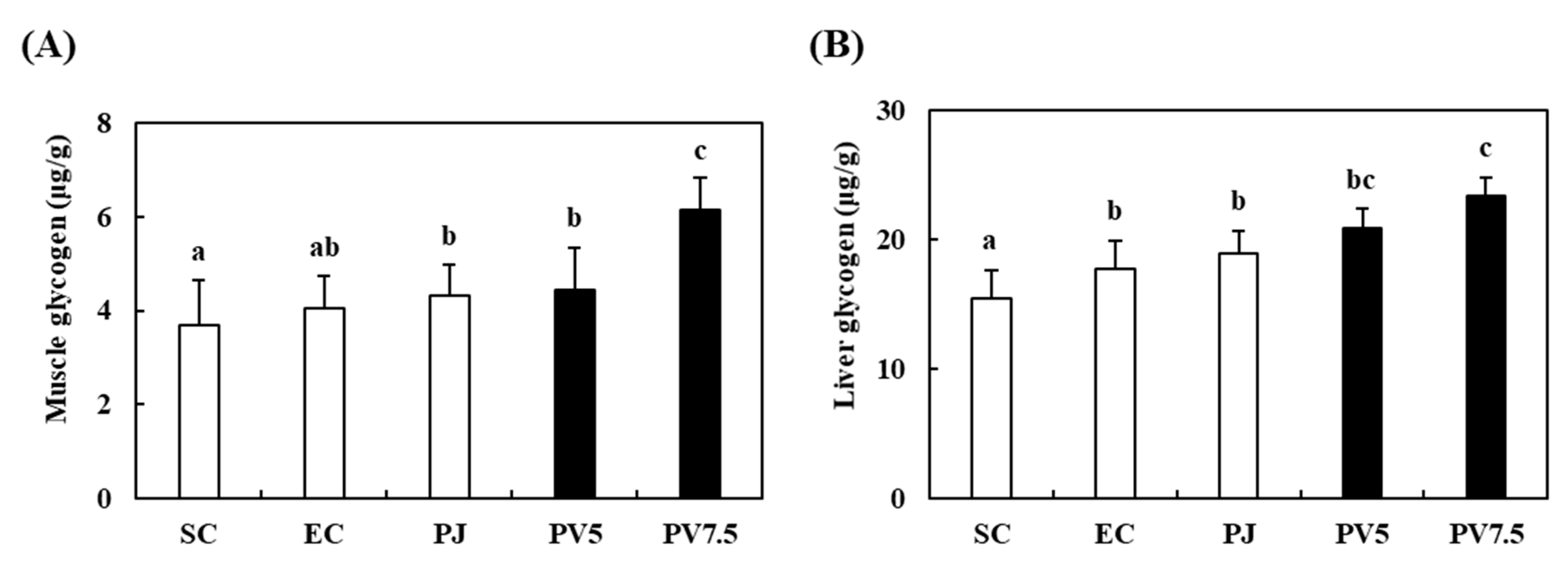
3.6. Effects of PV on Changes of Glycogen Accumulation
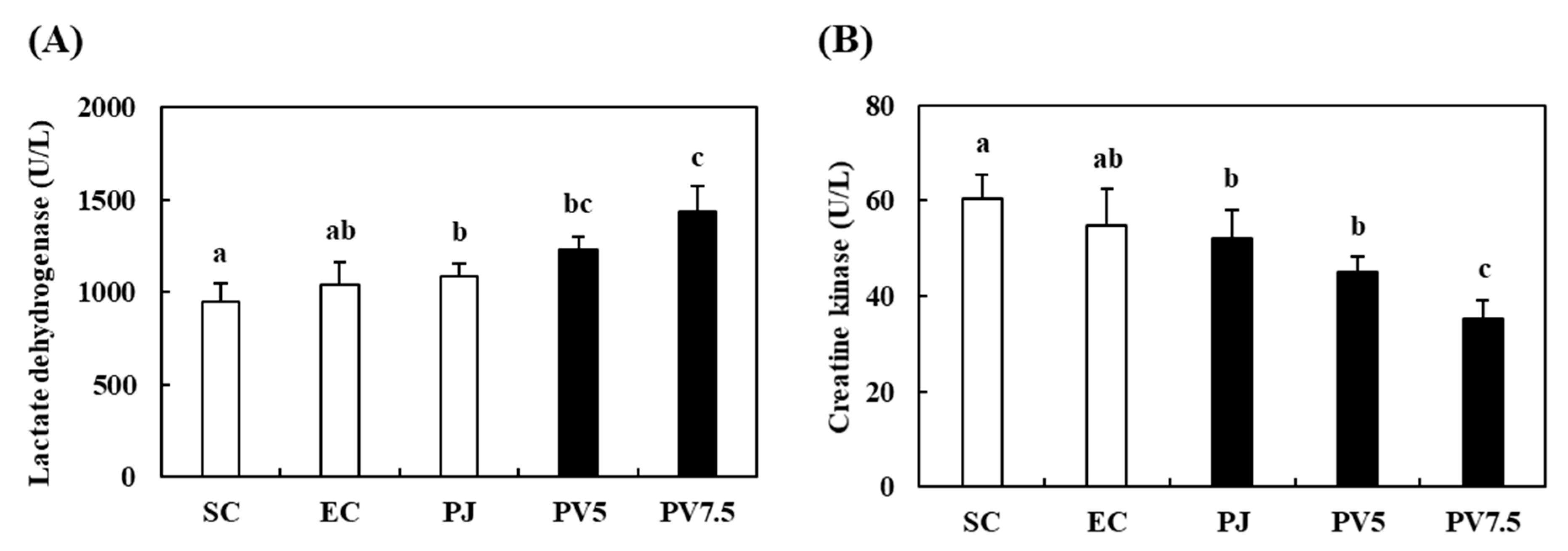
3.7. Effects of PV on Changes of LDH and CK Activities
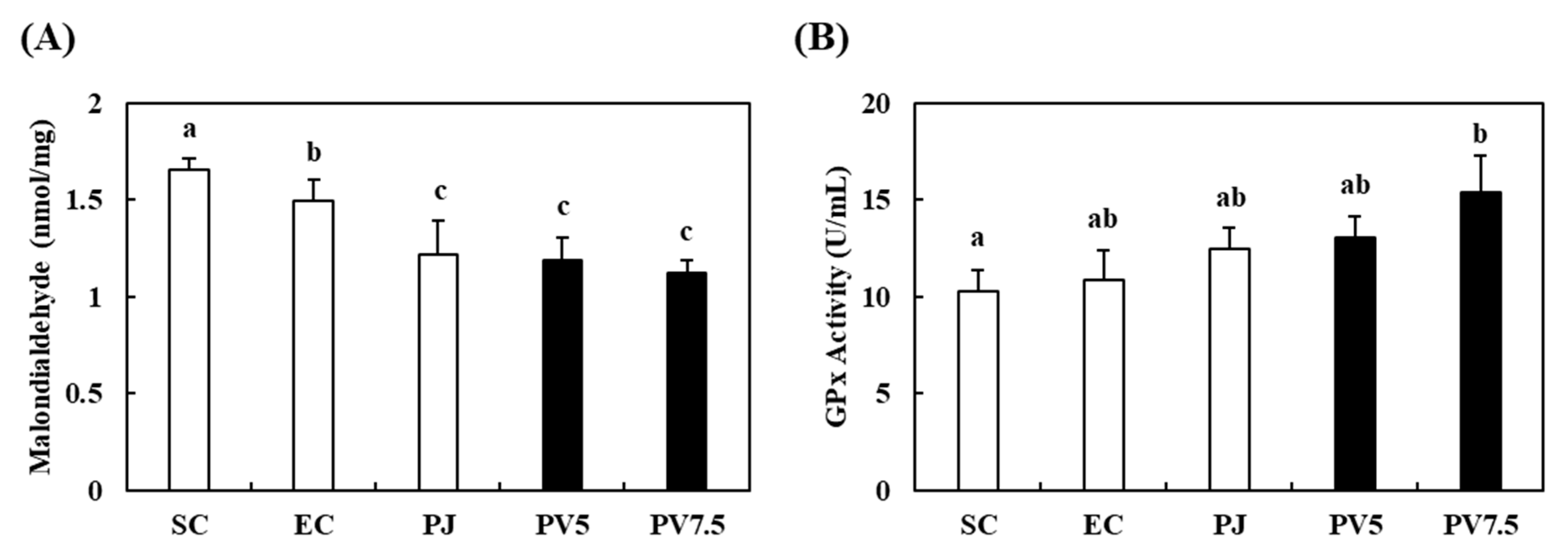
3.8. Effects of PV on Changes in MDA Level and GPx Activity in the Liver
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hwang, J.Y.; Ham, J.W.; Nam, S.H. The antioxidant activity of maesil (Prunus mume). Korean J. Food Sci. Technol. 2004, 36, 461–464. [Google Scholar]
- Paik, I.Y.; Chang, W.R.; Kwak, Y.S.; Cho, S.Y.; Jin, H.E. The effect of Prunus mume supplementation on energy substrate levels and fatigue induction factors. J. Life Sci. 2010, 20, 49–54. [Google Scholar] [CrossRef]
- Nakajima, S.; Fujita, K.; Inoue, Y.; Nishio, M.; Seto, Y. Effect of the folk remedy, Bainiku-ekisu, a concentrate of Prunus mume juice, on Helicobacter pylori infection in humans. Helicobacter 2006, 11, 589–591. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, S.H.; Lee, H.N.; Park, T.S. Prunus mume extract ameliorates exercise-induced fatigue in trained rats. J. Med. Food. 2008, 11, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.D.; Lee, J.H.; Jeong, J.H.; Kim, J.Y.; Yee, S.T.; Park, S.K.; Lee, M.K.; Seo, K.I. Production of novel vinegar having antioxidant and anti-fatigue activities from Salicornia herbacea L. J. Sci. Food Agric. 2016, 96, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zheng, Y.; Liu, X.; Cheng, C.; Zhang, X.; Xia, T.; Yu, S.; Wang, M. Antioxidant activity of Chinese shanxi aged vinegar and its correlation with polyphenols and flavonoids during the brewing process. J. Food Sci. 2017, 82, 2479–2486. [Google Scholar] [CrossRef]
- Kondo, S.; Tayama, K.; Tsukamoto, Y.; Ikeda, K.; Yamori, Y. Antihypertensive effects of acetic acid and vinegar on spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2001, 65, 2690–2694. [Google Scholar] [CrossRef]
- Sakakibara, S.; Yamauchi, T.; Oshima, Y.; Tsukamoto, Y.; Kadowaki, T. Acetic acid activates hepatic AMPK and reduces hyperglycemia in diabetic KK-A(y) mice. Biochem. Bioph. Res. Co. 2006, 344, 597–604. [Google Scholar] [CrossRef]
- Yagnik, D.; Serafin, V.; Shah, A.J. Antimicrobial activity of apple cider vinegar against Escherichia coli, Staphylococcus aureus and Candida albicans; downregulating cytokine and microbial protein expression. Sci. Rep. 2018, 8, 1732–1744. [Google Scholar] [CrossRef]
- Fushimi, T.; Tayama, K.; Fukaya, M.; Kitakoshi, K.; Nakai, N.; Tsukamoto, Y.; Sato, Y. The efficacy of acetic acid for glycogen repletion in rat skeletal muscle after exercise. Int. J. Sports Med. 2002, 23, 218–222. [Google Scholar] [CrossRef]
- Waller, A.P.; Geor, R.J.; Spriet, L.L.; Heigenhauser, G.J.F.; Lindinger, M.I. Oral acetate supplementation after prolonged moderate intensity exercise enhances early muscle glycogen resynthesis in horses. Exp. Physiol. 2009, 94, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.D.; Kim, J.H.; Lee, J.H.; Hong, S.M.; Yee, S.T.; Seo, K.I. Anti-fatigue effect of a cucumber vinegar beverage on rats after high-intensity exercise. Korean J. Food Sci. Technol. 2017, 49, 209–214. [Google Scholar] [CrossRef][Green Version]
- Blain, G.M.; Hureau, T.J. Limitation of fatigue and performance during exercise: The brain-muscle interaction. Exp. Physiol. 2017, 102, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Lv, J.; Lo, Y.M.; Cui, S.W.; Hu, X.; Fan, M. Effects of oat β–glucan on endurance exercise and its anti-fatigue properties in trained rats. Carbohydr. Polym. 2013, 92, 1159–1165. [Google Scholar] [CrossRef]
- Maes, M.; Twisk, F.N.M. Chronic fatigue syndrome: Harvey and Wessely’s (bio)psychosocial model versus a bio(psychosocial) model based on inflammatory and oxidative and nitrosative stress pathways. BMC Med. 2010, 8, 1–13. [Google Scholar] [CrossRef]
- Rittie, L.; Fisher, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress-implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, H.; Cheng, L.; Wang, L.; Wian, H.; Qi, X. In vitro and in vivo antioxidant activity of polyphenols extracted from black highland barley. Food Chem. 2016, 194, 1003–1012. [Google Scholar] [CrossRef]
- Cho, H.D.; Kim, J.H.; Won, Y.S.; Moon, K.D.; Seo, K.I. Inhibitory effects of pectinase-treated Prunus mume fruit concentrate on colorectal cancer proliferation and angiogenesis of endothelial cells. J. Food Sci. 2019, 84, 3284–3295. [Google Scholar] [CrossRef]
- Jung, K.M.; Lee, Y.S.; Kim, J.W.; Seol, J.M.; Jung, Y.H.; Kim, S.R. Low-temperature alcoholic fermentation for the production of high quality vinegar using peach. Korean Soc. Biotechnol. Bioeng. J. 2018, 33, 95–103. [Google Scholar]
- Kong, Y.; Zhang, L.L.; Sun, Y.; Zhang, Y.Y.; Sun, B.G.; Chen, H.T. Determination of the free amino acid, organic acid, and nucleotide in commercial vinegars. J. Food Sci. 2017, 82, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Na, H.S.; Choi, G.C.; Yang, S.I.; Lee, J.H.; Cho, J.Y.; Ma, S.J.; Kim, J.Y. Comparison of characteristics in commercial fermented vinegars made with different ingredients. Korean J. Food Preserv. 2013, 20, 482–487. [Google Scholar] [CrossRef]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural polyphenols for prevention and treatment of cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Merino, J.; Sun, Q.; Fitó, M.; Sales-Salvadó, J. Dietary polyphenols, Mediterranean diet, prediabetes, and Type 2 diabetes: A narrative review of the evidence. Oxid. Med. Cell. Longev. 2017, 2017, 6723931. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Wu, D.; Sun, K.; Tan, X.; Wang, J.; Ren, B.; Zhao, B.; Liu, Z.; Liu, X. Anti-fatigue activity of aqueous extracts of Sonchus arvensis L. in exercised trained mice. Molecules 2019, 24, 1168. [Google Scholar] [CrossRef]
- Xia, F.; Zhong, Y.; Li, M.; Chang, Q.; Liao, Y.; Liu, X.; Pan, R. Antioxidant and anti-fatigue constituents of okra. Nutrients 2015, 7, 8846–8858. [Google Scholar] [CrossRef]
- Reidy, P.T.; Rasmussen, B.B. Role of ingested amino acids and protein in the promotion of resistance exercise-induced muscle protein anabolism. J. Nutr. 2016, 146, 155–183. [Google Scholar] [CrossRef]
- Evans, G.H.; James, L.J.; Shirreffs, S.M.; Maughan, R.J. Optimizing the restoration and maintenance of fluid balance after exercise-induced dehydration. J. Appl. Physiol. 2017, 122, 945–951. [Google Scholar] [CrossRef]
- Robergs, R.A.; Ghiasvand, F.; Parker, D. Biochemistry of exercise-induced metabolic acidosis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R502–R516. [Google Scholar] [CrossRef]
- Fushimi, T.; Tayama, K.; Fukaya, M.; Kitakoshi, K.; Nakai, N.; Txukamoto, Y.; Sato, Y. Acetic acid feeding enhances glycogen repletion in liver and skeletal muscle of rats. J. Nutr. 2001, 131, 1973–1977. [Google Scholar] [CrossRef] [PubMed]
- Stephens, J.W.; Dikeman, M.E.; Unruh, J.A.; Haub, M.D.; Tokach, M.D.; Dritz, S.S. Effects of oral administration of sodium citrate or acetate to pigs on blood parameters, postmortem glycolysis, muscle pH decline, and quality attributes of pork. J. Anim. Sci. 2008, 86, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, W.C.; Wu, Z.Y.; Fu, C.X.; Hui, A.L.; Gao, H.; Chen, P.P.; Du, B.; Zhang, H.W. Two macamide extracts relieve physical fatigue by attenuating muscle damage in mice. J. Sci. Food Agric. 2018, 99, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Filho, L.F.S.; Menezes, P.P.; Santana, D.V.S.; Lima, B.S.; Saravanan, S.; Almeida, G.K.M.; Filho, J.E.R.M.; Santos, M.M.B.; Araujo, A.A.S.; de Oliveira, E.D. Effect of pulsed therapeutic ultrasound and diosmin on skeletal muscle oxidative parameter. Ultrasound Med. Biol. 2018, 44, 359–367. [Google Scholar] [CrossRef]
- Tojima, M.; Noma, K.; Torii, S. Changes in serum creatine kinase, leg muscle tightness, and delayed onset muscle soreness after a full marathon race. J. Sports Med. Phys Fitness. 2016, 56, 782–788. [Google Scholar]
- Pingitore, A.; Lima, G.P.; Mastorci, F.; Quinones, A.; Lervasi, G.; Vassalle, C. Exercise and oxidative stress: Potential effects of antioxidant dietary strategies in sports. Nutrition 2015, 31, 916–922. [Google Scholar] [CrossRef]
- Ranchordas, M.K.; Rogerson, D.; Soltani, H.; Costello, J.T. Antioxidants for preventing and reducing muscle soreness after exercise. Cochrane Database Syst. Rev. 2017, 12, CD009789. [Google Scholar] [CrossRef]
- Chen, J.; Tian, J.; Ge, H.; Liu, R.; Xiao, J. Effects of tetramethylpyrazine from Chinese black vinegar on antioxidant and hypolipidemia activities in HepG2 cells. Food Chem. Toxicol. 2017, 109 Pt 2, 930–940. [Google Scholar] [CrossRef]
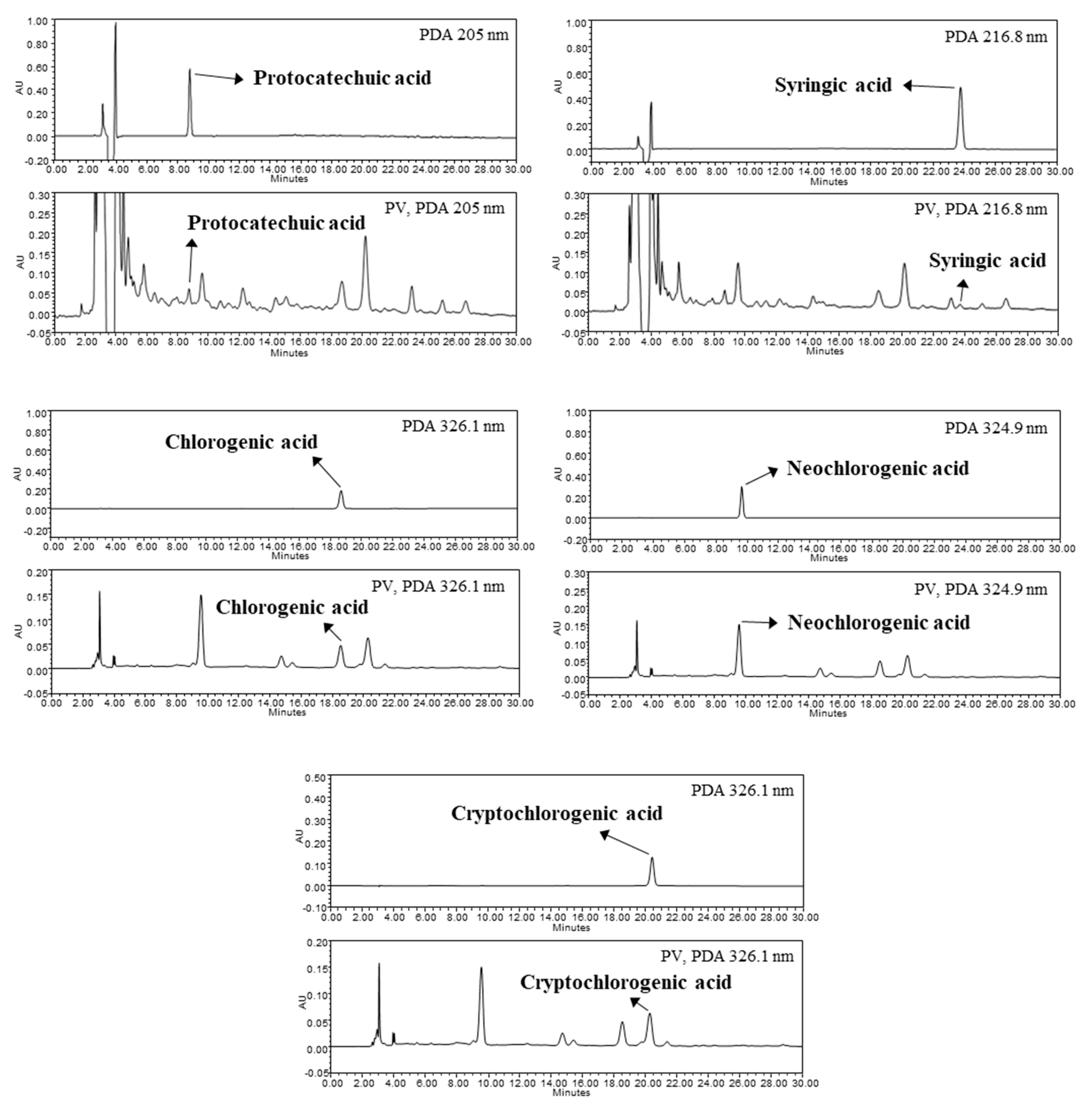
| Organic acids (mg%) | PJ | PV | Free amino acids (ppm) | PJ | PV |
|---|---|---|---|---|---|
| Acetic acid | N.D. | 4034.46 ± 114.37 | Aspartic acid | 19.66 ± 4.19 | 7.56 ± 0.48 |
| Oxalic acid | N.D. | 72.76 ± 4.61 | Tyrosine | 1.12 ± 0.23 | 5.46 ± 0.16 |
| Citric acid | 969.23 ± 37.38 | 1530.65 ± 79.36 | Phenylalanine | 0.33 ± 0.16 | 4.43 ± 0.24 |
| Succinic acid | N.D. | 1075.51 ± 61.72 | Histidine | 0.23 ± 0.11 | 32.93 ± 3.51 |
| Malic acid | 352.83 ± 51.34 | 140.95 ± 10.58 | Lysine | 0.21 ± 0.14 | 4.11 ± 0.37 |
| Lactic acid | N.D. | 390.87 ± 26.34 | Arginine | N.D. | 20.76 ± 1.39 |
| Total organic acids | 1,321.06 ± 73.45 | 7,259.24 ± 211.93 | Total free amino acid | 42.78 ± 8.55 | 83.32 ± 5.62 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Cho, H.-D.; Won, Y.-S.; Hong, S.-M.; Moon, K.-D.; Seo, K.-I. Anti-Fatigue Effect of Prunus Mume Vinegar in High-Intensity Exercised Rats. Nutrients 2020, 12, 1205. https://doi.org/10.3390/nu12051205
Kim J-H, Cho H-D, Won Y-S, Hong S-M, Moon K-D, Seo K-I. Anti-Fatigue Effect of Prunus Mume Vinegar in High-Intensity Exercised Rats. Nutrients. 2020; 12(5):1205. https://doi.org/10.3390/nu12051205
Chicago/Turabian StyleKim, Jeong-Ho, Hyun-Dong Cho, Yeong-Seon Won, Seong-Min Hong, Kwang-Deog Moon, and Kwon-Il Seo. 2020. "Anti-Fatigue Effect of Prunus Mume Vinegar in High-Intensity Exercised Rats" Nutrients 12, no. 5: 1205. https://doi.org/10.3390/nu12051205
APA StyleKim, J.-H., Cho, H.-D., Won, Y.-S., Hong, S.-M., Moon, K.-D., & Seo, K.-I. (2020). Anti-Fatigue Effect of Prunus Mume Vinegar in High-Intensity Exercised Rats. Nutrients, 12(5), 1205. https://doi.org/10.3390/nu12051205





