CYP27B1 Gene Polymorphism rs10877012 in Patients Diagnosed with Colorectal Cancer
Abstract
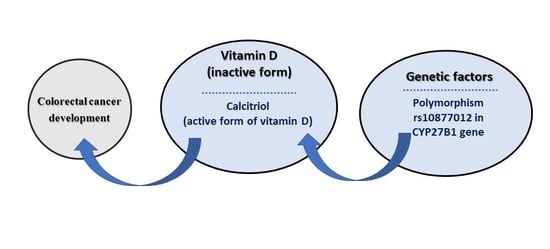
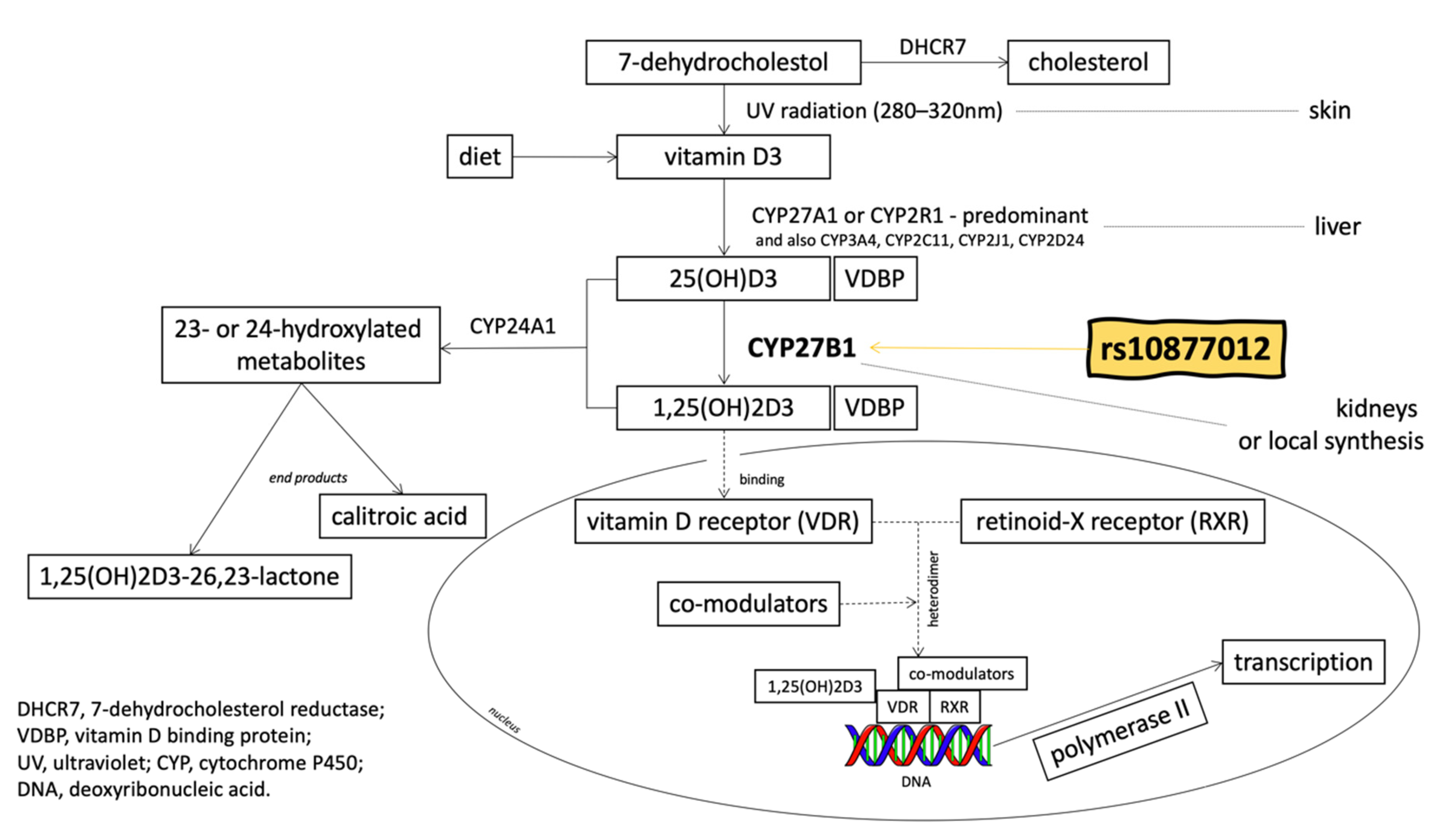
1. Introduction
2. Materials and Methods
2.1. Material Analysis
2.2. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Wroński, K.; Bocian, R. The patients knowledge about the role of calcium in the primary prevention of colorectal cancer. Contemp. Oncol. 2012, 16, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Deeb, K.; Trump, D.; Johnson, C. Vitamin D signalling pathways in cancer: Potential for anticancer therapeutics. Nat. Rev. Cancer 2007, 7, 684–700. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H.; Torikai, S.; Kurokawa, K. Calcitonin selectively stimulates 25-hydroxyvitamin D3-1 alpha-hydroxylase in the proximal straight tubule of the rat kidney. Nature 1981, 291, 327–329. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H.; Kurokawa, K. Unique hormonal regulation of vitamin D metabolism in the mammalian kidney. Miner. Electrolyte Metab. 1983, 9, 227–235. [Google Scholar] [PubMed]
- Tangpricha, V.; Flanagan, J.N.; Whitlatch, L.W.; Tseng, C.C.; Chen, T.C.; Holt, P.R.; Lipkin, M.S.; Holick, M.F. 25-hydroxyvitamin D-1alpha-hydroxylase in normal and malignant colon tissue. Lancet 2001, 357, 1673–1674. [Google Scholar] [CrossRef]
- Sun, J. The role of vitamin D and vitamin D receptors in colon cancer. Clin. Transl. Gastroenterol. 2017, 8, e103. [Google Scholar] [CrossRef]
- Jenkinson, C. The vitamin D metabolome: An update on analysis and function. Cell Biochem. Funct. 2019, 37, 408–423. [Google Scholar] [CrossRef]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef]
- Pereira, F.; Larriba, M.J.; Munoz, A. Vitamin D and colon cancer. Endocr. Relat. Cancer 2012, 19, R51–R71. [Google Scholar] [CrossRef]
- Munemitsu, S.; Albert, I.; Souza, B.; Rubinfeld, B.; Polakis, P. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc. Natl. Acad. Sci. USA 1995, 92, 3046–3050. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Honma, T.; Matsuda, Y.; Suzuki, Y.; Narisawa, R.; Ajioka, Y.; Asakura, H. Nuclear translocation of beta-catenin in colorectal cancer. Br. J. Cancer 2000, 82, 1689–1693. [Google Scholar] [PubMed]
- Cano, A.; Pérez-Moreno, M.; Rodrigo, I.; Locascio, A.; Blanco, M.J.; del Barrio, M.G.; Portillo, F.; Nieto, M.A. The transcription factor Snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2000, 2, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Larriba, M.J.; Munoz, A. SNAIL vs. vitamin D receptor expression in colon cancer: Therapeutics implications. Br. J. Cancer 2005, 92, 985–989. [Google Scholar] [CrossRef]
- Bises, G.; Kállay, E.; Weiland, T.; Wrba, F.; Wenzl, E.; Bonner, E.; Kriwanek, S.; Obrist, P.; Cross, H.S. 25-hydroxyvitamin D3-1α-hydroxylase expression in normal and malignant human colon. J. Histochem. Cytochem. 2004, 52, 985–989. [Google Scholar] [CrossRef]
- Genetic Home Refences. Available online: https://ghr.nlm.nih.gov/gene/CYP27B1 (accessed on 24 February 2020).
- Kong, X.F.; Zhu, X.H.; Pei, Y.L.; Jackson, D.M.; Holick, M.F. Molecular cloning, characterization, and promoter analysis of the human 25-hydroxyvitamin D(3)-1-alpha-hydroxylase gene. Proc. Natl. Acad. Sci. USA 1999, 96, 6988–6993. [Google Scholar] [CrossRef]
- Fu, G.K.; Portale, A.A.; Miller, W.L. Complete structure of the human gene for the vitamin D 1-alpha-hydroxylase, P450cl-alpha. DNA Cell Biol. 1997, 16, 1499–1507. [Google Scholar] [CrossRef]
- dbSNP (The Single Nucleotide Polymorphism Database). Available online: https://www.ncbi.nlm.nih.gov/snp/rs10877012 (accessed on 24 February 2020).
- Vidigal, V.M.; Silva, T.D.; de Oliveira, J.; Pimenta, C.; Felipe, A.V.; Forones, N.M. Genetic polymorphisms of vitamin D receptor (VDR), CYP27B1 and CYP24A1 genes and the risk of colorectal cancer. Int. J. Biol. Markers 2017, 32, e224–e230. [Google Scholar] [CrossRef]
- Gong, C.; Long, Z.; Yu, Y.; Zhu, L.; Tian, J.; Li, S.; Li, J.; Yu, H.; Chi, Q.; Piao, D.; et al. Dietary factors and polymorphisms in vitamin D metabolism genes: The risk and prognosis of colorectal cancer in northeast China. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Marques Vidigal, V.; Aguiar Junior, P.N.; Donizetti Silva, T.; de Oliveira, J.; Marques Pimenta, C.A.; Vitor Felipe, A.; Manoukian Forones, N. Genetic polymorphisms of vitamin D metabolism genes and serum level of vitamin D in colorectal cancer. Int. J. Biol. Markers 2017, 32, e441–e446. [Google Scholar] [CrossRef]
- McGrath, J.J.; Saha, S.; Burne, T.H.; Eyles, D.W. A systematic review of the association between common single nucleotide polymorphisms and 25-hydroxyvitamin D concentrations. J. Steroid. Biochem. Mol. Biol. 2010, 121, 471–477. [Google Scholar] [CrossRef]
- Bu, F.X.; Armas, L.; Lappe, J.; Zhou, Y.; Gao, G.; Wang, H.W.; Recker, R.; Zhao, L.J. Comprehensive association analysis of nine candidate genes with serum 25-hydroxy vitamin D levels among healthy Caucasian subjects. Hum. Genet. 2010, 128, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Pibiri, F.; Kittles, R.; Sandler, R.S.; Keku, T.O.; Kupfer, S.S.; Xicola, R.M.; Llor, X.; Ellis, N.A. Genetic variation in vitamin D-related genes and risk of colorectal cancer in African Americans. Cancer Causes Control 2014, 25, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Zhang, F.; Richards, J.B.; Kestenbaum, B.; van Meurs, J.B.; Berry, D.; Kiel, D.P.; Streeten, E.A.; Ohlsson, C.; Koller, D.L.; et al. Common genetic determinants of vitamin D insufficiency: A genome-wide association study. Lancet 2010, 376, 180–188. [Google Scholar] [CrossRef]
- O’Brien, K.M.; Sandler, D.P.; Taylor, J.A.; Weinberg, C.R. Serum vitamin D and risk of breast cancer within five years. Environ. Health Perspect. 2017, 125, 077004. [Google Scholar] [CrossRef] [PubMed]
- Cusato, J.; Boglione, L.; De Nicolò, A.; Favata, F.; Ariaudo, A.; Mornese Pinna, S.; Guido, F.; Avataneo, V.; Cantù, M.; Carcieri, C.; et al. Vitamin D pathway gene polymorphisms and hepatocellular carcinoma in chronic hepatitis C-affected patients treated with new drugs. Cancer Chemother. Pharmacol. 2018, 81, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Xu, F.; Qu, J.; Wang, Y.; Gao, M.; Yu, H.; Qian, B. Genetic polymorphisms in the vitamin D pathway in relation to lung cancer risk and survival. Oncotarget 2015, 6, 2573–2582. [Google Scholar] [CrossRef]
- Dong, L.M.; Ulrich, C.M.; Hsu, L.; Duggan, D.J.; Benitez, D.S.; White, E.; Slattery, M.L.; Farin, F.M.; Makar, K.W.; Carlson, C.S.; et al. Vitamin D related genes, CYP24A1 and CYP27B1, and colon cancer risk. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2540–2548. [Google Scholar] [CrossRef]
- Hibler, E.A.; Klimentidis, Y.C.; Jurutka, P.W.; Kohler, L.N.; Lance, P.; Roe, D.J.; Thompson, P.A.; Jacobs, E.T. CYP24A1 and CYP27B1 polymorphisms, concentrations of vitamin D metabolites, and odds of colorectal adenoma recurrence. Nutr. Cancer 2015, 67, 1131–1141. [Google Scholar] [CrossRef]
- Falleti, E.; Cmet, S.; Fabris, C.; Fattovich, G.; Cussigh, A.; Bitetto, D.; Ceriani, E.; Lenisa, I.; Dissegna, D.; Ieluzzi, D.; et al. Genetic polymorphisms of vitamin D pathway predict antiviral treatment outcome in slow responder naïve patients with chronic hepatitis C. PLoS ONE 2013, 8, e80764. [Google Scholar] [CrossRef]
- American Cancer Society. Available online: https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/staged.html (accessed on 24 February 2020).
- Signorello, L.B.; Shi, J.; Cai, Q.; Zheng, W.; Williams, S.M.; Long, J.; Cohen, S.S.; Li, G.; Hollis, B.W.; Smith, J.R.; et al. Common variation in vitamin D pathway genes predicts circulating 25- hydroxyvitamin D levels among African Americans. PLoS ONE 2011, 6, e28623. [Google Scholar] [CrossRef] [PubMed]
- Hypponen, E.; Berry, D.J.; Wjst, M.; Power, C. Serum 25-hydroxyvitamin D and IgE—A significant but nonlinear relationship. Allergy 2009, 64, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, E.T.; Van Pelt, C.; Forster, R.E.; Zaidi, W.; Hibler, E.A.; Galligan, M.A.; Haussler, M.R.; Jurutka, P.W. CYP24A1 and CYP27B1 polymorphisms modulate vitamin D metabolism in colon cancer cells. Cancer Res. 2013, 73, 2563–2573. [Google Scholar] [CrossRef] [PubMed]
- Fedirko, V.; Mandle, H.B.; Zhu, W.; Hughes, D.J.; Siddiq, A.; Ferrari, P.; Romieu, I.; Riboli, E.; Bueno-de-Mesquita, B.; van Duijnhoven, F.; et al. Vitamin D-Related Genes, Blood Vitamin D Levels and Colorectal Cancer Risk in Western European Populations. Nutrients 2019, 11, 1954. [Google Scholar] [CrossRef]
- Pan, Z.; Chen, M.; Hu, X.; Wang, H.; Yang, J.; Zhang, C.; Pan, F.; Sun, G. Associations between VDR gene polymorphisms and colorectal cancer susceptibility: An updated meta-analysis based on 39 case-control studies. Oncotarget 2018, 9, 13068–13076. [Google Scholar] [CrossRef][Green Version]
- Barry, E.L.; Peacock, J.L.; Rees, J.R.; Bostick, R.M.; Robertson, D.J.; Bresalier, R.S.; Baron, J.A. Vitamin D receptor genotype, vitamin D3 supplementation, and risk of colorectal adenomas: A randomized clinical trial. JAMA Oncol. 2017, 3, 628–635. [Google Scholar] [CrossRef]
- Giovannucci, E.; Liu, Y.; Rimm, E.B.; Hollis, B.W.; Fuchs, C.S.; Stampfer, M.J.; Willett, W.C. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J. Natl. Cancer Inst. 2006, 98, 451–459. [Google Scholar] [CrossRef]
- DeLuca, H.F.; Zierold, C. Mechanisms and functions of vitamin D. Nutr. Rev. 1998, 56, S4–S10. [Google Scholar] [CrossRef]
- Ryan-Harshman, M.; Aldoori, W. Diet and colorectal cancer. Can. Fam. Physician 2007, 53, 1913–1920. [Google Scholar]
- Lucas, C.; Barnich, N.; Nguyen, H. Microbiota, inflammation and colorectal cancer. Int. J. Mol. Sci. 2017, 18, 1310. [Google Scholar] [CrossRef]
- Rodriguez-Antona, C.; Ingelman-Sundberg, M. Cytochrome P450 pharmacogenetics and cancer. Oncogene 2006, 25, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- McFadyen, M.C.E.; Melvin, W.T.; Murray, G.I. Cytochrome P450 enzymes: Novel options for cancer therapeutics. Mol. Cancer Ther. 2004, 3, 363–371. [Google Scholar] [PubMed]
- Ingelman-Sundberg, M.; Rodriguez-Antona, C. Pharmacogenetics of drug-metabolizing enzymes: Implications for a safer and more effective drug therapy. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2005, 360, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Ingelman-Sundberg, M. Human drug metabolising cytochrome P450 enzymes: Properties and polymorphisms. Naunyn Schmiedeberg Arch. Pharmacol. 2004, 369, 89–104. [Google Scholar] [CrossRef]
- Savoie, M.B.; Paciorek, A.; Zhang, L.; Van Blarigan, E.L.; Sommovilla, N.; Abrams, D.; Atreya, C.E.; Bergsland, E.K.; Chern, H.; Kelley, R.K.; et al. Vitamin D Levels in Patients with Colorectal Cancer Before and After Treatment Initiation. J. Gastrointest. Cancer 2019, 50, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R. The metabolism and mechanism of action of 1.25-dihydroxyvitamin D3. Kidney Int. 1986, 30, 793–803. [Google Scholar] [CrossRef]
| CRC Patients Group (n = 106) | Control Group (n = 219) | p-Value | |
|---|---|---|---|
| Age: years (mean ± SEM) | 64.0 ± 1.8 | 54.6 ± 0.7 | <0.0001 |
| Gender, n (%) | |||
| Female | 38 (35.8) | 153 (69.8) | |
| Male | 68 (64.1) | 66 (30.1) | <0.0001 |
| Tumor stage, n (%) | |||
| I | 23 (21.7) | ||
| II | 31 (29.2) | ||
| III | 29 (27.4) | ||
| IV | 23 (21.7) | ||
| Pathological tumor stage (pT), n (%) | |||
| T1 | 0 (0) | ||
| T2 | 22 (20.8) | ||
| T3 | 64 (60.4) | ||
| T4 | 20 (18.8) | ||
| Pathological nodal status (pN), n (%) | |||
| no lymph node metastasis | 50 (47.2) | ||
| lymph node metastasis | 56 (52.8) | ||
| Pathological metastasis status (pM), n (%) | |||
| no distant metastasis | 66 (62.3) | ||
| metastasis to distant organs | 40 (37.7) |
| Genotype/Allele | CRC n (%) | Control n (%) | OR (95% CI) Control vs. CRC | p-Value |
|---|---|---|---|---|
| TT | 11 (10) | 54 (25) | - | - |
| GT | 50 (47) | 96 (44) | 2.56 (1.23–5.32) | 0.01 |
| GG | 45 (42) | 69 (32) | 3.20 (1.51–6.77) | 0.002 |
| G | 140 (66) | 234 (53) | ||
| T | 72 (34) | 204 (47) | ||
| Control vs. CRC | ||||
| TT vs. | 22 (14) | 108 (32) | ||
| GT + GG | 140 (86) | 234 (68) | 2.94 (1.77–4.86) | <0.0001 |
| For Females | ||||
| Genotype/Allele | CRC n (%) | Control n (%) | OR (95% CI) Control vs. CRC | p-Value |
| TT | 3 (8) | 40 (26) | - | - |
| GT | 20 (53) | 59 (39) | 4.52 (1.26–16.23) | 0.02 |
| GG | 15 (39) | 54 (35) | 3.70 (1.00–13.66) | 0.049 |
| Control vs. CRC | ||||
| TT vs. | 6 (11) | 80 (32) | ||
| GT + GG | 50 (89) | 167 (68) | 3.99 (1.64–9.70) | 0.0022 |
| For Males | ||||
| Genotype/Allele | CRC n (%) | Control n (%) | OR (95% CI) Control vs. CRC | p-Value |
| TT | 8 (12) | 14 (21) | - | - |
| GT | 30 (44) | 37 (56) | 1.42 (0.53–3.83) | 0.49 |
| GG | 30 (44) | 15 (23) | 3.50 (1.20–10.17) | 0.02 |
| Control vs. CRC | ||||
| TT vs. | 16 (15) | 28 (29) | ||
| GT + GG | 90 (85) | 67 (71) | 1.31 (0.64–2.67) | 0.46 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latacz, M.; Snarska, J.; Kostyra, E.; Wroński, K.; Fiedorowicz, E.; Savelkoul, H.; Jarmołowska, B.; Płomiński, J.; Grzybowski, R.; Cieślińska, A. CYP27B1 Gene Polymorphism rs10877012 in Patients Diagnosed with Colorectal Cancer. Nutrients 2020, 12, 998. https://doi.org/10.3390/nu12040998
Latacz M, Snarska J, Kostyra E, Wroński K, Fiedorowicz E, Savelkoul H, Jarmołowska B, Płomiński J, Grzybowski R, Cieślińska A. CYP27B1 Gene Polymorphism rs10877012 in Patients Diagnosed with Colorectal Cancer. Nutrients. 2020; 12(4):998. https://doi.org/10.3390/nu12040998
Chicago/Turabian StyleLatacz, Maria, Jadwiga Snarska, Elżbieta Kostyra, Konrad Wroński, Ewa Fiedorowicz, Huub Savelkoul, Beata Jarmołowska, Janusz Płomiński, Roman Grzybowski, and Anna Cieślińska. 2020. "CYP27B1 Gene Polymorphism rs10877012 in Patients Diagnosed with Colorectal Cancer" Nutrients 12, no. 4: 998. https://doi.org/10.3390/nu12040998
APA StyleLatacz, M., Snarska, J., Kostyra, E., Wroński, K., Fiedorowicz, E., Savelkoul, H., Jarmołowska, B., Płomiński, J., Grzybowski, R., & Cieślińska, A. (2020). CYP27B1 Gene Polymorphism rs10877012 in Patients Diagnosed with Colorectal Cancer. Nutrients, 12(4), 998. https://doi.org/10.3390/nu12040998