Food, Eating, and the Gastrointestinal Tract
Abstract
1. Introduction
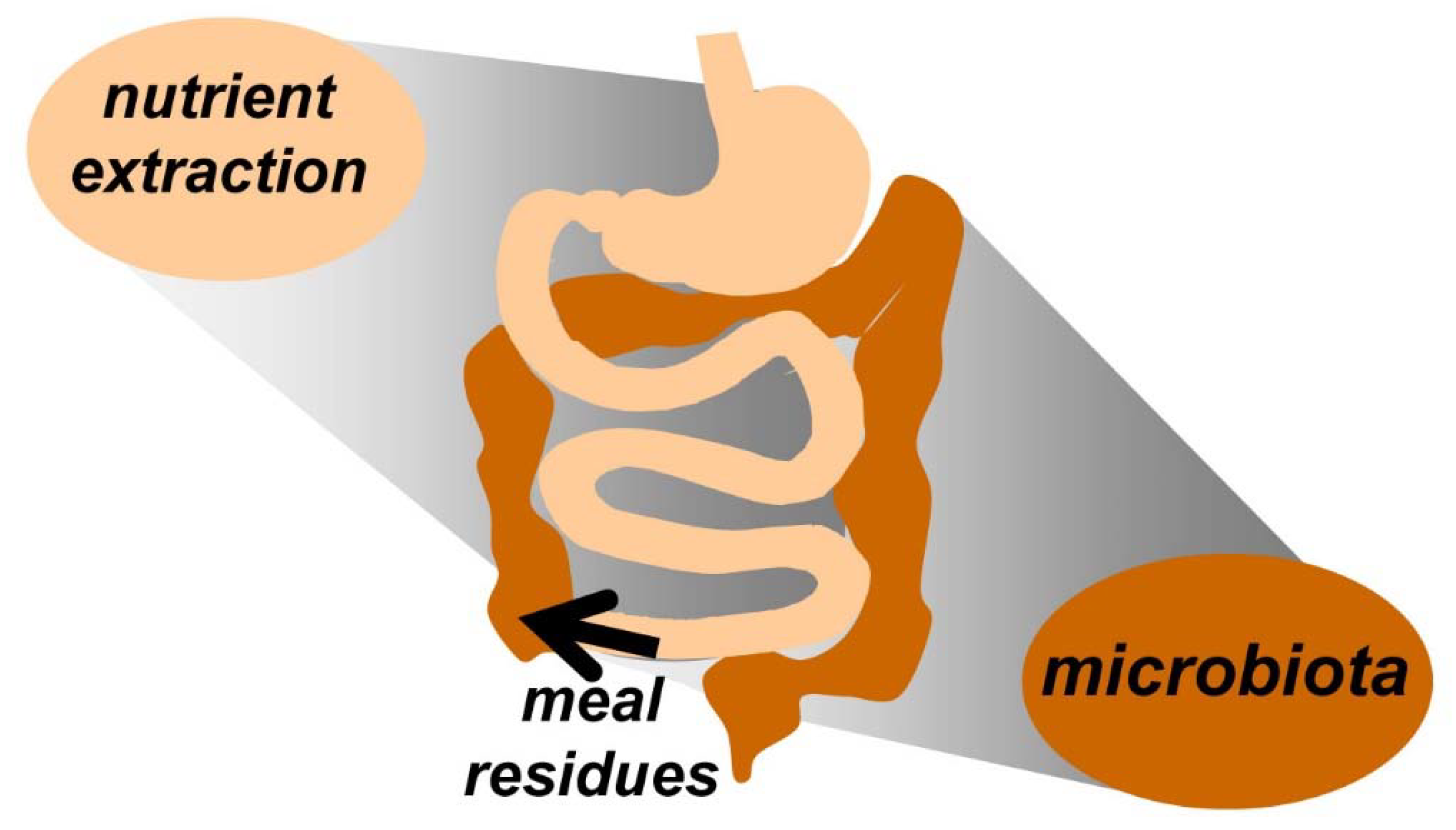
2. Physiological Responses to Meal Ingestion
3. Food Ingestion and the Brain–Gut Axis
4. Sensations before and during Food Ingestion
4.1. Taste
4.2. Flavor
4.3. Palatability
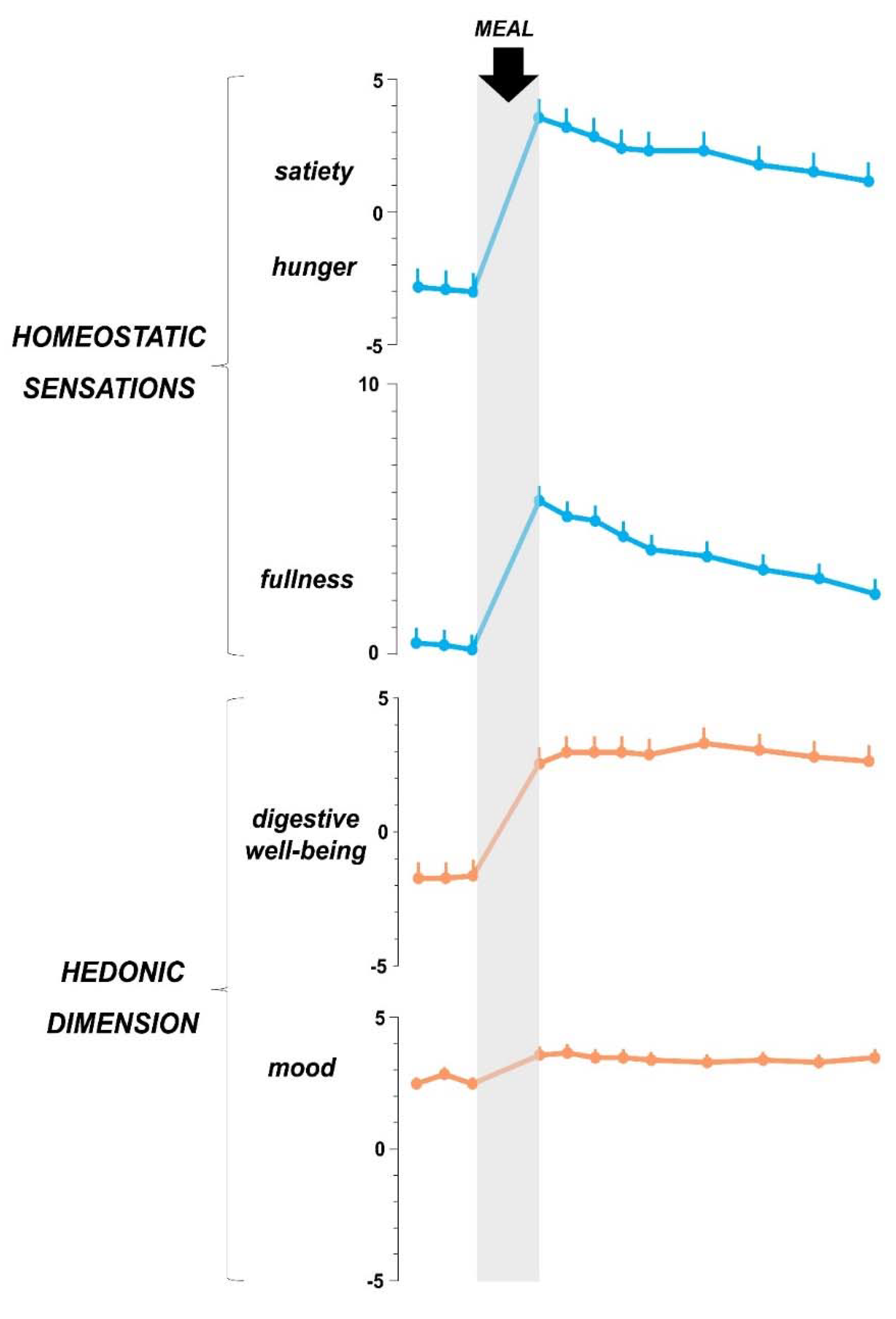
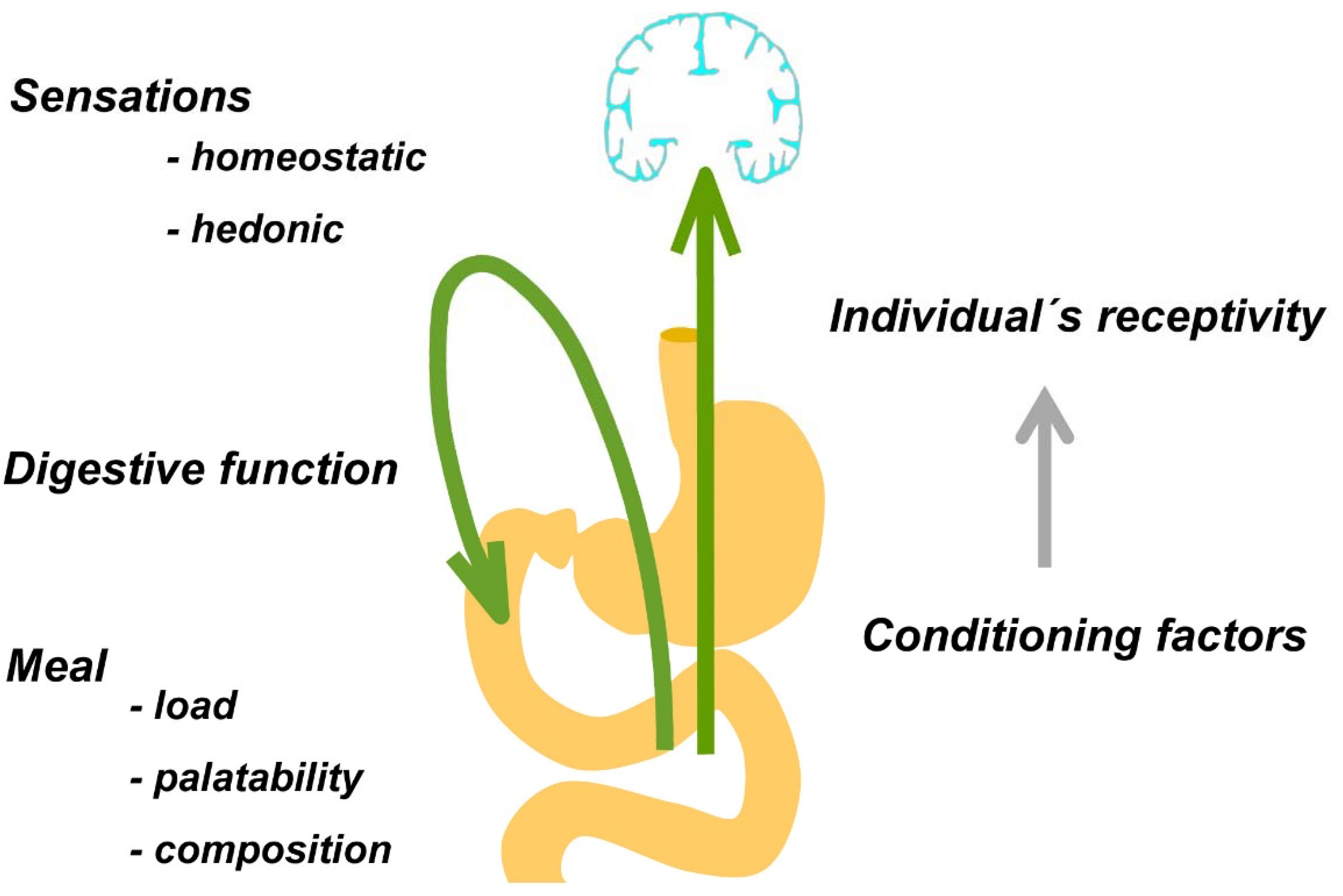
5. The Postprandial Experience
5.1. Digestive Function
5.2. Characteristics of the Meal
5.2.1. Meal Load
5.2.2. Meal Palatability
5.2.3. Meal Composition
5.3. The Individual’s Receptivity
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pribic, T.; Azpiroz, F. Biogastronomy: Factors that determine the biological response to meal ingestion. Neurogastroenterol. Motil. 2018, 30, e13309. [Google Scholar] [CrossRef] [PubMed]
- Deloose, E.; Tack, J. Redefining the functional roles of the gastrointestinal migrating motor complex and motilin in small bacterial overgrowth and hunger signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G228–G233. [Google Scholar] [CrossRef] [PubMed]
- Azpiroz, F.; Feinle, C.; Grundy, D.; Tack, J. Gastric sensitivity and reflexes: Basic mechanism underlying clinical problems. J. Gastroenterol. 2014, 49, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Boeckxstaens, G.; Camilleri, M.; Sifrim, D.; Houghton, L.A.; Elsenbruch, S.; Lindberg, G.; Azpiroz, F.; Parkman, H.P. Fundamentals of Neurogastroenterology: Physiology/Motility—Sensation. Gastroenterology 2016, 150, 1292–1304. [Google Scholar] [CrossRef] [PubMed]
- Bendezu, R.A.; Mego, M.; Monclus, E.; Merino, X.; Accarino, A.; Malagelada, J.R.; Navazo, I.; Azpiroz, F. Colonic content: Effect of diet, meals, and defecation. Neurogastroenterol. Motil. 2017, 29, e12930. [Google Scholar] [CrossRef]
- Azpiroz, F. Intestinal gas. In Pathophysiology, Diagnosis, Management, 10th ed.; Feldman, M., Friedman, L.S., Brand, L.J., Eds.; Elsevier: Philadelphia, PA, USA, 2015; pp. 242–250. [Google Scholar]
- Manichanh, C.; Eck, A.; Varela, E.; Roca, J.; Clemente, J.C.; Gonzalez, A.; Knights, D.; Knight, R.; Estrella, S.; Hernandez, C.; et al. Anal gas evacuation and colonic microbiota in patients with flatulence: Effect of diet. Gut 2013, 63, 401–408. [Google Scholar] [CrossRef]
- Mego, M.; Accarino, A.; Malagelada, J.R.; Guarner, F.; Azpiroz, F. Accumulative effect of food residues on intestinal gas production. Neurogastroenterol. Motil. 2015, 27, 1621–1628. [Google Scholar] [CrossRef]
- Burri, E.; Cisternas, D.; Villoria, A.; Accarino, A.; Soldevilla, A.; Malagelada, J.R.; Azpiroz, F. Abdominal accommodation induced by meal ingestion: Differential responses to gastric and colonic volume loads. Neurogastroenterol. Mot. 2013, 25, 339-e253. [Google Scholar] [CrossRef]
- Burri, E.; Barba, E.; Huaman, J.W.; Cisternas, D.; Accarino, A.; Soldevilla, A.; Malagelada, J.R.; Azpiroz, F. Mechanisms of postprandial abdominal bloating and distension in functional dyspepsia. Gut 2014, 63, 395–400. [Google Scholar] [CrossRef]
- Vanis, L.; Gentilcore, D.; Lange, K.; Gilja, O.H.; Rigda, R.S.; Trahair, L.G.; Feinle-Bisset, C.; Rayner, C.K.; Horowitz, M.; Jones, K.L. Effects of variations in intragastric volume on blood pressure and splanchnic blood flow during intraduodenal glucose infusion in healthy older subjects. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R391–R399. [Google Scholar] [CrossRef]
- Malagelada, C.; Accarino, A.; Molne, L.; Mendez, S.; Campos, E.; Gonzalez, A.; Malagelada, J.R.; Azpiroz, F. Digestive, cognitive and hedonic responses to a meal. Neurogastroenterol. Motil. 2015, 27, 389–396. [Google Scholar] [CrossRef]
- Malagelada, C.; Barba, I.; Accarino, A.; Molne, L.; Mendez, S.; Campos, E.; Gonzalez, A.; Alonso-Cotoner, C.; Santos, J.; Malagelada, J.R.; et al. Cognitive and hedonic responses to meal ingestion correlate with changes in circulating metabolites. Neurogastroenterol. Motil. 2016, 28, 1806–1814. [Google Scholar] [CrossRef]
- Ciccantelli, B.; Pribic, T.; Malagelada, C.; Accarino, A.; Azpiroz, F. Relation between cognitive and hedonic responses to a meal. Neurogastroenterol. Motil. 2017, 29, e13011. [Google Scholar] [CrossRef]
- Camilleri, M. Peripheral mechanisms in appetite regulation. Gastroenterology 2015, 148, 1219–1233. [Google Scholar] [CrossRef]
- Blundell, J.; de Graaf, C.; Hulshof, T.; Jebb, S.; Livingstone, B.; Lluch, A.; Mela, D.; Salah, S.; Schuring, E.; van der Knaap, H.; et al. Appetite control: Methodological aspects of the evaluation of foods. Obes. Rev. 2010, 11, 251–270. [Google Scholar] [CrossRef]
- Feinle-Bisset, C. Upper gastrointestinal sensitivity to meal-related signals in adult humans—Relevance to appetite regulation and gut symptoms in health, obesity and functional dyspepsia. Physiol. Behav. 2016, 162, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Egecioglu, E.; Skibicka, K.P.; Hansson, C.; Alvarez-Crespo, M.; Friberg, P.A.; Jerlhag, E.; Engel, J.A.; Dickson, S.L. Hedonic and incentive signals for body weight control. Rev. Endocr. Metab. Disord. 2011, 12, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Weltens, N.; Zhao, D.; Van Oudenhove, L. Where is the comfort in comfort foods? Mechanisms linking fat signaling, reward, and emotion. Neurogastroenterol Motil. 2014, 26, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Pribic, T.; Kilpatrick, L.; Ciccantelli, B.; Malagelada, C.; Accarino, A.; Rovira, A.; Pareto, D.; Mayer, E.; Azpiroz, F. Brain networks associated with cognitive and hedonic responses to a meal. Neurogastroenterol. Motil. 2017, 29, e13031. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; An, R.; Zhang, Y.; Li, X.; Wang, S. Correlations of macronutrient-induced functional magnetic resonance imaging signal changes in human brain and gut hormone responses. Am. J. Clin. Nutr. 2012, 96, 275–282. [Google Scholar] [CrossRef]
- Page, K.A.; Chan, O.; Arora, J.; Belfort-Deaguiar, R.; Dzuira, J.; Roehmholdt, B.; Cline, G.W.; Naik, S.; Sinha, R.; Constable, R.T.; et al. Effects of fructose vs glucose on regional cerebral blood flow in brain regions involved with appetite and reward pathways. JAMA 2013, 309, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.; Linder, K.; Kullmann, S.; Heni, M.; Ketterer, C.; Cavusoglu, M.; Krzeminski, A.; Fritsche, A.; Häring, H.U.; Preissl, H.; et al. Fat intake modulates cerebral blood flow in homeostatic and gustatory brain areas in humans. Am. J. Clin. Nutr. 2012, 95, 1342–1349. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Francis, S.T.; Eldeghaidy, S. Imaging methodologies and applications for nutrition research: What can functional MRI offer? Proc. Nutr. Soc. 2015, 74, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.S.; Preissl, H.; Enck, P. How to Perform and Interpret Functional Magnetic Resonance Imaging Studies in Functional Gastrointestinal Disorders. J. Neurogastroenterol. Motil. 2017, 23, 197–207. [Google Scholar] [CrossRef]
- Ly, H.G.; Dupont, P.; Van Laere, K.; Depoortere, I.; Tack, J.; Van Oudenhove, L. Differential brain responses to gradual intragastric nutrient infusion and gastric balloon distension: A role for gut peptides? Neuroimage 2017, 144, 101–112. [Google Scholar] [CrossRef]
- Simon, J.J.; Wetzel, A.; Sinno, M.H.; Skunde, M.; Bendszus, M.; Preissl, H.; Enck, P.; Herzog, W.; Friederich, H.C. Integration of homeostatic signaling and food reward processing in the human brain. JCI Insight 2017, 2. [Google Scholar] [CrossRef]
- Zanchi, D.; Depoorter, A.; Egloff, L.; Haller, S.; Mahlmann, L.; Lang, U.E.; Drewe, J.; Beglinger, C.; Schmidt, A.; Borgwardt, S. The impact of gut hormones on the neural circuit of appetite and satiety: A systematic review. Neurosci. Biobehav. Rev. 2017, 80, 457–475. [Google Scholar] [CrossRef]
- Astarita, G.; Langridge, J. An emerging role for metabolomics in nutrition science. J. Nutr. Nutr. 2013, 6, 181–200. [Google Scholar] [CrossRef]
- Bondia-Pons, I.; Canellas, N.; Abete, I.; Rodriguez, M.A.; Perez-Cornago, A.; Navas-Carretero, S.; Zulet, M.A.; Correig, X.; Martínez, J.A. Nutri-metabolomics: Subtle serum metabolic differences in healthy subjects by NMR-based metabolomics after a short-term nutritional intervention with two tomato sauces. Omics 2013, 17, 611–618. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, A.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef]
- Lyte, M. Microbial endocrinology: Host-microbiota neuroendocrine interactions influencing brain and behavior. Gut Microbes. 2014, 5, 381–389. [Google Scholar] [CrossRef]
- Mayer, E.A.; Hsiao, E.Y. The Gut and Its Microbiome as Related to Central Nervous System Functioning and Psychological Well-being: Introduction to the Special Issue of Psychosomatic Medicine. Psychosom. Med. 2017, 79, 844–846. [Google Scholar] [CrossRef]
- Smeets, P.A.; Erkner, A.; De Graaf, C. Cephalic phase responses and appetite. Nutr. Rev. 2010, 68, 643–655. [Google Scholar] [CrossRef]
- Berridge, K.C. ‘Liking’ and ‘wanting’ food rewards: Brain substrates and roles in eating disorders. Physiol. Behav. 2009, 97, 537–550. [Google Scholar] [CrossRef]
- Finlayson, G.; King, N.; Blundell, J. The role of implicit wanting in relation to explicit liking and wanting for food: Implications for appetite control. Appetite 2008, 50, 120–127. [Google Scholar] [CrossRef]
- Phan, U.T.; Chambers, E.T. Motivations for choosing various food groups based on individual foods. Appetite 2016, 105, 204–211. [Google Scholar] [CrossRef]
- Mela, D.J. Determinants of food choice: Relationships with obesity and weight control. Obes. Res. 2001, 9, 249S–255S. [Google Scholar] [CrossRef]
- Mela, D.J. Eating for pleasure or just wanting to eat? Reconsidering sensory hedonic responses as a driver of obesity. Appetite 2006, 47, 10–17. [Google Scholar] [CrossRef]
- Lutter, M.; Nestler, E.J. Homeostatic and hedonic signals interact in the regulation of food intake. J. Nutr. 2009, 139, 629–632. [Google Scholar] [CrossRef]
- Schultes, B.; Ernst, B.; Wilms, B.; Thurnheer, M.; Hallschmid, M. Hedonic hunger is increased in severely obese patients and is reduced after gastric bypass surgery. Am. J. Clin. Nutr. 2010, 92, 277–283. [Google Scholar] [CrossRef]
- Witt, A.A.; Lowe, M.R. Hedonic hunger and binge eating among women with eating disorders. Int. J. Eat. Disord. 2014, 47, 273–280. [Google Scholar] [CrossRef]
- Small, D.M. Flavor is in the brain. Physiol. Behav. 2012, 107, 540–552. [Google Scholar] [CrossRef]
- Auvray, M.; Spence, C. The multisensory perception of flavor. Conscious. Cogn. 2008, 17, 1016–1031. [Google Scholar] [CrossRef]
- Lindemann, B. Receptors and transduction in taste. Nature 2001, 413, 219–225. [Google Scholar] [CrossRef]
- Pandurangan, M.; Hwang, I. Systemic mechanism of taste, flavour and palatability in brain. Appl. Biochem. Biotechnol. 2015, 175, 3133–3147. [Google Scholar] [CrossRef]
- Chaudhari, N.; Roper, S.D. The cell biology of taste. J. Cell Biol. 2010, 190, 285–296. [Google Scholar] [CrossRef]
- Lawless, H.T.; Stevens, D.A.; Chapman, K.W.; Kurtz, A. Metallic taste from electrical and chemical stimulation. Chem. Senses 2005, 30, 185–194. [Google Scholar] [CrossRef]
- Reed, D.R. Birth of a new breed of supertaster. Chem. Senses 2008, 33, 489–491. [Google Scholar] [CrossRef]
- Robino, A.; Mezzavilla, M.; Pirastu, N.; Dognini, M.; Tepper, B.J.; Gasparini, P. A population-based approach to study the impact of PROP perception on food liking in populations along the Silk Road. PLoS ONE 2014, 9, e91716. [Google Scholar] [CrossRef]
- Deloose, E.; Corsetti, M.; Van Oudenhove, L.; Depoortere, I.; Tack, J. Intragastric infusion of the bitter tastant quinine suppresses hormone release and antral motility during the fasting state in healthy female volunteers. Neurogastroenterol. Motil. 2018, 30, e13171. [Google Scholar] [CrossRef]
- Avau, B.; Rotondo, A.; Thijs, T.; Andrews, C.N.; Janssen, P.; Tack, J.; Depoortere, I. Targeting extra-oral bitter taste receptors modulates gastrointestinal motility with effects on satiation. Sci. Rep. 2015, 5, 15985. [Google Scholar] [CrossRef]
- Cvijanovic, N.; Isaacs, N.J.; Rayner, C.K.; Feinle-Bisset, C.; Young, R.L.; Little, T.J. Lipid stimulation of fatty acid sensors in the human duodenum: Relationship with gastrointestinal hormones, BMI and diet. Int. J. Obes. 2017, 41, 233–239. [Google Scholar] [CrossRef]
- Depoortere, I. Taste receptors of the gut: Emerging roles in health and disease. Gut 2014, 63, 179–190. [Google Scholar] [CrossRef]
- Delwiche, J. The impact of perceptual interactions on perceived flavor. Food Qual. Prefer. 2004, 15, 137–146. [Google Scholar] [CrossRef]
- Doty, R.L. Olfaction. Annu. Rev. Psychol. 2001, 52, 423–452. [Google Scholar] [CrossRef]
- Barham, P.; Skibsted, L.H.; Bredie, W.L.; Frost, M.B.; Moller, P.; Risbo, J.; Snitkjær, P.; Mortensen, L.M. Molecular gastronomy: A new emerging scientific discipline. Chem. Rev. 2010, 110, 2313–2365. [Google Scholar] [CrossRef]
- Gilad, Y.; Lancet, D. Population differences in the human functional olfactory repertoire. Mol. Biol. Evol. 2003, 20, 307–314. [Google Scholar] [CrossRef]
- DuBose, C.; Cardello, A.; Maller, O. Effects of colorants and flavorants on identification, perceived flavor intensity, and hedonic quality of fruit-flavored beverages and cake. J. Food Sci. 1980, 45, 1393–1399. [Google Scholar] [CrossRef]
- Yeomans, M.R. Taste, palatability and the control of appetite. Proc. Nutr. Soc. 1998, 57, 609–615. [Google Scholar] [CrossRef]
- Sauer, H.; Ohla, K.; Dammann, D.; Teufel, M.; Zipfel, S.; Enck, P.; Mack, I. Changes in Gustatory Function and Taste Preference Following Weight Loss. J. Pediatr. 2017, 182, 120–126. [Google Scholar] [CrossRef]
- Sorensen, L.B.; Moller, P.; Flint, A.; Martens, M.; Raben, A. Effect of sensory perception of foods on appetite and food intake: A review of studies on humans. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 1152–1166. [Google Scholar] [CrossRef]
- Monrroy, H.; Pribic, T.; Galan, C.; Nieto, A.; Amigo, N.; Accarino, A.; Correig, X.; Azpiroz, F. Meal Enjoyment and Tolerance in Women and Men. Nutrients 2019, 11, 119. [Google Scholar] [CrossRef]
- Pribic, T.; Hernandez, L.; Nieto, A.; Malagelada, C.; Accarino, A.; Azpiroz, F. Effects of meal palatability on postprandial sensations. Neurogastroenterol. Motil. 2018, 30, e13197. [Google Scholar] [CrossRef]
- Pribic, T.; Vilaseca, H.; Nieto, A.; Hernandez, L.; Monrroy, H.; Malagelada, C.; Accarino, A.; Roca, J.; Azpiroz, F. Meal composition influences postprandial sensations independently of valence and gustation. Neurogastroenterol. Motil. 2018, 30, e13337. [Google Scholar] [CrossRef]
- Masihy, M.; Monrroy, H.; Borghi, G.; Pribic, T.; Galan, C.; Nieto, A.; Accarino, A.; Azpiroz, F. Influence of Eating Schedule on the Postprandial Response: Gender Differences. Nutrients 2019, 11, 401. [Google Scholar] [CrossRef]
- Pribic, T.; Nieto, A.; Hernandez, L.; Malagelada, C.; Accarino, A.; Azpiroz, F. Appetite influences the responses to meal ingestion. Neurogastroenterol. Motil. 2017, 29. [Google Scholar] [CrossRef]
- Pribic, T.; Vilaseca, H.; Nieto, A.; Hernandez, L.; Malagelada, C.; Accarino, A.; Roca, J.; Azpiroz, F. Education of the postprandial experience by a sensory-cognitive intervention. Neurogastroenterol. Motil. 2018, 30. [Google Scholar] [CrossRef]
- Tack, J.; Deloose, E.; Ang, D.; Scarpellini, E.; Vanuytsel, T.; Van Oudenhove, L.; Depoortere, I. Motilin-induced gastric contractions signal hunger in man. Gut 2016, 65, 214–224. [Google Scholar] [CrossRef]
- Halawi, H.; Camilleri, M.; Acosta, A.; Vazquez-Roque, M.; Oduyebo, I.; Burton, D.; Busciglio, I.; Zinsmeister, A.R. Relationship of gastric emptying or accommodation with satiation, satiety, and postprandial symptoms in health. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G442–G447. [Google Scholar] [CrossRef]
- Feinle, C.; Azpiroz, F. Dietary and life-style factors in funcional dyspepsia. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 150–157. [Google Scholar] [CrossRef]
- Feinle, C.; Azpiroz, F. Dietary lipids and functional gastrointestinal disorders. Am. J. Gastroenterol. 2013, 108, 737–747. [Google Scholar] [CrossRef]
- Hajishafiee, M.; Bitarafan, V.; Feinle-Bisset, C. Gastrointestinal Sensing of Meal-Related Signals in Humans, and Dysregulations in Eating-Related Disorders. Nutrients 2019, 11, 1298. [Google Scholar] [CrossRef]
- Monrroy, H.; Borghi, G.; Pribic, T.; Galan, C.; Nieto, A.; Amigo, N.; Accarino, A.; Correig, X.; Azpiroz, F. Biological Response to Meal Ingestion: Gender Differences. Nutrients 2019, 11, 702. [Google Scholar] [CrossRef]
| Reference | Design and Outcomes | Aim | Participants | Interventions | Main Results | Conclusions |
|---|---|---|---|---|---|---|
| Malagelada et al., 2015 [12] | Randomized, crossover trial Responses to test meal (a) sensations by scales (b) gastric tone by barostat | Effect of digestive function on perception | Healthy volunteers: 25 women 17 men | Distortion of digestive function by gastric distention or duodenal lipids | Experimental distortion of digestive function affects independently homeostatic and hedonic sensations after a meal | The digestive function determines the postprandial experience; homeostatic and hedonic sensations are independent |
| Malagelada et al., 2016 [13] | Open label study Responses to test meal (a) sensations by scales (b) metabolomic analysis | Metabolomic biomarkers of postprandial sensations | Healthy volunteers: 9 women 9 men | Ingestion of a test meal at the rate of 50 mL/min at until maximum satiation | (a) satiation correlated with increase in glucose and valine; (b) well-being and decrease in choice eating correlated with increase in triglycerides; (c) abdominal discomfort inversely correlated with increase in lipids | Postprandial sensations correlate with changes in circulating metabolites |
| Pribic et al., 2017 [20] | Open label study Responses to probe meal (a) sensations by scales (b) fMRI scans before and after probe meal | Brain networks related to postprandial sensations | 38 healthy males | Probe meal on two days with and without fMRI | (a) sensations were similar with and without fMRI; (b) Ingestion was associated with increase in thalamo-cortical connectivity and decrease in insular-cortical connectivity; (c) a larger decrease in insular-anterior cingulate cortex connectivity and was associated with higher satiety, fullness, and digestive well-being | Postprandial sensations correlate with changes in brain connectivity functional networks |
| DuBose et al., 1980 [59] | Open label study Identification of flavor of test foods | Influence of food color on flavor perception | Healthy volunteers | Test foods with colorants and flavorants: (a) masking of color; (b) color-flavor incongruence (e.g., green colored -orange flavor). | Color masking or distortion impaired flavor identification | Flavor perception is influenced by color of food |
| Monrroy et al., 2019 [63] | Randomized parallel trial. Sensations in response to comfort meal by scales | Role of gender on the responses to a comfort meal | Healthy volunteers: 10 women 10 men | Comfort meal ingested stepwise until full satiation | In women the meal loads required to achieve maximal satisfaction and full satiation were smaller than in men. Hence women enjoyed and tolerated smaller meal loads than men | Gender is a constitutive factor that determines the meal experience |
| Pribic et al., 2018 [64] | Randomized crossover trial. Sensations in response to test meals by scales | Effect of palatability on postprandial sensations | 22 healthy men | 2 meals with identical composition and physical characteristics but different palatability: (a) conventional (potato cream followed by vanilla cream); (b) unconventional meal (mixture of both creams). | The unconventional was found less palatable and meal produced more fullness and less satisfaction than the conventional meal | Food palatability bears a direct relation to hedonic but inverse relation to homeostatic sensations. |
| Pribic et al., 2018 [65] | Randomized crossover trial. Sensations in response to test meals by scales | Influence of meal composition independently of palatability on postprandial sensations | 12 healthy men | 2 meals with the same physical and organoleptic characteristics (taste, smell, texture, color, and temperature) but different composition: (a) low-fat; (b) high-fat test meal | While palatability was similar, the high-fat mal induced more satisfaction than the low-fat meal, without significant differences in homeostatic sensations | Meal composition determines the postprandial experience independently of palatability. |
| Masihy et al., 2019 [66] | Randomized parallel trial. Responses to probe meal: (a) sensations by scales (b) physiological measures | Influence of eating schedule on postprandial responses: gender effects | Healthy volunteers: 10 women 10 men | Lunch-type meal eaten at: (a) habitual afternoon schedule; (b) unconventional morning schedule | No schedule effect on physiological responses to probe meal in women and men were detected. However, in contrast to men, in women, the probe meal at unconventional time induced less satisfaction than at the conventional time | Women are more susceptible to the influence of eating schedule on the postprandial experience than men. |
| Pribic et al., 2017 [67] | Randomized cross over. Sensations in response to test meals by scales | Influence of appetite on postprandial experience | 12 healthy men | Probe meal consumed two hours after: (a) low-calorie breakfast; (b) high-calorie breakfast | As compared to the low-calorie breakfast, with the high-calorie breakfast subjects were less hungry before the probe meal and experienced more postprandial fullness and less satisfaction | Appetite before the meal influences the postprandial experience |
| Pribic et al., 2018 [68] | Randomized, parallel study. Sensations in response to probe meal by scales. | Influence of education on postprandial experience | Healthy men: 14 per group | Administration of probe meal on 2 days without and with prior educational intervention. One group received a sensory-cognitive intervention and the other a sham intervention | The sensory-cognitive intervention enhanced homeostatic and hedonic sensations after the probe meal, whereas the sham intervention had no effect | The receptiveness of the subject and the postprandial experience can be conditioned by education |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Livovsky, D.M.; Pribic, T.; Azpiroz, F. Food, Eating, and the Gastrointestinal Tract. Nutrients 2020, 12, 986. https://doi.org/10.3390/nu12040986
Livovsky DM, Pribic T, Azpiroz F. Food, Eating, and the Gastrointestinal Tract. Nutrients. 2020; 12(4):986. https://doi.org/10.3390/nu12040986
Chicago/Turabian StyleLivovsky, Dan M, Teorora Pribic, and Fernando Azpiroz. 2020. "Food, Eating, and the Gastrointestinal Tract" Nutrients 12, no. 4: 986. https://doi.org/10.3390/nu12040986
APA StyleLivovsky, D. M., Pribic, T., & Azpiroz, F. (2020). Food, Eating, and the Gastrointestinal Tract. Nutrients, 12(4), 986. https://doi.org/10.3390/nu12040986






