Weight-Reducing Effect of Lactobacillus Plantarum ZJUFT17 Isolated from Sourdough Ecosystem
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of L. Plantarum ZJUFT17
2.2. Animals and Treatment
2.3. Histological Analysis
2.4. Serum Biochemical Analysis
2.5. Gut Microbiota Analysis
2.6. Statistical Analysis
3. Results
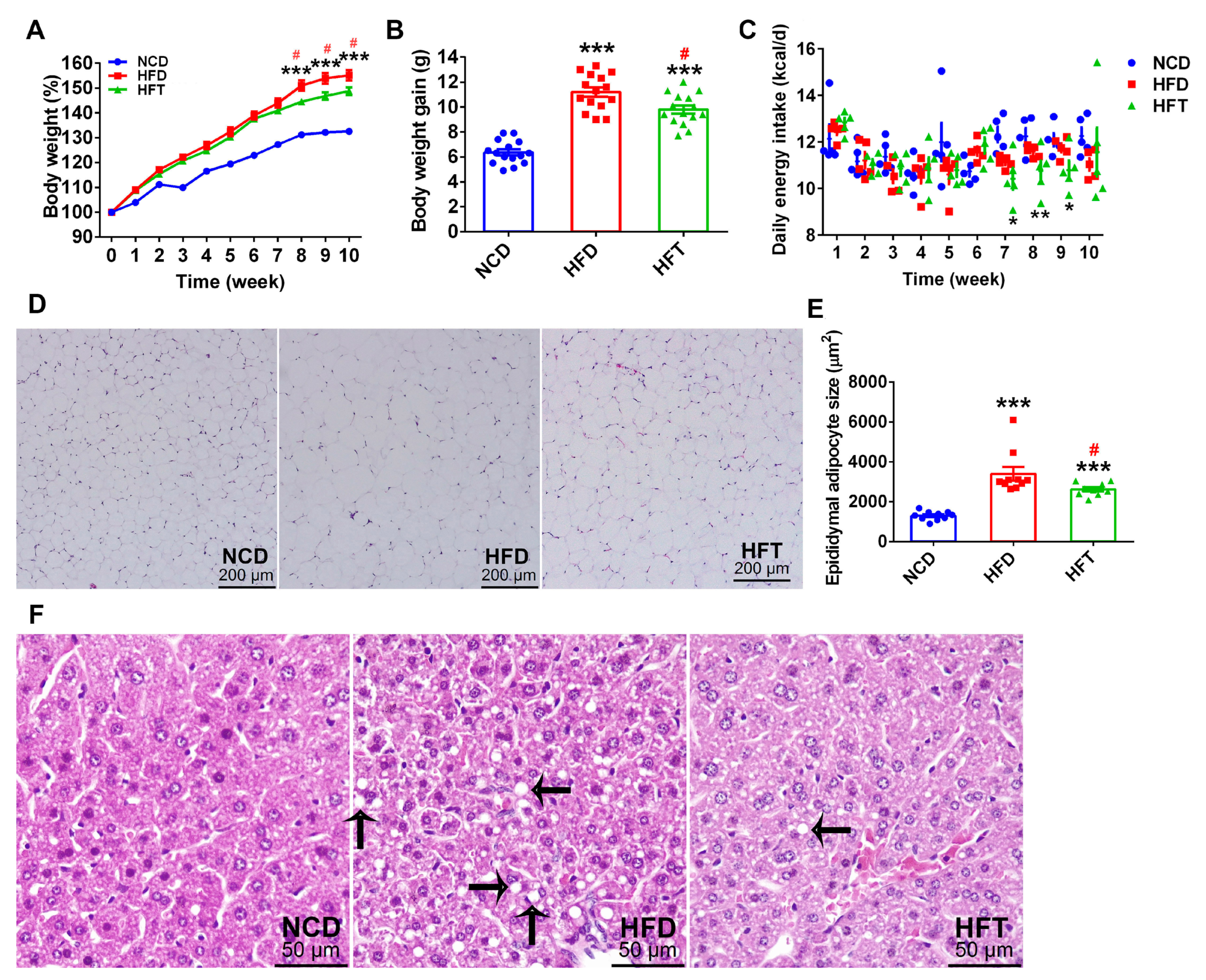
3.1. L. Plantarum ZJUFT17 Alleviated HFD-Induced Obesity and Lipid Accumulation
3.2. L. Plantarum ZJUFT17 Improved Serum Lipid Profiles
3.3. L. Plantarum ZJUFT17 Modulated the Serum Levels of Insulin and Adipokines
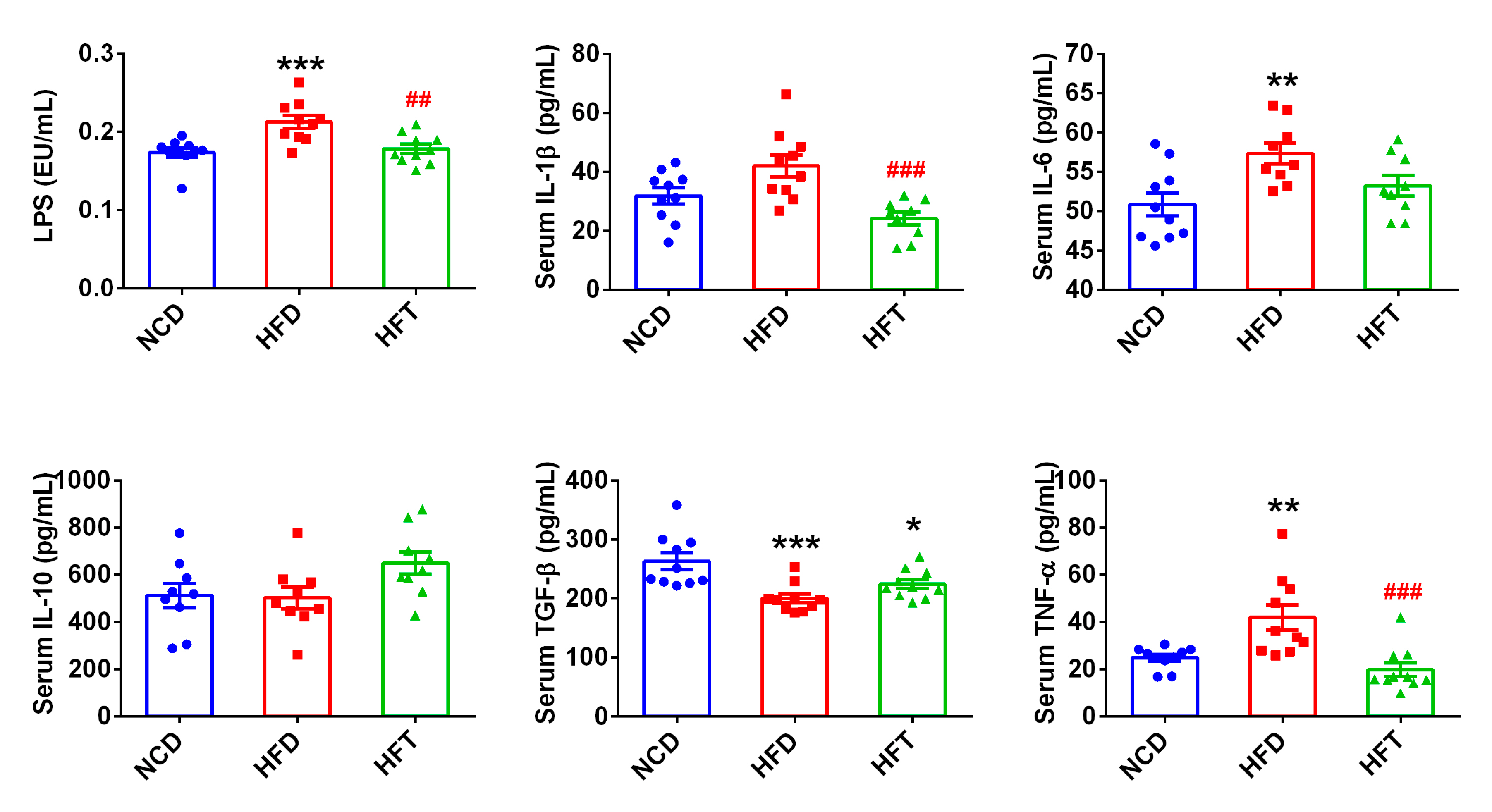
3.4. L. Plantarum ZJUFT17 Attenuated Systemic Inflammation Induced by HFD
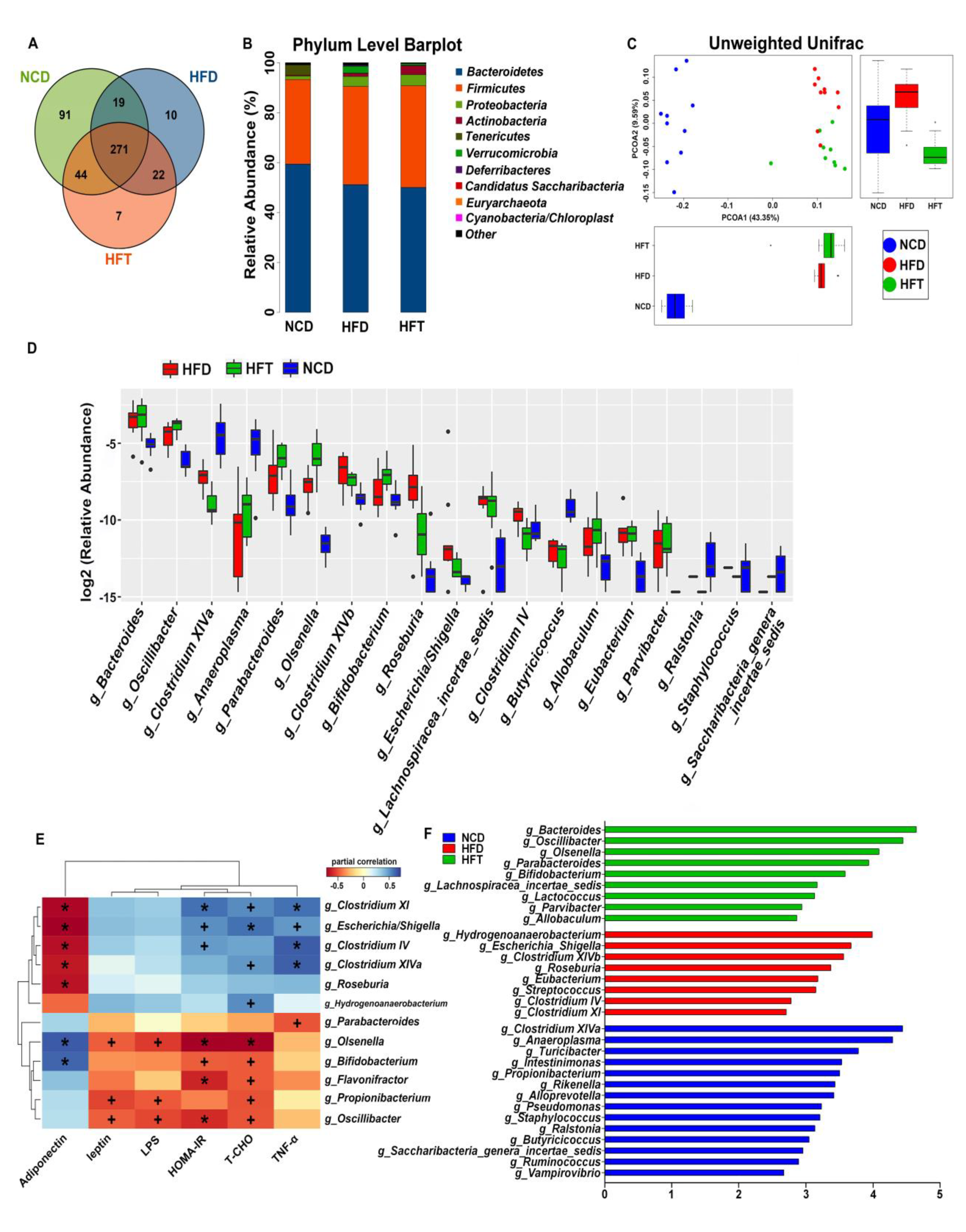
3.5. High-Fat Diet and L. Plantarum ZJUFT17 Administration Altered Gut Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethics Statement
References
- De Ferranti, S.; Mozaffarian, D. The perfect storm: Obesity, adipocyte dysfunction, and metabolic consequences. Clin. Chem. 2008, 54, 945–955. [Google Scholar] [CrossRef]
- Cox, A.J.; West, N.P.; Cripps, A.W. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015, 3, 207–215. [Google Scholar] [CrossRef]
- Peyton, K.J.; Liu, X.M.; Shebib, A.R.; Johnson, F.K.; Johnson, R.A.; Durante, W. Arginase inhibition prevents the development of hypertension and improves insulin resistance in obese rats. Amino Acids 2018, 50, 747–754. [Google Scholar] [CrossRef]
- Swinburn, B.A.; Kraak, V.I.; Allender, S.; Atkins, V.J.; Baker, P.I.; Bogard, J.R.; Brinsden, H.; Calvillo, A.; De Schutter, O.; Devarajan, R.; et al. The global syndemic of obesity, undernutrition, and climate change: The lancet commission report. Lancet 2019, 393, 791–846. [Google Scholar] [CrossRef]
- World Health Organization. World Health Statistics 2018: Monitoring Health for the SDGs; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.M.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Patterson, E.; Ryan, P.M.; Cryan, J.F.; Dinan, T.G.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Gut microbiota, obesity and diabetes. Postgrad. Med. J. 2016, 92, 286–300. [Google Scholar] [CrossRef]
- Jang, H.M.; Jang, H.M.; Han, S.K.; Kim, J.K.; Oh, S.J.; Jang, H.B.; Kim, D.H. Lactobacillus sakei alleviates high-fat-diet-induced obesity and anxiety in mice by inducing AMPK activation and SIRT1 expression and inhibiting gut microbiota-mediated NF-κB activation. Mol. Nutr. Food Res. 2019, 63, 1800978. [Google Scholar] [CrossRef]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Izzo, A.A.; et al. Gut microbiota and obesity: A role for probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef]
- Boulange, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016, 8, 42. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Schneeberger, M.; Everard, A.; Gómez-Valadés, A.G.; Matamoros, S.; Ramírez, S.; Delzenne, N.M.; Gomis, R.; Claret, M.; Cani, P.D. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci. Rep. 2015, 5, 16643. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Dahiya, D.K.; Puniya, M.; Shandilya, U.K.; Dhewa, T.; Kumar, N.; Kumar, S.; Puniya, A.K.; Shukla, P. Gut Microbiota Modulation and Its Relationship with Obesity Using Prebiotic Fibers and Probiotics: A Review. Front Microbiol. 2017, 8, 563. [Google Scholar] [CrossRef]
- Svane, M.S.; Jørgensen, N.B.; Bojsen-Møller, K.N.; Dirksen, C.; Nielsen, S.; Kristiansen, V.B.; Toräng, S.; Wewer Albrechtsen, N.J.; Rehfeld, J.F.; Hartmann, B.; et al. Peptide YY and glucagon-like peptide-1 contribute to decreased food intake after Roux-en-Y gastric bypass surgery. Int. J. Obes. (Lond.) 2016, 40, 1699–1706. [Google Scholar] [CrossRef]
- Yadav, H.; Lee, J.H.; Lloyd, J.; Walter, P.; Rane, S.G. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J. Biol. Chem. 2013, 288, 25088–25097. [Google Scholar] [CrossRef]
- Kim, K.-A.; Jeong, J.-J.; Kim, D.-H. Lactobacillus brevis OK56 ameliorates high-fat diet-induced obesity in mice by inhibiting NF-κB activation and gut microbial LPS production. J. Funct. Foods 2015, 13, 183–191. [Google Scholar] [CrossRef]
- De Vuyst, L.; Van Kerrebroeck, S.; Harth, H.; Huys, G.; Daniel, H.M.; Weckx, S. Microbial ecology of sourdough fermentations: Diverse or uniform? Food Microbiol. 2014, 37, 11–29. [Google Scholar] [CrossRef]
- Gobbetti, M.; Angelis, M.D.; Cagno, R.D.; Calasso, M.; Archetti, G.; Rizzello, C.G. Novel insights on the functional/nutritional features of the sourdough fermentation. Int. J. Food Microbiol. 2018, 302, 103–113. [Google Scholar] [CrossRef]
- Li, Y.; Liu, T.; Zhang, X.; Zhao, M.; Zhang, H.; Feng, F. Lactobacillus plantarum helps to suppress body weight gain, improve serum lipid profile and ameliorate low-grade inflammation in mice administered with glycerol monolaurate. J. Funct. Foods 2019, 53, 54–61. [Google Scholar] [CrossRef]
- Li, Y.; Liu, T.; Zhang, X.; Zhao, M.; Zhang, H.; Feng, F. In vitro and in vivo investigations of probiotic properties of lactic acid bacteria isolated from Chinese traditional sourdough. Appl. Microbiol. Biotechnol. 2019, 103, 1893–1903. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, M.; Zhang, H.; Li, Y.; Liu, M.; Feng, F. Antimicrobial Emulsifier-Glycerol Monolaurate Induces Metabolic Syndrome, Gut Microbiota Dysbiosis, and Systemic Low-Grade Inflammation in Low-Fat Diet Fed Mice. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef]
- Toral, M.; Gómez-Guzmán, M.; Jiménez, R.; Romero, M.; Sánchez, M.; Utrilla, M.P.; Garrido-Mesa, N.; Rodríguez-Cabezas, M.E.; Olivares, M.; Gálvez, J.; et al. The probiotic Lactobacillus coryniformis CECT5711 reduces the vascular pro-oxidant and pro-inflammatory status in obese mice. Clin. Sci. 2014, 127, 33–45. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Barathikannan, K.; Chelliah, R.; Rubab, M.; Daliri, E.B.; Elahi, F.; Kim, D.H.; Agastian, P.; Oh, S.Y.; Oh, D.H. Gut Microbiome Modulation Based on Probiotic Application for Anti-Obesity: A Review on Efficacy and Validation. Microorganisms 2019, 7, 456. [Google Scholar] [CrossRef]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Kubota, N.; Terauchi, Y.; Miki, H.; Tamemoto, H.; Yamauchi, T.; Komeda, K.; Satoh, S.; Nakano, R.; Ishii, C.; Sugiyama, T.; et al. PPARγ mediates high-fat diet–induced adipocyte hypertrophy and insulin resistance. Mol. Cell 1999, 4, 597–609. [Google Scholar] [CrossRef]
- Roden, M.; Petersen, K.; Shulman, G. Insulin resistance in type 2 diabetes. Textb. Diabetes 2017, 61, 174–186. [Google Scholar]
- Kadowaki, T.; Yamauchi, T. Adiponectin and adiponectin receptors. Endocr. Rev. 2005, 26, 439–451. [Google Scholar] [CrossRef]
- Wang, Z.V.; Scherer, P.E. Adiponectin, the past two decades. J. Mol. Cell Biol. 2016, 8, 93–100. [Google Scholar] [CrossRef]
- Karimi, G.; Jamaluddin, R.; Mohtarrudin, N.; Ahmad, Z.; Khazaai, H.; Parvaneh, M. Single-species versus dual-species probiotic supplementation as an emerging therapeutic strategy for obesity. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 910–918. [Google Scholar] [CrossRef]
- Rather, S.A.; Pothuraju, R.; Sharma, R.K.; De, S.; Mir, N.A.; Jangra, S. Anti-obesity effect of feeding probiotic dahi containingLactobacillus caseiNCDC 19 in high fat diet-induced obese mice. Int. J. Dairy Technol. 2014, 67, 504–509. [Google Scholar] [CrossRef]
- Pan, H.; Guo, J.; Su, Z. Advances in understanding the interrelations between leptin resistance and obesity. Physiol. Behav. 2014, 130, 157–169. [Google Scholar] [CrossRef]
- Hersoug, L.G.; Moller, P.; Loft, S. Gut microbiota-derived lipopolysaccharide uptake and trafficking to adipose tissue: Implications for inflammation and obesity. Obes. Rev. 2016, 17, 297–312. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Wang, J.; Tang, H.; Zhang, C.; Zhao, Y.; Derrien, M.; Rocher, E.; van-Hylckama Vlieg, J.E.; Strisse, l.K.; Zhao, L.; Obin, M.; et al. Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME J. 2015, 9, 1–15. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, K.; Sun, Y.; Kuo, S.M. Dietary or supplemental fermentable fiber intake reduces the presence of Clostridium XI in mouse intestinal microbiota: The importance of higher fecal bacterial load and density. PLoS ONE 2018, 13, e0205055. [Google Scholar] [CrossRef]
- Kverka, M.; Zakostelska, Z.; Klimesova, K.; Sokol, D.; Hudcovic, T.; Hrncir, T.; Rossmann, P.; Mrazek, J.; Kopecny, J.; Verdu, E.F.; et al. Oral administration of Parabacteroides distasonis antigens attenuates experimental murine colitis through modulation of immunity and microbiota composition. Clin. Exp. Immunol. 2011, 163, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.R.; Lin, C.S.; Chang, C.J.; Lin, T.L.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Lu, C.C.; Young, J.D.; Lai, H.C. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut 2019, 68, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 2019, 26, 222–235.e5. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, G.; Yao, Y.; Guo, S.; Lu, K.; Sheng, Z. The role of bifidobacteria in gut barrier function after thermal injury in rats. J. Trauma Acute Care Surg. 2006, 61, 650–657. [Google Scholar] [CrossRef]
- Neves, A.L.; Coelho, J.; Couto, L.; Leite-Moreira, A.; Roncon-Albuquerque, R., Jr. Metabolic endotoxemia: A molecular link between obesity and cardiovascular risk. J. Mol. Endocrinol. 2013, 51, R51–R64. [Google Scholar] [CrossRef] [PubMed]
- Le, T.K.C.; Hosaka, T.; LE, T.T.; Nguyen, T.G.; Tran, Q.B.; LE, T.H.; Pham, X.D. Oral administration of Bifidobacterium spp. improves insulin resistance, induces adiponectin, and prevents inflammatory adipokine expressions. Biomed. Res. 2014, 35, 303–310. [Google Scholar] [CrossRef]
- Yang, J.Y.; Lee, Y.S.; Kim, Y.; Lee, S.H.; Ryu, S.; Fukuda, S.; Hase, K.; Yang, C.S.; Lim, H.S.; Kim, M.S.; et al. Gut commensal Bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol. 2017, 10, 104–116. [Google Scholar] [CrossRef] [PubMed]


| Ingredients (g/100 g Diet) | Normal Chow Diet | High Fat Diet |
|---|---|---|
| Casein | 18.96 | 23.31 |
| L-Cystine | 0.28 | 0.35 |
| Corn Starch | 29.86 | 8.48 |
| Maltodextrin | 3.32 | 11.65 |
| Sucrose | 33.17 | 20.14 |
| Cellulose | 4.74 | 5.83 |
| Soybean Oil | 2.37 | 2.91 |
| Lard | 1.90 | 20.68 |
| Mineral Mix | 2.68 | 3.31 |
| Potassium Citrate, 1 H2O | 1.56 | 1.92 |
| Vitamin Mix | 0.95 | 1.16 |
| Choline Bitartrate | 0.19 | 0.23 |
| Calories supplementation (kcal %) | ||
| Proteins | 20 | 20 |
| Carbohydrates | 70 | 35 |
| Fats | 10 | 45 |
| Total calories (kcal/100 g diet) | 385 | 473 |
| Organs and Tissues | Weight of Organs and Adipose Tissue (% of Final Body Weight) | ||
|---|---|---|---|
| NCD | HFD | HFT | |
| Liver | 3.57 ± 0.28 a | 3.26 ± 0.23 b | 3.22 ± 0.16 b |
| Kidney | 1.38 ± 0.06 a | 1.19 ± 0.07 b | 1.19 ± 0.08 b |
| Spleen | 0.26 ± 0.03 | 0.25 ± 0.03 | 0.23 ± 0.02 |
| Epididymal fat | 1.71 ± 0.31 a | 4.12 ± 0.78 b | 3.75 ± 0.85 b |
| α-Diversity | Mean Value | p Value | ||
|---|---|---|---|---|
| NCD | HFD | HFT | ||
| Chao1 | 300.2 a | 227.3 b | 236.0 b | 0.00019 |
| Observed_species | 263.7 a | 202.2 b | 213.3 b | 0.00015 |
| PD_whole_tree | 18.03 a | 14.49 b | 15.26 b | 0.00007 |
| Shannon | 5.568 a | 5.104 b | 5.158 b | 0.01402 |
| Simpson | 0.9576 a | 0.9404 b | 0.9444 ab | 0.01292 |
| Goods_coverage | 0.9983 a | 0.9989 b | 0.9987 b | 0.00207 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Li, Y.; Zhao, M.; Mo, Q.; Feng, F. Weight-Reducing Effect of Lactobacillus Plantarum ZJUFT17 Isolated from Sourdough Ecosystem. Nutrients 2020, 12, 977. https://doi.org/10.3390/nu12040977
Liu T, Li Y, Zhao M, Mo Q, Feng F. Weight-Reducing Effect of Lactobacillus Plantarum ZJUFT17 Isolated from Sourdough Ecosystem. Nutrients. 2020; 12(4):977. https://doi.org/10.3390/nu12040977
Chicago/Turabian StyleLiu, Tongjie, Yang Li, Minjie Zhao, Qiufen Mo, and Fengqin Feng. 2020. "Weight-Reducing Effect of Lactobacillus Plantarum ZJUFT17 Isolated from Sourdough Ecosystem" Nutrients 12, no. 4: 977. https://doi.org/10.3390/nu12040977
APA StyleLiu, T., Li, Y., Zhao, M., Mo, Q., & Feng, F. (2020). Weight-Reducing Effect of Lactobacillus Plantarum ZJUFT17 Isolated from Sourdough Ecosystem. Nutrients, 12(4), 977. https://doi.org/10.3390/nu12040977








